Abstract
Depletion of the Bacillus subtilis GTPase CpgA produces abnormal cell shapes, nonuniform deposition of cell wall, and five- to sixfold accumulation of peptidoglycan precursors. Nevertheless, the inherent structure of the cell wall appeared mostly unchanged. The results are consistent with CpgA being involved in coordinating normal peptidoglycan deposition.
The gene cpgA, encoding a multidomain GTPase, is located in a conserved gene cluster, downstream of and cotranscribed with two genes prpC and prkC, encoding, respectively, a Ser/Thr phosphatase and a sensor kinase (18, 27, 28, 31). PrkC contains an external domain with three PASTA repeats that are likely involved in binding peptidoglycan (34), while its Mycobacterium tuberculosis homologue, PknB, has been implicated in cell wall synthesis (20). We have recently shown that cells depleted for CpgA display marked morphological changes, including swollen cells and a variety of bizarre forms (8). Production of the peptidoglycan sacculus and rod shape morphology in B. subtilis is dependent on penicillin-binding proteins (PBPs) with transglycosylase and transpeptidase activities (17). However, recent evidence indicates that the actin-like proteins MreB, Mbl, and MreBH form a cytoskeletal structure under the membrane that is also required for the assembly of peptidoglycan (5, 6, 10, 14). We now suggest that, while prkC and prpC are involved in monitoring the status of peptidoglycan, CpgA (YloQ), based on its structure (23, 24), low abundance, and probable association with ribosomes (4, 8, 9, 16), is a translation factor affecting production of certain proteins involved in morphology determination. We examined in detail here the possible nature of the cell wall defect in cells depleted of CpgA.
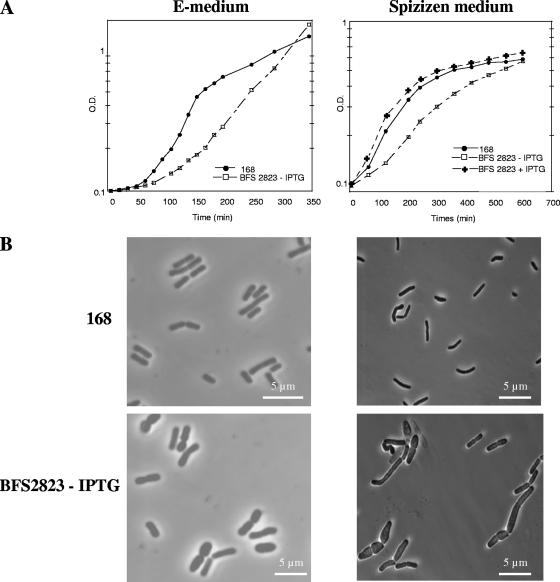
We used a strain (BFS2823) with a copy of cpgA controlled by a pspac promoter in the chromosome (8, 15), allowing depletion of CpgA (confirmed by reverse transcription-PCR [data not shown]) in exponentially growing cells in E-medium, in the absence of IPTG (isopropyl-β-d-thiogalactopyranoside). Under these conditions, as shown in Fig. 1A, we observed a reduced growth rate (generation time of 56 min compared to 31 min for the nondepleted strain) and major morphological changes, with many swollen and irregular-shaped cells, as shown previously (8). The minimal E-medium (25) contains a low concentration of phosphate, but supplementation with increased phosphate had no effect on growth or cellular morphology. A significant increase in generation time (170 min compared to 110 min for the parental strain) and abnormal shapes were also observed (Fig. 1B) after depletion of CpgA in Spizizen medium (1). In contrast, in cultures of BFS2823 in medium grown with IPTG (Fig. 1) the cells appeared normal. However, as we observed previously (8), growth of depleted cultures in LB was not affected, although a small number of longer cells were observed (all data not shown). Therefore, the effect on growth rate and the greatly perturbed morphology, after depletion of CpgA, may be expressed in media supporting slow growth rates, indicating that CpgA is essential for normal growth under these conditions (see also reference 21).
FIG. 1.
