Abstract
Two surface organelles of uropathogenic Escherichia coli (UPEC), flagella and type 1 fimbriae, are critical for colonization of the urinary tract but mediate opposite actions. Flagella propel bacteria through urine and along mucus layers, while type 1 fimbriae allow bacteria to adhere to specific receptors present on uroepithelial cells. Constitutive expression of type 1 fimbriae leads to repression of motility and chemotaxis in UPEC strain CFT073, suggesting that UPEC may coordinately regulate motility and adherence. To identify genes involved in this regulation of motility by type 1 fimbriae, transposon mutagenesis was performed on a phase-locked type 1 fimbrial ON variant of strain CFT073 (CFT073 fim L-ON), followed by a screen for restoration of motility in soft agar. Functions of the genes identified included attachment, metabolism, transport, DNA mismatch repair, and transcriptional regulation, and a number of genes had hypothetical function. Isogenic deletion mutants of these genes were also constructed in CFT073 fim L-ON. Motility was partially restored in six of these mutants, including complementable mutations in four genes encoding known transcriptional regulators, lrhA, lrp, slyA, and papX; a mismatch repair gene, mutS; and one hypothetical gene, ydiV. Type 1 fimbrial expression in these mutants was unaltered, and the majority of these mutants expressed larger amounts of flagellin than the fim L-ON parental strain. Our results indicate that repression of motility in CFT073 fim L-ON is not solely due to the constitutive expression of type 1 fimbriae on the surfaces of the bacteria and that multiple genes may contribute to this repression.
Motility and adherence are two integral aspects of bacterial pathogenesis. Adherence, often mediated by fimbriae, permits bacteria to adhere to a cell surface and establish an infection, whereas flagellum-driven motility allows bacteria to move to more-advantageous sites for colonization. Both fimbriae and flagella have been shown to be important in the pathogenesis of uropathogenic Escherichia coli (UPEC). Type 1 fimbriae, expressed on the surfaces of E. coli and most of the Enterobacteriaceae (11), have been shown to be critical for colonization of the bladder by UPEC (27), and accordingly, genes encoding these fimbriae are highly expressed in vivo (53). While type 1 fimbriae allow UPEC to bind mannose-containing glycoprotein receptors, such as uroplakin (46, 61), and establish infection in the bladder, our laboratory (33) and another (51) have recently shown that flagella allow UPEC to ascend from the bladder to the kidneys. Furthermore, flagellum production contributes to the fitness of UPEC during murine urinary tract colonization (34, 60).
Flagella are complex organelles that are encoded by over 40 genes (reviewed in references 14 and 55). Genes for flagellum synthesis form a complex and tightly regulated transcriptional hierarchy consisting of three classes. The class I genes flhDC encode the master regulator FlhD2C2, required for transcription of the class II genes. Transcription of flhDC is modulated by a number of physiological and environmental signals, such as temperature (2), osmolarity (52), histone-like nucleoid-structuring protein (H-NS) (5), and autoregulation (31). Class II genes encode structural and assembly proteins needed for synthesis of the hook-basal body. In addition, two regulatory proteins, FliA (σ28) and FlgM (anti-σ28), are transcribed from class II promoters. FliA is the transcription factor required for expression from the class III promoters (41). These class III genes encode the flagellar filament proteins and other proteins needed for motility and the chemotaxis system.
Expression of type 1 fimbriae is phase variable and controlled by a promoter situated on an invertible element (IE) upstream of the type 1 fimbrial operon (fimAICDFGH) (1). The IE alternates between two orientations, phase ON and phase OFF. When the IE is positioned so that the promoter faces the fim operon, type 1 fimbriae are produced and the bacteria are considered phase ON. In the phase OFF conformation, the promoter faces away and transcription is blocked, resulting in no production of type 1 fimbriae. Switching of the IE between these two phases is mediated by the FimB and FimE recombinases (7, 30, 44) as well as the recently identified FimB- and FimE-like recombinases IpuA and IpbA (10). FimB promotes phase variation between both promoter orientations, while FimE promotes primarily ON-to-OFF switching of the IE (24, 30).
Reciprocal regulation is one mechanism by which bacteria reconcile the contradictory, yet necessary, actions of adherence and motility. It would not be beneficial for an organism tethered to a surface to suddenly attempt swimming or swarming. Also, high-level expression of fimbriae by a swimming organism could sabotage motility. Therefore, it is expected that a strongly fimbriated bacterium would not be highly motile and that a motile organism would not express large numbers of fimbriae. Accordingly, reciprocal regulation of motility and adherence has been observed in a number of pathogenic bacterial groups, including Bordetella pertussis (3), Vibrio cholerae (26), Salmonella enterica serovar Typhimurium (16), and Proteus mirabilis (37). In the uropathogens P. mirabilis and UPEC, proteins encoded within fimbrial operons have been shown to regulate motility. For example, overexpression of MrpJ, a protein encoded by the last gene of the MR/P fimbrial operon, inhibits both swimming and swarming motility in P. mirabilis (37). Also, PapX of UPEC, a homolog of MrpJ encoded by the pheV-associated but not the pheU-associated pap allele of CFT073, caused reduced motility when overexpressed in both P. mirabilis (37) and UPEC (A. Simms and H. L. T. Mobley, unpublished).
Recently, our laboratory (35) and another (10) have shown that constitutive production of type 1 fimbriae by a phase-locked ON variant of UPEC strain CFT073 (CFT073 fim L-ON) leads to a decrease in swimming motility in soft agar. Western blot analysis indicated that this repression of motility is due to a decrease in the level of flagella produced; also, real-time PCR studies demonstrated that constitutive expression of type 1 fimbriae results in decreased transcription of the major flagellin subunit gene, fliC (35). Deletion of the entire region of DNA carrying the structural and accessory genes of type 1 fimbriae partially restored motility in CFT073 fim L-ON (35), suggesting that repression of motility in this strain is not solely due to the constitutive expression of type 1 fimbriae on the surfaces of the bacteria. Here, we used transposon mutagenesis and isogenic deletion mutants of CFT073 fim L-ON to examine other possible mechanisms of regulation that repress motility when type 1 fimbriae are expressed.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
E. coli CFT073 was initially isolated from the blood and urine of a patient with acute pyelonephritis (45). Additionally, the genome of E. coli CFT073 has been sequenced and annotated (59). All relevant E. coli strains and plasmids used in this study are listed in Table 1. E. coli was cultured on Luria-Bertani (LB) agar or in LB broth incubated at 37°C. Antibiotics were added as needed at the following concentrations: nalidixic acid (Nal), 50 μg/ml; ampicillin (Amp), 100 μg/ml; and kanamycin (Kan), 25 μg/ml. In vitro growth curves of E. coli in LB broth were generated in triplicate using a Microbiology Reader Bioscreen C (Oy Growth Curves AB Ltd.) in 0.2-ml volumes; optical density at 600 nm (OD600) was recorded every 15 min.
TABLE 1.
Relevant bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| E. coli | ||
| CFT073 | Wild-type pyelonephritis isolate (O6:K2:H1) | 45 |
| CFT073 fim L-ON | CFT073 fim IE phase locked ON; constitutive type 1 fimbrial expression | 27 |
| CFT073 fim L-ON Nalr | Nalr derivative of CFT073 fim L-ON | This study |
| CFT073 Δlrp | CFT073 Δlrp::kan; replacement of nucleotides 41 to 428 of lrp in CFT073 with the kanamycin resistance gene of pKD4 | This study |
| CFT073 ΔlrhA | CFT073 ΔlrhA::kan; replacement of nucleotides 41 to 889 of lrhA in CFT073 with the kanamycin resistance gene of pKD4 | This study |
| CFT073 ΔmutS | CFT073 ΔmutS::kan; replacement of nucleotides 99 to 2332 of mutS in CFT073 with the kanamycin resistance gene of pKD4 | This study |
| CFT073 ΔydiV | CFT073 ΔydiV::kan; replacement of nucleotides 91 to 552 of ydiV in CFT073 with the kanamycin resistance gene of pKD4 | This study |
| CFT073 ΔslyA | CFT073 ΔslyA::kan; replacement of nucleotides 46 to 345 of slyA in CFT073 with the kanamycin resistance gene of pKD4 | This study |
| CFT073 ΔpapX | CFT073 ΔpapX::kan; replacement of nucleotides 1 to 550 of papX in CFT073 with the kanamycin resistance gene of pKD4 | This study |
| CFT073 fim L-ON Δlrp | CFT073 fim L-ON Δlrp::kan; replacement of lrp (as in CFT073 Δlrp) in CFT073 fim L-ON | This study |
| CFT073 fim L-ON ΔlrhA | CFT073 fim L-ON ΔlrhA::kan; replacement of lrhA (as in CFT073 ΔlrhA) in CFT073 fim L-ON | This study |
| CFT073 fim L-ON ΔmutS | CFT073 fim L-ON ΔmutS::kan; replacement of mutS (as in CFT073 ΔmutS) in CFT073 fim L-ON | This study |
| CFT073 fim L-ON ΔydiV | CFT073 fim L-ON ΔydiV::kan; replacement of ydiV (as in CFT073 ΔydiV) in CFT073 fim L-ON | This study |
| CFT073 fim L-ON ΔslyA | CFT073 fim L-ON ΔslyA::kan; replacement of slyA (as in CFT073 ΔslyA) in CFT073 fim L-ON | This study |
| CFT073 fim L-ON ΔpapX | CFT073 fim L-ON ΔpapX::kan; replacement of papX, as in CFT073 ΔpapX, in CFT073 fim L-ON | This study |
| Plasmids | ||
| pUT/mini-Tn5Km2 | Mini-Tn5 Km2 transposon in pUT suicide vector (Kanr Ampr) | 18 |
| pKD4 | Lambda red recombinase system template plasmid (Kanr Ampr) | 17 |
| pGEN-MCS | Derivative of pGEN222 with multiple cloning site replacing gfpuv (Ampr) | 33 |
| pGEN-lrp | lrp (with native promoter) of CFT073 cloned into BamHI-SphI sites of pGEN-MCS (Ampr) | This study |
| pGEN-lrhA | lrhA (with native promoter) of CFT073 cloned into BamHI-SphI sites of pGEN-MCS (Ampr) | This study |
| pGEN-mutS | mutS (with native promoter) of CFT073 cloned into BamHI-SphI sites of pGEN-MCS (Ampr) | This study |
| pGEN-ydiV | ydiV (with native promoter) of CFT073 cloned into BamHI-SphI sites of pGEN-MCS (Ampr) | This study |
| pGEN-slyA | slyA (with native promoter) of CFT073 cloned into BamHI-SphI sites of pGEN-MCS (Ampr) | This study |
| pLX3607 | pQE60 cloning vector with lacIq cloned into XhoI site (Ampr) | 37 |
| pDRM001 | papX of CFT073 cloned into NcoI-HindIII sites of pLX3607 (Ampr) | 37 |
Transposon mutagenesis of E. coli CFT073 fim L-ON and motility of the resultant Tn5 mutants.
Transposon mutagenesis was performed via a filter-mating conjugation protocol. Both donor [E. coli S17 λ pir (pUT/mini-Tn5Km2)] and recipient (E. coli CFT073 fim L-ON Nalr) strains were cultured overnight in static LB broth cultures with appropriate antibiotics. No difference was observed in growth rate between the nalidixic acid-marked strain CFT073 fim L-ON Nalr and CFT073 fim L-ON (data not shown). Cultures were gently mixed at a 1:4 donor/recipient ratio and placed onto one 0.2-mm-pore-size nitrocellulose filter on an LB agar plate. After 2 hours of incubation at 37°C, the filter was removed and bacteria on the filter were suspended by vigorous vortexing in a small amount of LB broth. This suspension was plated on LB agar containing nalidixic acid and kanamycin to select for the recovery of kanamycin-resistant transposon mutants of CFT073 fim L-ON Nalr.
To evaluate the motilities of kanamycin-resistant transposon mutants of CFT073 fim L-ON Nalr, colonies from the initial mutagenesis were passaged to new LB agar plates containing nalidixic acid and kanamycin. Following overnight growth at 37°C, patched colonies were stabbed, using sterile toothpicks, into soft-agar plates (1% tryptone, 0.5% NaCl, 0.25% agar) without antibiotics. Care was taken to avoid touching the bottom of the plate to avoid possible spread by twitching motility. After 16 h of incubation at 30°C, the diameters of motility were measured for each strain. Motile strains were identified as those in which the diameter of motility was larger than that of the E. coli CFT073 fim L-ON parental control. Wild-type (WT) E. coli CFT073 and CFT073 fim L-ON were always included as controls, and three independent motility experiments for each motile transposon mutant were performed.
Identification of the location of the Tn5 insertion in motile transposon mutants of CFT073 fim L-ON.
Sites of transposon insertion were amplified by arbitrary primed PCR, as described by Burall et al. (12). Arbitrary primed PCR was performed in two sequential amplification steps, with primers complementary to mini-Tn5 ends reading outward and arbitrarily designed primers reading inward from the unknown sequence. For arbitrary PCR 1, primer Arb1 paired with primer 1955 was used. Primer sequences are listed in Table S1 in the supplemental material. The template for these PCRs consisted of a single-colony suspension of mutant E. coli in 25 μl of double-distilled water (ddH2O) boiled for 10 min. PCR amplifications were completed in a DNA Engine PTC-200 thermal cycler (MJ Research). After the initial denaturation for 5 min at 95°C, amplification was completed by cycling 29 times at 95°C for 30 seconds, followed by variable annealing at 53° to 60°C for 30 seconds and 72°C for 90 seconds. The reaction was completed with a final elongation step at 72°C for 5 min. For arbitrary PCR 2, the reagents and conditions were the same, except that the template was a 2-μl sample from arbitrary PCR 1 and different primer sets were used. For arbitrary PCR 2, primers complementary to sequences nested outside the PCR 1 primers, primer 1954 paired with Arb2 (the constant region of primer Arb1), were used (12). Arbitrary PCR 2 products were analyzed by electrophoresis on a 1% (wt/vol) agarose gel, and PCR products were directly cloned into pCR2.1-TOPO (Invitrogen) per the manufacturer's protocols. These reaction products were then transformed into electrocompetent E. coli Top10 (Invitrogen). Plasmid DNA was extracted using a QIAprep Spin mini prep kit (Qiagen), and nucleotide sequencing using the M13 universal forward and reverse primers was performed by the University of Michigan DNA Sequencing Core on purified plasmid DNA.
Construction and complementation of isogenic mutants of CFT073 and CFT073 fim L-ON.
Deletion mutants of genes identified in the transposon screen or other genes of interest were generated in CFT073 or CFT073 fim L-ON, using the lambda red recombinase system designed by Datsenko and Wanner (17). Primers containing sequences homologous to the 5′ and 3′ regions of the targeted genes were designed (H1P1 and H2P2) and used to amplify the kanamycin resistance gene from the template plasmid pKD4 (see Table S1 in the supplemental material). Lambda red-mediated recombination then replaced the specific gene(s) with this resulting PCR product. Kanamycin was used for selection of all mutants. Primers that flank the target gene sequence were designed (see Table S1 in the supplemental material) and used to confirm by PCR whether the kanamycin resistance cassette recombined within the target gene site as described previously (34).
To complement selected mutants of CFT073 and CFT073 fim L-ON, the lrp, lrhA, mutS, ydiV, and slyA genes, with associated promoters, were amplified using primers listed in Table S1 in the supplemental material. Each PCR fragment was digested with BamHI and SphI, followed by ligation into BamHI-SphI-cut pGEN-MCS. pGEN-MCS is a derivative of plasmid pGEN222 (22) in which the gfpuv gene is replaced with a multiple cloning site (33). The resultant plasmid constructs were used to transform their respective mutants, with selection for ampicillin resistance. The constructs were also confirmed by isolation of plasmid DNA, followed by both digestion with the above-mentioned restriction enzymes and PCR using primers outside the BamHI and SphI sites of pGEN-MCS (see Table S1 in the supplemental material). Both papX mutants of CFT073 and CFT073 fim L-ON were complemented by transformation with the plasmid pDRM001 (37). This plasmid contains papX under the control of an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible promoter. However, no IPTG was used in our assays presented here, as we have determined that the background expression of papX from this promoter is sufficient for repression of motility in CFT073 (A. Simms and H. L. T. Mobley, unpublished). Complementation was assessed by restoration of repression of motility in each of the mutants, using the phenotypic assays described below.