(A) Effect of depletion of CpgA on growth. Cultures were grown at 37°C for strain 168 trpC (•), its derivative BFS2823 without IPTG (□) or BFS2823 with 1 mM IPTG (✚) in E-medium or Spizizen medium. BFS2823 expresses cpgA from a pspac promoter. (B) Morphology defect of CpgA-depleted cells. Phase-contrast micrographs of strain 168 (wild type) and CpgA-depleted cells (BFS2823) grown either in E-medium or Spizizen medium, sampled at and optical density (OD) of 0.4, are shown.
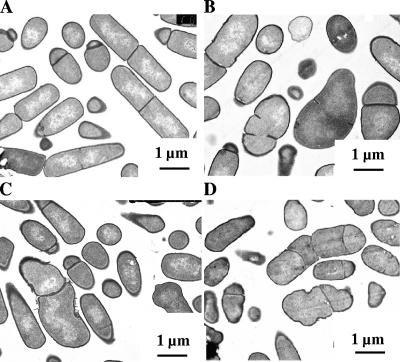
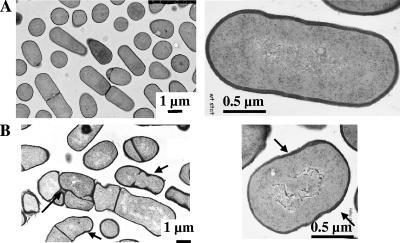
To obtain more information on the nature of the abnormal morphology in CpgA depleted cells, we carried out a detailed study of electron microscopic (EM) sections of the normal and depleted cells in E-medium with sampling for EM sectioning and staining with uranyl acetate as described previously (26). In the absence of IPTG, up to 70% of cells displayed a range of marked abnormalities throughout the growth phase (Fig. 2 and 3), including swollen, coccoid-like cells and a variety of bizarre shapes. Importantly, in many cells, there appeared to be an irregular deposition of cell wall, producing various levels of thickness (see arrows in Fig. 3), compared to the wild type. This suggested impairment of the expansion of the cell wall, normally involving uniform deposition or distribution of the multilayered cell wall throughout the cell. Finally, up to 15% of the depleted cells showed multiple or otherwise abnormal septal cleavage planes (Fig. 3B). We confirmed that these shape changes were abolished in the presence of IPTG or when depleted cells of strain OMG501, derived from BFS2823 but ectopically expressing cpgA from a xylose dependent copy of cpgA (8), were used (data not shown).
FIG. 2.
EM sections of CpgA-depleted cells throughout the growth phase. Strain 168 (A) and strain BFS2823 (B to D), both grown in E-medium without IPTG, are shown. EM sections (samples were fixed with 2.5% glutaraldehyde in cacodylate buffer and stained with 1% uranyl acetate, and ultrathin sections were contrasted with lead acetate) were prepared as described previously (26) and are shown at different stages of the growth phase. (A) Mid-exponential phase, OD = 0.4; (B) OD = 0.4; (C) OD = 0.8; (D) stationary phase. In contrast to strain 168, a variety of misshapen cells with some abnormal division planes are visible throughout the growth phase with depleted cells. BFS2823 with IPTG or a derivative of BFS2823, ectopically expressing cpgA from a xyl promoter, produces largely normal cells (data not shown).
FIG. 3.
EM sections of CpgA-depleted cells reveal nonuniform deposition of cell wall. (A) Wild-type strain 168; (B) BFS2823 grown without IPTG in E-medium at 37°C, with nonuniform deposition of cell wall (arrows) and multiple aberrant division planes in a minority of cells.
These results indicated that in depleted cells, the nature of the cell envelope, probably the peptidoglycan, the major shape determinant, and/or its assembly, were perturbed. However, with depleted cells grown in E-medium, plus or minus IPTG (1 mM), in plate and liquid tests, we found no change in sensitivity (exponential- or stationary-phase cells) to ampicillin (PBP target), vancomycin (binds to late peptidoglycan precursors), or fosfomycin (inhibitor of MurA, first enzyme in the biosynthesis of peptidoglycan) (19). However, with cefuroxime or cefotaxime (cephalosporins also targeting PBPs) the depleted cells were more sensitive, with a reduced MIC in Spizizen medium, 0.25 μg/ml compared to 1 μg/ml for nondepleted cells. The depleted cells, in contrast, displayed a level of sensitivity to osmotic shock (4% NaCl), Triton X (0.01 to 0.5%), or EGTA (25 mM) identical to that of the nondepleted cells or wild-type strain 168 (data not shown).
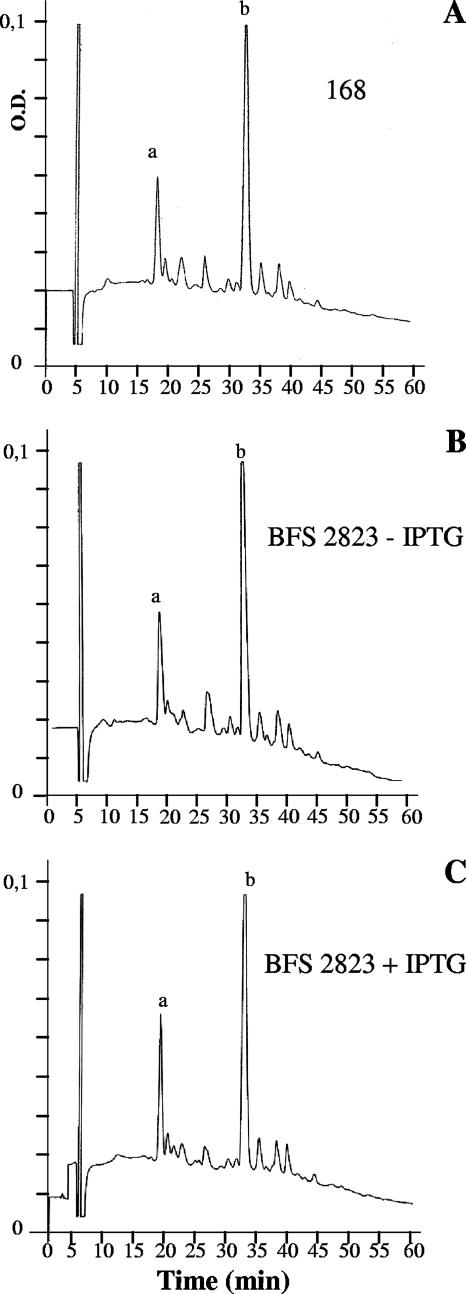
We also measured in depleted cells the level of teichoic acids (from cell wall phosphorus content, as described previously [11]), the major PBPs 1 to 5 (measured as described previously [12, 35]), the cytoskeleton protein Mbl and its association with the membrane (using Western blotting and microscopy analysis of an Mbl-GFP fusion as described in reference 18), peptidoglycan (diaminopimelic acid content [3]) and, as shown in Fig. 4, the muropeptide composition (determined by high-pressure liquid chromatography [HPLC] analysis). In every case, the depleted cells were essentially normal, compared to nondepleted cells or the wild type. Finally, in contrast to cells depleted of Mbl, MreB, MreBH (7, 14), or MreC/D, implicated in morphogenesis through the coupling of Mbl cables to sites of extracellular cell wall synthesis (22), we found that the shape changes in CpgA-depleted cells were not reversed by 10 mM magnesium (data not shown), providing more evidence that morphogenetic factors, compromised in some way in CpgA-depleted cells, did not involve specific reductions in the major cytoskeleton components. Interestingly, however, EM sections of cells depleted of MreD, were also previously reported to show irregular deposition of cell wall material (22), indicating that MreD and CpgA may function in the same pathway.
FIG. 4.
HPLC analysis of the muropeptides in peptidoglycan from depleted and nondepleted strains. (A) Strain 168; (B) BFS2823 without IPTG; (C) BFS2823 with IPTG. Purified peptidoglycan (2) was digested with cellosyl N-acetylmuramidase, and the resulting muropeptides were reduced as described previously (3). The muropeptide mixtures were applied to a 5-μm Vydac C18 reversed-phase column and eluted as described previously (3) a, main monomer; b, main dimer.