Motility of isogenic mutants of CFT073 and CFT073 fim L-ON.
Motility was evaluated using soft-agar plates as described previously (34). Briefly, a sample of overnight culture of each mutant was used to inoculate 5 ml of sterile LB broth, followed by incubation at 37°C with aeration (200 rpm) to an OD600 of 1.0 to 1.2. Cultures were standardized to an OD600 of 1.0 and stabbed into the middles of soft-agar plates by using a sterile inoculating needle. Care was taken to avoid touching the bottom of the plate to avoid possible spreading from twitching motility. The plates were incubated for 16 h at 30°C, after which the diameter of motility was measured. Our laboratory has previously shown that CFT073 fim L-ON is heavily fimbriated and abundantly expresses FimH at 30°C throughout the motility assays (35), as seen under regular growth conditions at 37°C. WT E. coli CFT073 and CFT073 fim L-ON were always included as controls. The motilities of the complemented mutants of CFT073 and CFT073 fim L-ON were determined as described above except that the cultures and soft-agar plates contained ampicillin for maintenance of the complementation plasmid. Results of the motility agar assay were confirmed for all mutants by phase contrast microscopy. Wet mounts of bacterial cultures grown to an OD600 of 0.3 to 0.4, corresponding to optimal WT motility (35), were viewed at ×400 magnification by using a Zeiss Axioplan microscope. In this assay, CFT073 fim L-ON exhibits reduced motility compared to the WT strain (35).
Detection of flagellum production.
Bacteria were cultured for optimal WT motility as mentioned previously (35), followed by standardization to an OD600 of 0.35. Aliquots of these standardized suspensions (1.5 ml) were centrifuged at 3,500 rpm for 10 min at room temperature to pellet the bacteria. Whole-cell lysates were prepared for electrophoresis by gentle resuspension of the bacterial pellets in 100 μl of ddH2O and 20 μl of 6× sodium dodecyl sulfate (SDS) sample buffer, followed by boiling for 10 min. Samples were electrophoresed under denaturing conditions on a 10% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corp.). The blot was incubated with a 1:40,000 dilution of rabbit polyclonal antiserum to H1 flagella (Statens Serum Institute; Copenhagen, Denmark), followed by a 1:25,000 dilution of peroxidase-conjugated goat anti-rabbit immunoglobulin G (Sigma). The blot was developed using chemiluminescence according to the manufacturer's instructions (Amersham ECL Plus; GE Healthcare Life Sciences).
Detection of type 1 fimbrial expression.
Type 1 fimbrial expression was first examined in all mutants of CFT073 fim L-ON, using a mannose-sensitive hemagglutination assay as described previously (54). Briefly, approximately 1 × 109 CFU of bacteria, cultured statically overnight in LB broth, were resuspended in phosphate-buffered saline (PBS) and serially diluted twofold in round-bottom 96-well microtiter plates. An equal amount of a 3% (vol/vol) solution of guinea pig erythrocytes (Rockland Immunochemicals) with or without 25 μg α-methyl mannoside was mixed with the bacterial suspension. A diffuse mat of cells across the bottom of the well indicated positive hemagglutination.
For Western blotting, bacteria were cultured for optimal WT motility as described above, followed by standardization to an OD600 of 0.35. Aliquots of these standardized suspensions (1.5 ml) were centrifuged at 10,000 rpm for 10 min at room temperature to pellet the bacteria. Whole-cell lysates were prepared for electrophoresis by gentle resuspension of the bacterial pellets in 100 μl of acidified ddH2O (pH 1.8), followed by boiling for 10 min. After boiling, 20 μl of 6× SDS sample buffer was added to each fimbrial sample, followed by neutralization with 1 N NaOH. Samples were electrophoresed under denaturing conditions on a 10% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corp.). The blot was incubated with a 1:5,000 dilution of mouse polyclonal antiserum against FimC and FimH (courtesy of S. Langermann), followed by a 1:100,000 dilution of peroxidase-conjugated goat anti-mouse immunoglobulin G (Sigma). The blot was developed using chemiluminescence according to the manufacturer's instructions (Amersham ECL Plus; GE Healthcare Life Sciences).
Indirect fluorescent antibody staining.
Detection of H1 flagellin on the surfaces of CFT073, CFT073 fim L-ON, and the six isogenic deletion mutants of CFT073 fim L-ON was performed by a sequential staining procedure. Bacteria, cultured to optimal motility in LB (35), were spotted onto Teflon-printed indirect fluorescent-antibody slides (Electron Microscopy Sciences), dried, and fixed in PBS with 4% formaldehyde at 4°C. Fixative was washed off the slides with PBS. The slides were incubated with a polyclonal rabbit serum raised against purified FliC protein of CFT073 (1:1,000). Goat anti-rabbit IgG (Alexa 488 conjugate; Molecular Probes) (1:5,000) was used as the secondary antibody. All antisera were diluted in PBS containing 4% fetal bovine serum, and all incubations were done for 1 h at room temperature. The slides were rinsed three times with PBS after each incubation. Propidium iodide was used to stain bacterial nucleic acids by diluting a 1.0-mg/ml solution (Sigma) 1:10,000 in the first PBS wash after incubation with the secondary antibody. The slides were mounted with ProLong Gold Antifade mounting medium (Invitrogen). The slides were examined at ×1,000 magnification with an Olympus BX60 system microscope equipped for fluorescence with filters for fluorescein isothiocyanate and Texas Red detection. All images were obtained and analyzed with an Olympus DP70 color digital video camera and DP Controller/Manager software.
Statistical analysis.
For motility assays, a paired Student t test was used to determine significant differences in motility between strains (InStat; GraphPad Software).
RESULTS
Screening for genes involved in repression of motility in CFT073 constitutively expressing type 1 fimbriae (fim L-ON).
Our laboratory (35) and another (10) have previously shown that constitutive expression of type 1 fimbriae leads to a repression of motility and chemotaxis in UPEC strain CFT073. This regulation appears to occur at the transcriptional level; it was demonstrated by real-time PCR studies that constitutive expression of type 1 fimbriae results in decreased transcription of the major flagellin subunit gene, fliC, leading to a subsequent decrease in the level of flagella produced (35). Therefore, we hypothesized that UPEC possesses defined regulatory pathways by which it controls the transition from adherent to motile states and vice versa. To test this hypothesis, transposon mutagenesis was performed on a phase-locked type 1 fimbrial ON variant of UPEC strain CFT073 (CFT073 fim L-ON), followed by a screen for restoration of swimming motility in soft agar (Fig. 1). CFT073 fim L-ON contains a mutation in the left inverted repeat of the type 1 fimbrial IE that blocks DNA inversion by FimB, FimE, or other recombinases, thus locking the type 1 fimbrial promoter in an ON conformation (27). CFT073 fim L-ON constitutively expresses type 1 fimbriae on its surface.
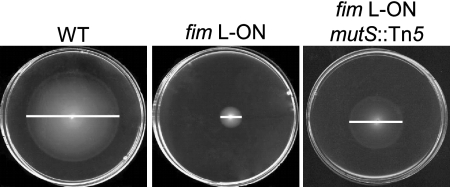
FIG. 1.
Screen for restoration of swimming motility in transposon mutants of E. coli CFT073 constitutively expressing type 1 fimbriae (fim L-ON). Motilities of the WT, fim L-ON, and a representative motile transposon mutant of fim L-ON (fim L-ON mutS::Tn5) in soft agar are shown. White bars indicate the diameter of motility of each strain (WT, 44 mm; fim L-ON, 10 mm; fim L-ON mutS::Tn5, 25 mm).
The genome of UPEC CFT073 has been fully sequenced and annotated and contains 5,379 open reading frames (59). Consequently, to achieve ≥90% genome saturation as calculated using the formula of Zilsel et al. (62), the swimming motilities of 12,000 Kanr Tn5 mutants of CFT073 fim L-ON Nalr in 0.25% tryptone agar were examined. There was no detectable difference in growth or motility between CFT073 fim L-ON Nalr and CFT073 fim L-ON. Forty-eight of 12,000 Kanr Tn5 mutants of CFT073 fim L-ON Nalr exhibited reproducible motility phenotypes greater than that of the CFT073 fim L-ON parent strain. Interestingly, however, all of these mutants were less motile than the CFT073 WT strain (data not shown). The locations of the Tn5 insertion in all 48 of these mutants were identified (Table 2). In 22 of the 48 mutants, the transposon inserted itself into the type 1 fimbrial operon, specifically within fimA, fimI, fimC, and fimD (see below). Other classes of genes disrupted in the remaining 26 mutants included those involved with metabolism, DNA mismatch repair, transcriptional regulation, and transport (Table 2). A large number of mutants contained the transposon in hypothetical genes. Also, multiple mutants in which the transposon had inserted itself in the same gene or region of the chromosome were isolated (Table 2) (mutS, hypothetical gene c1234, and the intergenic region between hypothetical genes c1204 and c1205).
TABLE 2.
Location of Tn5 insertions in motile CFT073 fim L-ON Nalr transposon mutants
| Function | Gene(s) or locationsa |
|---|---|
| Attachment | fimA (2), fimI (4), fimC (5), fimD (11) |
| Metabolism | tyrB, nrdF, c3405 |
| DNA mismatch repair | mutS (2) |
| Transcriptional regulation | lrp, lrhA, slyA, ybdO, yjjQ |
| Transport | yrbE |
| Hypothetical | yjdA, yjdM, ydiV, ykgI, c0322, c1234 (3), c4376, c2400, c2295, region upstream of c1204 and c1205 (3), region upstream of yhbT and yhbU |
The numbers in parenthesis represent the numbers of motile transposon mutants of CFT073 fim L-ON Nalr in which the Tn5 cassette had inserted itself in that particular gene or location.
Disruption of type 1 fimbrial genes partially restored motility in CFT073 fim L-ON Nalr.
Our laboratory showed previously that deletion of the type 1 fimbrial operon in CFT073 fim L-ON partially restored motility and flagellum expression (35). In this earlier study, the major structural and assembly genes of type 1 fimbriae were deleted to create a fim-null mutant of CFT073 fim L-ON, CFT073 ΔfimAICDFGH. The motility of CFT073 fim L-ON ΔfimAICDFGH was significantly higher than that of its CFT073 fim L-ON parent but not of the same level as that of WT CFT073 (35). In the study presented here, 22 of 48 motile Tn5 mutants of CFT073 fim L-ON Nalr analyzed contained the transposon insertion in the type 1 fimbrial operon (Table 2). In all of these mutants, the disruption occurred downstream of the IE containing the type 1 fimbrial promoter. A PCR-based IE assay described previously (39) was used to determine that the type 1 fimbrial promoter was unaffected and remained in the locked ON orientation in all of the type 1 fimbrial Tn5 mutants (data not shown). Also, these mutants were shown, via mannose-sensitive hemagglutination and Western blots, to express no detectable type 1 fimbriae (data not shown). The increased motilities of these Tn5 fimbrial mutants of CFT073 fim L-ON compared to that of the parent strain, as well as our previous observation that deletion of the type 1 fimbrial operon partially restores motility to CFT073 fim L-ON (35), indicated that the production of type 1 fimbriae in CFT073 fim L-ON partially inhibits motility. This partial inhibition of motility, along with the isolation of motile Tn5 mutants with insertions in nonfimbrial genes, suggested that factors other than the production of type 1 fimbriae also contribute to the repression of motility in CFT073 fim L-ON.
Six nonfimbrial genes are involved in repression of motility in CFT073 fim L-ON.
As presented above, this 90% saturation transposon screen identified 21 nonfimbrial loci that contributed to the regulation of motility and type 1 fimbrial expression. The level of flagellin protein produced by these mutants was different in each strain, in direct correlation with the motility results (data not shown). Also, in all mutants, the IE containing the type 1 fimbrial promoter remained in the ON position. The majority of these Tn5 mutants were shown to express type 1 fimbriae by mannose-sensitive hemagglutination and Western blotting (data not shown). Two mutants that were shown not to produce any detectable type 1 fimbriae contained the transposon in either c2295 or the region in between c1204 and c1205. However, the other two mutants isolated in which the transposon had also inserted itself into the region in between c1204 and c1205 were shown to produce type 1 fimbriae (data not shown).
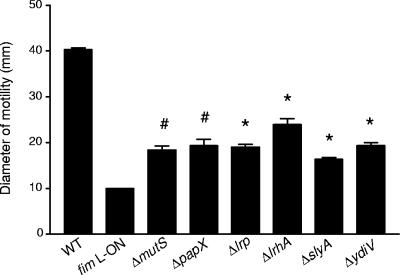
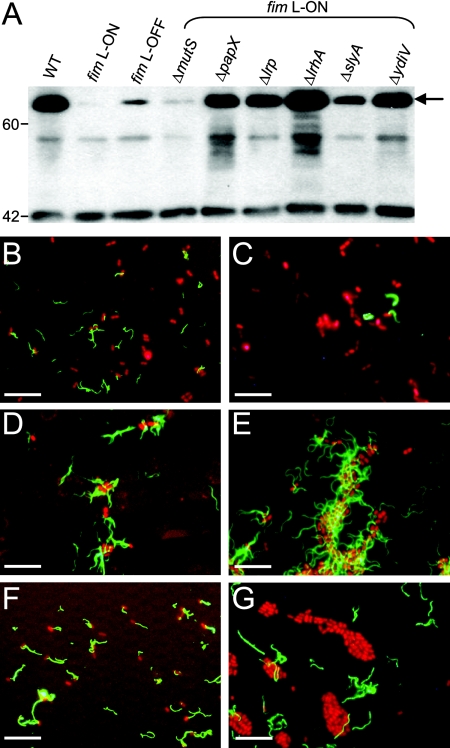
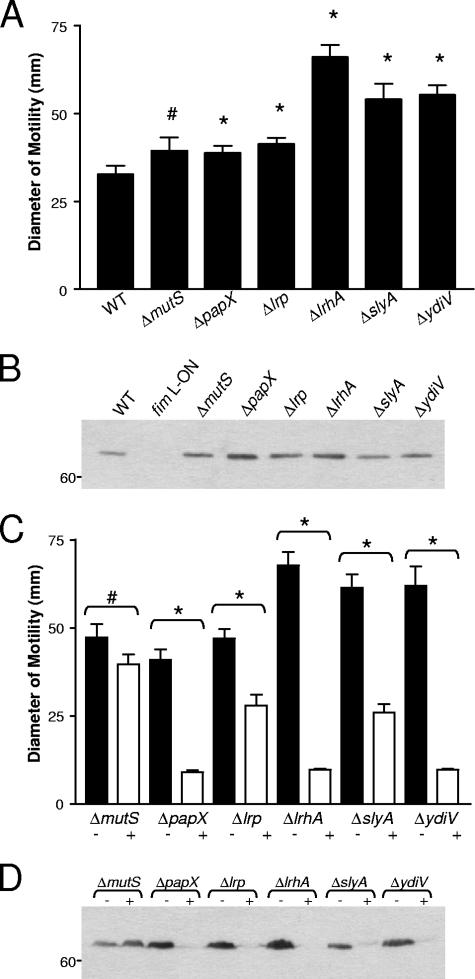
This observation, as well as the known polarity of mini-Tn5 transposon insertions (18), led us to construct isogenic in-frame deletion mutants of CFT073 fim L-ON in genes identified in the transposon screen as well as in other genes of interest known to affect flagellum expression. In all mutants constructed, the IE containing the type 1 fimbrial promoter remained in the ON position and these mutants were shown to express type 1 fimbriae by mannose-sensitive hemagglutination and Western blotting (data not shown). Examination of the motilities of these 34 isogenic deletion mutants of CFT073 fim L-ON revealed six nonfimbrial genes important for repression of motility during constitutive expression of type 1 fimbriae: mutS, papX, lrp, lrhA, slyA, and ydiV. As seen with the type 1 fimbrial mutant (35), single-gene-deletion mutants of these six candidate genes in CFT073 fim L-ON exhibited increased motility compared to the fim L-ON parent strain (Fig. 2). However, motility was not fully restored to WT levels (Fig. 2). Also, when the strains were cultured in broth to optimal motility (35), the amounts of flagellin protein produced in the papX, lrp, lrhA, slyA, and ydiV mutants were greater than that in the fim L-ON parent (Fig. 3A). As expected based on the motility results, these mutants expressed greater numbers of flagella on their surfaces than the fim L-ON strain (Fig. 3C to F). Interestingly, the Western blot analysis indicated that the level of flagellin made by CFT073 fim L-ON ΔmutS was comparable to that seen in the fim L-ON parental strain (Fig. 3A) and, accordingly, this mutant appeared to express less surface flagella than the other mutants of CFT073 fim L-ON (Fig. 3G). These results indicate that the increase in motility seen in this mutant may be due to factors other than a simple increase in the production of flagella.