From all of these above studies, the obvious defect in normal cell wall deposition (EM sections and precursor accumulation) could not be correlated, with the exception of some sensitivity to cephalosporins, with chemical defects in the assembled peptidoglycan. Finally, we therefore determined whether the normal flow of peptidoglycan precursors was maintained in cells depleted of CpgA. As before, cells from strain 168 or strain BFS2823, in E-medium, with or without 1 mM IPTG, were harvested, and peptidoglycan precursors were extracted from the cytoplasm and quantified as described previously (3, 13, 29, 30). Assembly of B. subtilis peptidoglycan monomer units proceeds by a linear sequence of reactions via a series of UDP nucleotide precursors and lipid intermediates (33). The cytoplasmic steps culminate in the formation of the UDP-MurNAc pentapeptide precursor from UDP-N-acetylglucosamine (UDP-GlcNAc). In fact, as shown in Table 1, CpgA depletion resulted in a reproducible five- to sixfold increase in the level of UDP-MurNAc pentapeptide, with a similar increase in the immediate upstream precursors, the di- and tripeptides. In contrast, there was no significant change in the pool of the early precursor, UDP-GlcNAc. This indicated a bottleneck in the depleted cells, causing precursors to accumulate as the coordinated distribution of precursors into the expanding cell wall to ensure uniform construction of the multilayered sacculus was disrupted. Such a bottleneck would be consistent with the observed nonuniform deposition of cell wall material in the depleted strain, revealed by the EM analysis.
TABLE 1.
Peptidoglycan nucleotide precursor pools in cells from strain 168 and strain BFS2823 with or without IPTG
| Strain | Pool level (pmol/mg of cell protein)a
|
|||
|---|---|---|---|---|
| UDP-MurNAc pentapeptide | UDP-MurNAc tripeptide | UDP-MurNAc dipeptide | UDP- GlcNAc | |
| 168 | 90 | 3 | 8 | 162 |
| BFS2823 (without IPTG) | 530 | 23 | 114 | 187 |
| BFS2823 (with IPTG) | 60 | 5 | ND | 196 |
The peptidoglycan precursor fraction was obtained from a 2-liter exponential culture in E-medium as previously described (3). After a gel filtration step (Sephadex G-25); excluded fractions absorbing at 262 nm were pooled, lyophilized, and analyzed by HPLC as described previously (29, 30). Values are the average of two measurements. ND, not determined.
In conclusion, we suggest that CpgA acts as a translation factor to control the synthesis of certain factors required for a late step in biogenesis of peptidoglycan involving coordinated deposition of a uniform cell wall layer. When CpgA is depleted, we envisage that such factors (although not apparently Mbl or a major PBP) become limiting. Then, although peptidoglycan precursors are apparently largely inserted and cross-linked normally, a final step in the coordinated spatial deposition throughout the cell is perturbed, resulting in the accumulation of precursors, a cell wall of varying thickness and abnormal cell morphology. Importantly, we now have evidence that CpgA is a substrate, at least in vitro, for the kinase PrkC and the phosphatase PrpC (unpublished data), providing further support for functional linkage of both proteins in peptidoglycan biogenesis and homeostatic control.
Acknowledgments
We thank Frederic Sapunaric for PBP analysis. We also appreciate the contributions of ERASMUS students Ania Kucharska and Michal Kuzdahl.
We acknowledge the support of the CNRS and the University of Paris-Sud. C.A. received support from the Fondation pour la Recherche Médicale. R.C.-L. acknowledges the support of INRA.
Footnotes
Published ahead of print on 14 March 2008.