FIG. 2.
Diameters of swimming motility of motile deletion mutants of CFT073 fim L-ON. Data represent the averages of three separate experiments. Error bars represent the standard errors of the means (SEM). Significant differences in motility from the fim L-ON parent were determined using a paired Student t test. #, P < 0.05; *, P < 0.01.
FIG. 3.
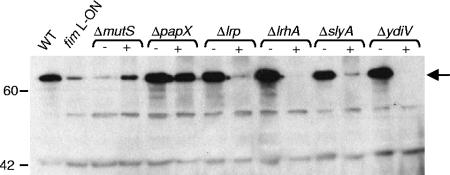
Flagellum production in motile deletion mutants of CFT073 fim L-ON. (A) Western blot analysis of flagellin production. Whole-cell lysates from standardized mid-log-phase cultures were prepared, electrophoresed onto SDS-polyacrylamide gel electrophoresis gels, and subjected to Western blot analysis with antiserum specific to H1 flagella. The WT and the phase-locked type 1 fimbrial mutants CFT073 fim L-ON and fim L-OFF are included as controls. The arrow indicates the 60.9-kDa FliC protein. Molecular mass markers are indicated on the left in kDa. (B-G) Indirect immunofluorescence analysis of flagellum expression. CFT073 (B), CFT073 fim L-ON (C), CFT073 fim L-ON ΔlrhA (D), CFT073 fim L-ON ΔslyA (E), CFT073 fim L-ON ΔydiV (F), and CFT073 fim L-ON ΔmutS (G) were spotted onto microscope slides and stained with antibody specific to H1 flagellin of CFT073 (green). Bacterial nucleic acids were visualized by staining with propidium iodide (red). Scale bars indicate 10 μm. The flagellar phenotypes of the papX and lrp mutants of CFT073 fim L-ON as determined by this analysis were similar to that of CFT073 fim L-ON ydiV (F).
Complementation of mutants.
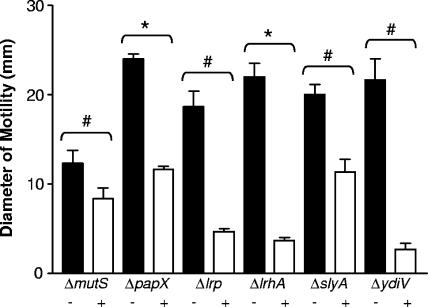
The MutS protein recognizes and binds to mismatched DNA sequences and is critical for DNA mismatch repair in prokaryotes (reviewed in reference 28). Therefore, it is possible that the deletion of mutS in CFT073 fim L-ON ΔmutS led to an increase in random mutation events during DNA replication and cell division. To address this possibility, as well as to determine whether the mutations made in the other mutants were nonpolar and disrupted only the genes of interest, mutS, papX, lrp, lrhA, slyA, and ydiV, with their respective promoters, were cloned into the low-copy-number plasmid pGEN-MCS and transformed into the respective mutant strains. Motility, flagellar, and type 1 fimbrial phenotypes were determined for each complemented mutant strain and compared to those from each mutant strain transformed with the vector alone. In each mutant examined, complementation with the plasmid-expressed gene led to a significant decrease in motility in soft agar compared to that of the vector-only controls (Fig. 4). In the papX, lrp, lrhA, slyA, and ydiV mutants, this decrease in motility after complementation coincided with a reduction in flagellin protein (Fig. 5), indicating that products of these genes directly affected production of flagella in UPEC. However, in the mutS mutant of CFT073 fim L-ON, complementation with WT mutS led to an increase in the amount of flagellin protein produced (Fig. 5).
FIG. 4.
Diameters of swimming motility of complemented mutants of CFT073 fim L-ON as well as mutants carrying the vector plasmid only. The + and − signs indicate mutants transformed with the complementation plasmid and the vector plasmid, respectively. Data represent the averages of three separate experiments. Error bars represent the SEM. Significant differences in motility between complemented mutants and mutants carrying the vector plasmid were determined using a paired Student t test. #, P < 0.05; *, P < 0.01.
FIG. 5.
Western blot analysis of flagellum production in complemented motile mutants of CFT073 fim L-ON. Whole-cell lysates from standardized mid-log-phase cultures were prepared, electrophoresed onto SDS-polyacrylamide gel electrophoresis gels, and subjected to Western blot analysis with antiserum specific to H1 flagella. The + and − signs indicate mutants transformed with the complementation plasmid and the vector plasmid, respectively. WT CFT073 and the parental phase-locked type 1 fimbrial mutant fim L-ON are included as controls. The arrow indicates the 60.9-kDa FliC protein. Molecular mass markers are indicated on the left in kDa.
Deletion of identified genes increases motility in WT CFT073.
We identified the contribution of six genes to motility in a variant of UPEC strain CFT073 constitutively expressing type 1 fimbriae (CFT073 fim L-ON). The remaining question involved examining the effect on the motilities of these genes in the WT strain, in which type 1 fimbrial genes can phase vary without restriction. Therefore, single-isogenic-deletion mutants of mutS, papX, lrp, lrhA, slyA, and ydiV were made in CFT073. The motility of each of these mutants was increased compared to that of CFT073 (Fig. 6A), indicating that the products of these genes repress motility in CFT073. However, in contrast to the results seen with the majority of the fim L-ON mutants, the levels of flagellin protein produced in these mutants of CFT073 were not largely increased compared to those in the WT parent (Fig. 6B). Only slight increases in flagellin protein are seen in the slyA and ydiV mutants, while the motilities were increased almost twofold. Also, the motility of CFT073 ΔpapX was only slightly increased compared to that of WT CFT073, but the level of flagellin protein appears to be much higher in the mutant strain. Complementation of the mutants of CFT073 further supported the observation that these genes repress motility. As expected from the results with the fim L-ON strains, complementation with the plasmid-expressed gene led to a significant decrease in motility in soft agar compared to those of the vector-only controls (Fig. 6C). Also, as seen with the mutants of CFT073 fim L-ON, in the papX, lrp, lrhA, slyA, and ydiV mutants, the decrease in motility after complementation coincided with a reduction in flagellin protein (Fig. 6D), while in the mutS mutant of CFT073, complementation with WT mutS led to an increase in the amount of flagellin protein produced (Fig. 6D).
FIG. 6.
Analysis of motility and flagellin production in mutants of WT CFT073. (A) Diameters of swimming motility of motile deletion mutants of CFT073. Data represent the averages of three separate experiments. Error bars represent the SEM. Significant differences in motility from the WT parent were determined using a paired Student t test. #, P < 0.05; *, P < 0.01. (B) Western blot analysis of flagellum production in motile deletion mutants of CFT073. The WT and the phase-locked type 1 fimbrial mutant CFT073 fim L-ON are included as controls. Molecular mass markers are indicated on the left in kDa. (C) Diameters of swimming motility of complemented mutants of CFT073 as well as mutants carrying the vector plasmid only. The + and − signs indicate mutants transformed with the complementation plasmid and the vector plasmid, respectively. Data represent the averages of three separate experiments. Error bars represent the SEM. Significant differences in motility between complemented mutants and mutants carrying the vector plasmid were determined using a paired Student t test. #, P < 0.05; *, P < 0.01. (D) Western blot analysis of flagellum production in complemented motile mutants of CFT073. Molecular mass markers are indicated on the left in kDa.
DISCUSSION
The abilities to adhere to host epithelia and to move via flagella are important yet opposite factors in the pathogenesis of UPEC. Previously, it was determined that constitutive expression of type 1 fimbriae leads to a repression of motility and chemotaxis in UPEC strain CFT073, suggesting that UPEC may coordinately regulate motility and adherence (10, 35). Furthermore, our laboratory showed that deletion of the entire region of DNA carrying the structural and accessory genes of type 1 fimbriae can only partially restore motility in this phase-locked fim ON mutant, CFT073 fim L-ON (35), suggesting that repression of motility in this strain is not solely due to the constitutive expression of these fimbriae on the surfaces of the bacteria. Here, we present studies that attempt to identify other genes involved in this regulation of motility by type 1 fimbriae. We initially identified 48 transposon mutants of CFT073 fim L-ON, corresponding to 27 genes, for which motility in soft agar was partially restored. Non-fimbria-encoding genes identified were knocked out in single-deletion mutants of CFT073 fim L-ON, and six genes that restored motility to CFT073 fim L-ON in our soft-agar screen were identified. These genes, all complementable, included the mismatch repair gene mutS; four known transcriptional regulators, lrhA, lrp, slyA, and papX; and one gene of hypothetical function, ydiV. BLASTx analysis revealed 95% amino acid identity between YdiV and a putative diguanylate phosphodiesterase (PDE) of E. coli strain B. This is interesting in that PDEs degrade the second messenger, cyclic di-GMP (c-di-GMP), which is known to regulate the transition between motility and biofilm formation (reviewed in reference 29). YdiV contains the prototypical EAL domain necessary for this hydrolysis of c-di-GMP. In many bacterial species, c-di-GMP inversely regulates adherence and motility in that low concentrations of c-di-GMP are associated with motile bacteria, whereas high concentrations of this second messenger promote adherence and biofilm formation. However, our results with the ydiV mutant of CFT073 are not consistent with these observations. In our study, we saw the opposite in that deletion of ydiV in both WT CFT073 and CFT073 fim L-ON increased motility. Reasons for this discrepancy between our study and the previous literature could be that the EAL domain of ydiV is nonfunctional or that YdiV may have other, as-yet-unidentified interactions with other protein systems. Further studies are needed to determine whether ydiV acts as a functional PDE in CFT073 and what role it plays in regulation of motility of UPEC.
LrhA (LysR homolog A) has been shown to repress type 1 fimbrial expression and biofilm synthesis. LrhA acts upon the expression of type 1 fimbriae by binding the promoters of the recombinase genes fimE and fimB as well as the actual promoter of the type 1 fimbrial operon (fimAICDFGH) itself (8). FimB and FimE drive the inversion of the IE containing the promoter for the type 1 fimbrial operon, thus determining whether type 1 fimbriae are expressed by the bacterium. FimB can switch the IE in both directions, while FimE predominantly switches the IE from ON to OFF (24, 30). LrhA exhibits higher affinity for the fimE gene than fimB and therefore, by activating transcription of the fimE gene, represses synthesis of type 1 fimbriae (8). In our study, expression of type 1 fimbriae was locked ON, due to mutations generated in the IE, rendering it unsusceptible to inversion by the fim recombinases. However, it is not surprising that we isolated a mutant of this gene, given that LrhA has also been shown previously to repress motility in UPEC. LrhA represses expression of the global regulator FlhD2C2 by binding directly to the flhDC promoter (36). Similar to our results generated with an lrhA mutant constructed in the fim L-ON background of UPEC CFT073, an lrhA mutant of the laboratory E. coli strain K-12 exhibited greater swimming and swarming motility than the WT parent strain.
The transcriptional regulator SlyA was initially discovered in the chromosome of Salmonella enterica serovar Typhimurium and was presumed to encode a hemolytic and cytolytic protein (38); however, it was later shown to be a regulator of a cryptic hemolysin (HlyE/ClyA/SheA) in E. coli (42). In addition, SlyA has been shown to regulate the expression of a number of genes required for acid and heat resistance and also for survival of bacteria within phagocytic cells (56). The relationship between SlyA and motility in S. enterica and enteropathogenic E. coli has been examined. In S. enterica, production of flagellin was decreased in a slyA mutant, indicating a positive regulation of motility by SlyA in this organism (56). Deletion of hosA, a homolog of SlyA, in enteropathogenic E. coli also leads to a reduction in flagellin production (21). However, these results are opposite of what we observed in UPEC strain CFT073, where deletion of slyA led to an increase in flagellin production. As discussed above, SlyA regulates the expression of the pore-forming toxin HlyE (ClyA/SheA) (49). Expression of hlyE is activated by SlyA and repressed by H-NS. SlyA and H-NS both bind the promoter of hlyE at overlapping sites (40). This competition for binding to the promoter leads to the differential regulation. Interestingly, H-NS is a positive regulator of flagellum expression in E. coli. Deletion of hns confers a nonmotile phenotype in E. coli that can be partially complemented by the introduction of plasmid-borne hns (5). Taken together with our results, these data suggest that SlyA may compete with H-NS for binding sites present on the flhDC promoter in CFT073. Also, H-NS represses phase variation of type 1 fimbriae in that mutations in hns increase the expression of the fimB, fimE, and fimA genes (20, 48). Phase variation of type 1 fimbrial expression is sensitive to temperature. Switching from phase ON to phase OFF, mediated by fimE, declines as the temperature increases, while switching from phase OFF to phase ON (fimB) increases to its optimal level at 37°C (23). Deletion of hns abrogated this temperature sensitivity of both fimE and fimB expression (48). It remains to be seen if SlyA itself regulates the expression of type 1 fimbriae in UPEC or whether this regulation is mediated through antagonism of the function of H-NS.
The leucine-responsive regulatory protein (Lrp) has previously been implicated in phase variation of type 1 fimbriae of E. coli. Lrp is a global regulator that influences the transcription of over 40 genes, commonly known as the leucine-Lrp regulon (reviewed in references 13 and 47). Lrp is involved in the regulation of a number of fimbrial operons in E. coli, including S fimbriae (sfa) (58), P fimbriae (pap) (9), and type 1 fimbriae (fim) (6). In regulation of type 1 fimbriae, Lrp binds directly to the IE containing the fimA promoter, affecting both fimE- and fimB-mediated switching (25). The amino acids leucine, isoleucine, valine, and alanine also contribute to this lrp-stimulated inversion (23). However, in our study, the promoter of fimA was locked in the ON confirmation, negating any effect Lrp should have had on its transcription. As mentioned above, lrp is involved in the regulation of P fimbriae of UPEC. Lrp is required for transcription of the pap operon in that the pap operon is locked OFF in an lrp-null mutant of UPEC (57). Therefore, it is interesting to speculate that lrp may repress expression of motility through regulation of the pap operon. Contributing to this idea is our observation that a papX mutant of CFT073 fim L-ON is more motile than its parental strain. PapX is a protein encoded within the pheV-associated pap operon of CFT073. However, papX is not required for P fimbrial biogenesis (43). Also, it has been previously shown that overexpression of papX in P. mirabilis leads to a repression of motility (37). Furthermore, a papX mutant of UPEC CFT073 exhibits a slight but significant increase in motility and flagellin expression compared to the WT strain. We are currently investigating the role of PapX in the transcription of type 1 fimbriae and regulation of motility in UPEC.