REFERENCES
- 1.Anagnostopoulos, C., and J. Spizizen. 1961. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 81741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atrih, A., G. Bacher, G. Allmaier, M. P. Williamson, and S. J. Foster. 1999. Analysis of peptidoglycan structure from vegetative cells of Bacillus subtilis 168 and role of PBP 5 in peptidoglycan maturation. J. Bacteriol. 1813956-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billot-Klein, D., L. Gutmann, S. Sablé, E. Guittet, and J. van Heijenoort. 1994. Modification of peptidoglycan precursors is a common feature of the low-level vancomycin resistant VANB-type Enterococcus D366 and of the naturally glycopeptide-resistant species Lactobacillus casei, Pediococcus pentosaceus, Leuconostoc mesenteroides, and Enterococcus gallinarum. J. Bacteriol. 1762398-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell, T. L., D. M. Daigle, and E. D. Brown. 2005. Characterization of the Bacillus subtilis GTPase YloQ and its role in ribosome function. Biochem. J. 389843-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carballido-López, R. 2006. Orchestrating bacterial cell morphogenesis. Mol. Microbiol. 60815-819. [DOI] [PubMed] [Google Scholar]
- 6.Carballido-López, R., and J. Errington. 2003. The bacterial cytoskeleton: in vivo dynamics of the actin-like protein Mbl of Bacillus subtilis. Dev. Cell 419-28. [DOI] [PubMed] [Google Scholar]
- 7.Carballido-López, R., A. Formstone, Y. Li, S. D. Ehrlich, P. Noirot, and J. Errington. 2006. Actin homolog MreBH governs cell morphogenesis by localization of the cell wall hydrolase LytE. Dev. Cell 11399-409. [DOI] [PubMed] [Google Scholar]
- 8.Cladière, L., K. Hamze, E. Madec, V. M. Levdikov, A. J. Wilkinson, I. B. Holland, and S. J. Séror. 2006. The GTPase, CpgA(YloQ), a putative translation factor, is implicated in morphogenesis in Bacillus subtilis. Mol. Genet. Genomics 275409-420. [DOI] [PubMed] [Google Scholar]
- 9.Daigle, D. M., and E. D. Brown. 2004. Studies of the interaction of Escherichia coli YjeQ with the ribosome in vitro. J. Bacteriol. 1861381-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel, R. A., and J. Errington. 2003. Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell 113767-776. [DOI] [PubMed] [Google Scholar]
- 11.De Siervo, A. J. D. 1969. Alterations in the phospholipid composition of Escherichia coli B during growth at different temperatures. J. Bacteriol. 1001342-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eymann, C., A. Dreisbach, D. Albrecht, J. Bernhardt, D. Becher, S. Gentner, L. T. Tam, K. Büttner, G. Buurman, C. Scharf, S. Venz, U. Völker, and M. Hecker. 2004. A comprehensive proteome map of growing Bacillus subtilis cells. Proteomics 42849-2876. [DOI] [PubMed] [Google Scholar]
- 13.Flouret, B., D. Mengin-Lecreulx, and J. van Heijenoort. 1981. Reverse-phase high pressure liquid chromatography of uridine diphosphate N-acetylmuramyl peptide precursors of bacterial cell wall peptidoglycan. Anal. Biochem. 11459-63. [DOI] [PubMed] [Google Scholar]
- 14.Formstone, A., and J. Errington. 2005. A magnesium-dependent mreB null mutant: implications for the role of mreB in Bacillus subtilis. Mol. Microbiol. 551646-1657. [DOI] [PubMed] [Google Scholar]
- 15.Foulger, D., and J. Errington. 1998. A 28-kbp segment from the spoVM region of the Bacillus subtilis 168 genome. Microbiology 144801-805. [DOI] [PubMed] [Google Scholar]
- 16.Himeno, H., K. Hanawa-Suetsugu, T. Kimura, K. Takagi, W. Sugiyama, S. Shirata, T. Mikami, F. Odagiri, Y. Osanai, D. Watanabe, S. Goto, L. Kalachnyuk, C. Ushida, and A. Muto. 2004. A novel GTPase activated by the small subunit of ribosome. Nucleic Acids Res. 325303-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Höltje, J. V. 1998. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol. Mol. Biol. Rev. 62181-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwanicki, A., K. Hinc, S. Seror, G. Wegrzyn, and M. Obuchowski. 2005. Transcription in the prpC-yloQ region in Bacillus subtilis. Arch. Microbiol. 183421-430. [DOI] [PubMed] [Google Scholar]