With the exception of mutS, the levels of flagellin produced in the other five mutants were increased significantly compared to that in the fim L-ON parent strain, indicating that the regulation imparted by the products of these genes involved production of the flagellum. However, we do not know at what step in the flagellar gene cascade this regulation occurred in each mutant and if this regulation occurred during transcription or translation. In the mutS mutant, the level of flagellin protein was not changed compared to that of the WT and, interestingly, complementation with mutS on a low-copy-number plasmid led to an actual increase in the amount of flagella produced. These results suggest that the increase in motility seen in the mutS mutant is not due to direct actions of mutS on flagellum synthesis and that mutS may act further downstream in the flagellar cascade, perhaps by altering the flagellar motor or chemotaxis systems. MutS is a key part of the DNA mismatch repair system (reviewed in reference 28). It has been previously shown in the laboratory strain E. coli K-12 that deletion of mutS alone does not confer many differences in gene expression in this strain (50). However, the transcript levels of both fliC and the gene carrying the flagellar hook subunit flgE were increased in the mutS mutant compared to those in the WT strain (50). In our study, we did not see an increase in FliC protein, but did see an increase in motility in our fim L-ON mutS mutant. It is possible that a transient increase in the expression of fliC occurred in our mutS mutant, which we would not have identified by the methods used. There is a high frequency of strains possessing a defective DNA mismatch repair system among UPEC isolates (19). These mutator strains have an advantage in vivo in late infection of the murine urinary tract and in in vitro urine culture (32). If the increase in motility were due to random mutations created by loss of the mismatch repair system in our mutant, we would have expected the repression of the motility phenotype to not be restored by complementation with mutS. However, this was not the case, indicating that in some way, MutS itself is involved in regulation of motility in our constitutively expressing type 1 fimbrial strain.
In summary, our data strongly suggest that UPEC possesses several methods for coordinately regulating the expression of type 1 fimbriae and flagella. We have identified six genes that appear to regulate motility in UPEC when type 1 fimbriae are constitutively expressed. Deletion of these genes also increases motility in the WT strain, where phase variation of type 1 fimbriae can occur. It remains to be determined whether these genes regulate expression of type 1 fimbriae in UPEC. Our laboratory is currently examining this possibility as well as examining the mechanism(s) these genes employ to regulate motility in UPEC.
Supplementary Material
Acknowledgments
This study was supported in part by Public Health Service Grant AI059722 from the National Institutes of Health.
We thank Denise Kirschner and the members of her laboratory at the University of Michigan for their help in calculating genome saturation.
Footnotes
Published ahead of print on 21 March 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Abraham, J. M., C. S. Freitag, J. R. Clements, and B. I. Eisenstein. 1985. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc. Natl. Acad. Sci. USA 825724-5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler, J., and B. Templeton. 1967. The effect of environmental conditions on the motility of Escherichia coli. J. Gen. Microbiol. 46175-184. [DOI] [PubMed] [Google Scholar]
- 3.Akerley, B. J., P. A. Cotter, and J. F. Miller. 1995. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell 80611-620. [DOI] [PubMed] [Google Scholar]
- 4.Reference deleted.
- 5.Bertin, P., E. Terao, E. H. Lee, P. Lejeune, C. Colson, A. Danchin, and E. Collatz. 1994. The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. J. Bacteriol. 1765537-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blomfield, I. C., P. J. Calie, K. J. Eberhardt, M. S. McClain, and B. I. Eisenstein. 1993. Lrp stimulates phase variation of type 1 fimbriation in Escherichia coli K-12. J. Bacteriol. 17527-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomfield, I. C., D. H. Kulasekara, and B. I. Eisenstein. 1997. Integration host factor stimulates both FimB- and FimE-mediated site-specific DNA inversion that controls phase variation of type 1 fimbriae expression in Escherichia coli. Mol. Microbiol. 23705-717. [DOI] [PubMed] [Google Scholar]
- 8.Blumer, C., A. Kleefeld, D. Lehnen, M. Heintz, U. Dobrindt, G. Nagy, K. Michaelis, L. Emody, T. Polen, R. Rachel, V. F. Wendisch, and G. Unden. 2005. Regulation of type 1 fimbriae synthesis and biofilm formation by the transcriptional regulator LrhA of Escherichia coli. Microbiology 1513287-3298. [DOI] [PubMed] [Google Scholar]
- 9.Braaten, B. A., J. V. Platko, M. W. van der Woude, B. H. Simons, F. K. de Graaf, J. M. Calvo, and D. A. Low. 1992. Leucine-responsive regulatory protein controls the expression of both the pap and fan pili operons in Escherichia coli. Proc. Natl. Acad. Sci. USA 894250-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryan, A., P. Roesch, L. Davis, R. Moritz, S. Pellett, and R. A. Welch. 2006. Regulation of type 1 fimbriae by unlinked FimB- and FimE-like recombinases in uropathogenic Escherichia coli strain CFT073. Infect. Immun. 741072-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchanan, K., S. Falkow, R. A. Hull, and S. I. Hull. 1985. Frequency among Enterobacteriaceae of the DNA sequences encoding type 1 pili. J. Bacteriol. 162799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burall, L. S., J. M. Harro, X. Li, C. V. Lockatell, S. D. Himpsl, J. R. Hebel, D. E. Johnson, and H. L. Mobley. 2004. Proteus mirabilis genes that contribute to pathogenesis of urinary tract infection: identification of 25 signature-tagged mutants attenuated at least 100-fold. Infect. Immun. 722922-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvo, J. M., and R. G. Matthews. 1994. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiol. Rev. 58466-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chilcott, G. S., and K. T. Hughes. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64694-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.Clegg, S., and K. T. Hughes. 2002. FimZ is a molecular link between sticking and swimming in Salmonella enterica serovar Typhimurium. J. Bacteriol. 1841209-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 1726568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denamur, E., S. Bonacorsi, A. Giraud, P. Duriez, F. Hilali, C. Amorin, E. Bingen, A. Andremont, B. Picard, F. Taddei, and I. Matic. 2002. High frequency of mutator strains among human uropathogenic Escherichia coli isolates. J. Bacteriol. 184605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorman, C. J., and N. N. Bhriain. 1992. Thermal regulation of fimA, the Escherichia coli gene coding for the type 1 fimbrial subunit protein. FEMS Microbiol. Lett. 78125-130. [DOI] [PubMed] [Google Scholar]
- 21.Ferrándiz, M. J., K. Bishop, P. Williams, and H. Withers. 2005. HosA, a member of the SlyA family, regulates motility in enteropathogenic Escherichia coli. Infect. Immun. 731684-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galen, J. E., J. Nair, J. Y. Wang, S. S. Wasserman, M. K. Tanner, M. B. Sztein, and M. M. Levine. 1999. Optimization of plasmid maintenance in the attenuated live vector vaccine strain Salmonella typhi CVD 908-htrA. Infect. Immun. 676424-6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gally, D. L., J. A. Bogan, B. I. Eisenstein, and I. C. Blomfield. 1993. Environmental regulation of the fim switch controlling type 1 fimbrial phase variation in Escherichia coli K-12: effects of temperature and media. J. Bacteriol. 1756186-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gally, D. L., J. Leathart, and I. C. Blomfield. 1996. Interaction of FimB and FimE with the fim switch that controls the phase variation of type 1 fimbriae in Escherichia coli K-12. Mol. Microbiol. 21725-738. [DOI] [PubMed] [Google Scholar]
- 25.Gally, D. L., T. J. Rucker, and I. C. Blomfield. 1994. The leucine-responsive regulatory protein binds to the fim switch to control phase variation of type 1 fimbrial expression in Escherichia coli K-12. J. Bacteriol. 1765665-5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardel, C. L., and J. J. Mekalanos. 1996. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect. Immun. 642246-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunther, I. N., J. A. Snyder, V. Lockatell, I. Blomfield, D. E. Johnson, and H. L. Mobley. 2002. Assessment of virulence of uropathogenic Escherichia coli type 1 fimbrial mutants in which the invertible element is phase-locked on or off. Infect. Immun. 703344-3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh, P. 2001. Molecular mechanisms of DNA mismatch repair. Mutat. Res. 48671-87. [DOI] [PubMed] [Google Scholar]
- 29.Jenal, U., and J. Malone. 2006. Mechanisms of cyclic-di-GMP signaling in bacteria. Annu. Rev. Genet. 40385-407. [DOI] [PubMed] [Google Scholar]
- 30.Klemm, P. 1986. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 51389-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kutsukake, K. 1997. Autogenous and global control of the flagellar master operon, flhD, in Salmonella typhimurium. Mol. Gen. Genet. 254440-448. [DOI] [PubMed] [Google Scholar]
- 32.Labat, F., O. Pradillon, L. Garry, M. Peuchmaur, B. Fantin, and E. Denamur. 2005. Mutator phenotype confers advantage in Escherichia coli chronic urinary tract infection pathogenesis. FEMS Immunol. Med. Microbiol. 44317-321. [DOI] [PubMed] [Google Scholar]
- 33.Lane, M. C., C. J. Alteri, S. N. Smith, and H. L. Mobley. 2007. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc. Natl. Acad. Sci. USA 10416669-16674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane, M. C., V. Lockatell, G. Monterosso, D. Lamphier, J. Weinert, J. R. Hebel, D. E. Johnson, and H. L. Mobley. 2005. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect. Immun. 737644-7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lane, M. C., A. N. Simms, and H. L. Mobley. 2007. Complex interplay between type 1 fimbrial expression and flagellum-mediated motility of uropathogenic Escherichia coli. J. Bacteriol. 1895523-5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehnen, D., C. Blumer, T. Polen, B. Wackwitz, V. F. Wendisch, and G. Unden. 2002. LrhA as a new transcriptional key regulator of flagella, motility and chemotaxis genes in Escherichia coli. Mol. Microbiol. 45521-532. [DOI] [PubMed] [Google Scholar]
- 37.Li, X., D. A. Rasko, C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2001. Repression of bacterial motility by a novel fimbrial gene product. EMBO J. 204854-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Libby, S. J., W. Goebel, A. Ludwig, N. Buchmeier, F. Bowe, F. C. Fang, D. G. Guiney, J. G. Songer, and F. Heffron. 1994. A cytolysin encoded by Salmonella is required for survival within macrophages. Proc. Natl. Acad. Sci. USA 91489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim, J. K., N. W. Gunther IV, H. Zhao, D. E. Johnson, S. K. Keay, and H. L. T. Mobley. 1998. In vivo phase variation of Escherichia coli type 1 fimbrial genes in women with urinary tract infection. Infect. Immun. 663303-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lithgow, J. K., F. Haider, I. S. Roberts, and J. Green. 2007. Alternate SlyA and H-NS nucleoprotein complexes control hlyE expression in Escherichia coli K-12. Mol. Microbiol. 66685-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu, X., and P. Matsumura. 1995. An alternative sigma factor controls transcription of flagellar class-III operons in Escherichia coli: gene sequence, overproduction, purification and characterization. Gene 16481-84. [DOI] [PubMed] [Google Scholar]
- 42.Ludwig, A., C. Tengel, S. Bauer, A. Bubert, R. Benz, H. J. Mollenkopf, and W. Goebel. 1995. SlyA, a regulatory protein from Salmonella typhimurium, induces a haemolytic and pore-forming protein in Escherichia coli. Mol. Gen. Genet. 249474-486. [DOI] [PubMed] [Google Scholar]
- 43.Marklund, B. I., J. M. Tennent, E. Garcia, A. Hamers, M. Baga, F. Lindberg, W. Gaastra, and S. Normark. 1992. Horizontal gene transfer of the Escherichia coli pap and prs pili operons as a mechanism for the development of tissue-specific adhesive properties. Mol. Microbiol. 62225-2242. [DOI] [PubMed] [Google Scholar]
- 44.McClain, M. S., I. C. Blomfield, and B. I. Eisenstein. 1991. Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli. J. Bacteriol. 1735308-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mobley, H. L., D. M. Green, A. L. Trifillis, D. E. Johnson, G. R. Chippendale, C. V. Lockatell, B. D. Jones, and J. W. Warren. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 581281-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 2821494-1497. [DOI] [PubMed] [Google Scholar]
- 47.Newman, E. B., and R. Lin. 1995. Leucine-responsive regulatory protein: a global regulator of gene expression in E. coli. Annu. Rev. Microbiol. 49747-775. [DOI] [PubMed] [Google Scholar]
- 48.Olsen, P. B., M. A. Schembri, D. L. Gally, and P. Klemm. 1998. Differential temperature modulation by H-NS of the fimB and fimE recombinase genes which control the orientation of the type 1 fimbrial phase switch. FEMS Microbiol. Lett. 16217-23. [DOI] [PubMed] [Google Scholar]
- 49.Oscarsson, J., Y. Mizunoe, B. E. Uhlin, and D. J. Haydon. 1996. Induction of haemolytic activity in Escherichia coli by the slyA gene product. Mol. Microbiol. 20191-199. [DOI] [PubMed] [Google Scholar]
- 50.Robbins-Manke, J. L., Z. Z. Zdraveski, M. Marinus, and J. M. Essigmann. 2005. Analysis of global gene expression and double-strand-break formation in DNA adenine methyltransferase- and mismatch repair-deficient Escherichia coli. J. Bacteriol. 1877027-7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwan, W. R. Flagella allow uropathogenic Escherichia coli ascension into murine kidneys. Int J. Med. Microbiol., in press. [DOI] [PMC free article] [PubMed]
- 52.Shin, S., and C. Park. 1995. Modulation of flagellar expression in Escherichia coli by acetyl phosphate and the osmoregulator OmpR. J. Bacteriol. 1774696-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snyder, J. A., B. J. Haugen, E. L. Buckles, C. V. Lockatell, D. E. Johnson, M. S. Donnenberg, R. A. Welch, and H. L. Mobley. 2004. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 726373-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snyder, J. A., B. J. Haugen, C. V. Lockatell, N. Maroncle, E. C. Hagan, D. E. Johnson, R. A. Welch, and H. L. Mobley. 2005. Coordinate expression of fimbriae in uropathogenic Escherichia coli. Infect. Immun. 737588-7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soutourina, O. A., and P. N. Bertin. 2003. Regulation cascade of flagellar expression in Gram-negative bacteria. FEMS Microbiol. Rev. 27505-523. [DOI] [PubMed] [Google Scholar]
- 56.Spory, A., A. Bosserhoff, C. von Rhein, W. Goebel, and A. Ludwig. 2002. Differential regulation of multiple proteins of Escherichia coli and Salmonella enterica serovar Typhimurium by the transcriptional regulator SlyA. J. Bacteriol. 1843549-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Woude, M. W., L. S. Kaltenbach, and D. A. Low. 1995. Leucine-responsive regulatory protein plays dual roles as both an activator and a repressor of the Escherichia coli pap fimbrial operon. Mol. Microbiol. 17303-312. [DOI] [PubMed] [Google Scholar]
- 58.van der Woude, M. W., and D. A. Low. 1994. Leucine-responsive regulatory protein and deoxyadenosine methylase control the phase variation and expression of the sfa and daa pili operons in Escherichia coli. Mol. Microbiol. 11605-618. [DOI] [PubMed] [Google Scholar]
- 59.Welch, R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 9917020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright, K. J., P. C. Seed, and S. J. Hultgren. 2005. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect. Immun. 737657-7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu, X. R., T. T. Sun, and J. J. Medina. 1996. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc. Natl. Acad. Sci. USA 939630-9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zilsel, J., P. H. Ma, and J. T. Beatty. 1992. Derivation of a mathematical expression useful for the construction of complete genomic libraries. Gene 12089-92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.