- 19.Kahan, F. M., J. S. Kahan, P. J. Cassidy, and H. Kropp. 1974. The mechanism of action of fosfomycin (phosphonomycin). Ann. N. Y. Acad. Sci. 235364-386. [DOI] [PubMed] [Google Scholar]
- 20.Kang, C.-M., D. W. Abbott, S. T. Park, C. C. Dascher, L. C. Cantley, and R. N. Husson. 2005. The Mycobacterium tuberculosis serine/threonine kinases PknA and PknB: substrate identification and regulation of cell shape. Genes Dev. 191692-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi, K., S. D. Ehrlich, A. Albertini, G. Amati, K. K. Andersen, M. Arnaud, K. Asai, S. Ashikaga, S. Aymerich, et al. 2003. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA 1004678-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leaver, M., and J. Errington. 2005. Roles for MreC and MreD proteins in helical growth of the cylindrical cell wall in Bacillus subtilis. Mol. Microbiol. 571196-1209. [DOI] [PubMed] [Google Scholar]
- 23.Leipe, D. D., Y. I. Wolf, E. V. Koonin, and L. Aravind. 2002. Classification and evolution of P-loop GTPases and related ATPases. J. Mol. Biol. 31741-72. [DOI] [PubMed] [Google Scholar]
- 24.Levdikov, V. M., E. V. Blagova, J. A. Brannigan, L. Cladière, A. A. Antson, M. N. Isupov, S. J. Séror, and A. J. Wilkinson. 2004. The crystal structure of YloQ, a circularly permuted GTPase essential for Bacillus subtilis viability. J. Mol. Biol. 340767-782. [DOI] [PubMed] [Google Scholar]
- 25.Lévine, A., F. Vannier, C. Absalon, L. Kuhn, P. Jackson, E. Scrivener, J. V. Labas, J. Vinh, P. Courtney, J. Garin, and S. J. Séror. 2006. Analysis of the dynamic Bacillus subtilis Ser/Thr/Tyr phosphoproteome implicated in a wide variety of cellular processes. Proteomics 62157-2173. [DOI] [PubMed] [Google Scholar]
- 26.Londoño-Vallejo, J. A., C. Fréhel, and P. Stragier. 1997. SpoIIQ, a forespore expressed gene required for engulfment in Bacillus subtilis. Mol. Microbiol. 2429-39. [DOI] [PubMed] [Google Scholar]
- 27.Madec, E., A. Laszkiewicz, A. Iwanicki, M. Obuchowski, and S. Séror. 2002. Characterization of a membrane-linked Ser/Thr protein kinase in Bacillus subtilis, implicated in developmental processes. Mol. Microbiol. 46571-586. [DOI] [PubMed] [Google Scholar]
- 28.Madec, E., A. Stensballe, S. Kjellström, L. Cladière, M. Obuchowski, O. N. Jensen, and S. J. Séror. 2003. Mass spectrometry and site-directed mutagenesis identify several autophosphorylated residues required for the activity of PrkC, a Ser/Thr kinase from Bacillus subtilis. J. Mol. Biol. 330459-472. [DOI] [PubMed] [Google Scholar]
- 29.Mengin-Lecreulx, D., B. Flouret, and J. van Heijenoort. 1982. Cytoplasmic steps of peptidoglycan synthesis in Escherichia coli. J. Bacteriol. 1511109-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mengin-Lecreulx, D., B. Flouret, and J. van Heijenoort. 1983. Pool levels of UDP N-acetylglucosamine and UDP N-acetylglucosamine-enolpyruvate in Escherichia coli and correlation with peptidoglycan synthesis. J. Bacteriol. 1541284-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obuchowski, M., E. Madec, G. D. Delattre, G. Boël, A. Iwanicki, D. Foulger, and S. J. Séror. 2000. Characterization of PrpC from Bacillus subtilis, a member of the PPM phosphatase family. J. Bacteriol. 1825634-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenthal, S., and N. Sharon. 1964. The use of Sephadex G-25 for the isolation of nucleotide sugar derivatives from Micrococcus lysodeikticus. Biochim. Biophys. Acta 83378-380. [DOI] [PubMed] [Google Scholar]
- 33.van Heijenoort, J. 2001. Recent advances in the formation of the bacterial peptidoglycan monomer unit. Nat. Prod. Rep. 18503-519. [DOI] [PubMed] [Google Scholar]
- 34.Yeats, C., R. D. Finn, and A. Bateman. 2002. The PASTA domain: a beta-lactam binding domain. Trends Biochem. Sci. 27438. [DOI] [PubMed] [Google Scholar]
- 35.Zorzi, W., X. Y. Zhou, O. Dardenne, J. Lamotte, D. Raze, J. Pierre, L. Gutmann, and J. Coyette. 1996. Structure of the low-affinity penicillin-binding protein 5 PBP5fm in wild-type and highly penicillin-resistant strains of Enterococcus faecium. J. Bacteriol. 1784948-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]