Abstract
The two-component system SaeRS of Staphylococcus aureus is closely involved in the regulation of major virulence factors. However, little is known about the signals leading to saeRS activation. A total of four overlapping transcripts (T1 to T4) from three different transcription starting points are expressed in the sae operon. We used a β-galactosidase reporter assay to characterize the putative promoter regions within the saeRS upstream region. The main transcript T2 is probably generated by endoribonucleolytic processing of the T1 transcript. Only two distinct promoter elements (P1 and P3) could be detected within the saeRS upstream region. The P3 promoter, upstream of saeRS, generates the T3 transcript, includes a cis-acting enhancer element and is repressed by saeRS. The most distal P1 promoter is strongly autoregulated, activated by agr, and repressed by sigma factor B. In strain Newman a mutation within the histidine kinase SaeS leads to a constitutively activated sae system. Evaluation of different external signals revealed that the P1 promoter in strain ISP479R and strain UAMS-1 is inhibited by low pH and high NaCl concentrations but activated by hydrogen peroxide. The most prominent induction of P1 was observed at subinhibitory concentrations of α-defensins in various S. aureus strains, with the exception of strain ISP479R and strain COL. P1 was not activated by the antimicrobial peptides LL37 and daptomycin. In summary, the results indicate that the sensor molecule SaeS is activated by alteration within the membrane allowing the pathogen to react to phagocytosis related effector molecules.
Staphylococcus aureus asymptomatically colonizes the noses of healthy individuals but also causes a variety of infections in humans. Polymorphonuclear neutrophils (PMNs) provide an effective line of defense against infections. However, S. aureus has developed a variety of mechanisms to avoid being killed by PMNs, including the expression of a number of immune modulators and toxins (for a review, see reference 8). The expression of most virulence and adherence factors is directly or indirectly influenced by diverse regulators such as agr, the alternative sigma factor B (SigB), sarA homologues or sae (for reviews, see references 4, 6, 17, and 32). Within this network sae appears to be a central downstream regulator that controls the expression of major virulence genes such as hla (coding for alpha-hemolysin), coa (coding for coagulase), or fnbA (coding for fibronectin-binding protein A) (11, 33, 39). Microarray analysis and proteomics have revealed that most of the genes activated by sae are involved in bacterial adhesion, immune modulation, or toxicity (25, 29, 38). In addition, the importance of gene regulation by sae in vivo was shown in several animal models (3, 15, 16, 29, 43).
The sae locus consists of four open reading frames, two of which (saeR and saeS) show strong sequence homology to response regulators and to histidine kinases (HKs) of bacterial two-component regulators (10). Two additional open reading frames, saeP and saeQ, both located upstream of saeRS, are likely to be important for the functionality of the sae operon (33, 39). Four overlapping sae-specific transcripts (T1 to T4) can be detected: the T1 message (3.0 kb) initiates upstream of saeP, the T2 message (2.4 kb) initiates upstream of saeQ, and T3 (2.0 kb) initiates in front of saeR. T4 (0.7 kb) represents a monocistronic mRNA encompassing saeP only. Thus far, the signals leading to the activation of the saeRS system are only poorly understood. The T1 transcript seems to be activated in the post-exponential-growth phase (33, 39) but downregulated under low pH or osmotic upshift (33). Subinhibitory concentrations of several classes of antibiotics have also been implicated in sae activation (glycopeptides, β-lactams) (25, 26) or inhibition (clindamycin) (33). The results on interactions with other virulence regulators such as agr and SigB are partially contradictory, which may be due to strain differences (15, 25, 33).
We are able to show here that the sae upstream region contains two active promoter elements, P1 and P3. The main strongly autoregulated P1 promoter can be activated by H2O2 and by subinhibitory concentrations of α-defensins, the key players in the phagocytic killing of bacteria. Thus, sae activation may play an important role in the synthesis of virulence factors leading to the destruction of PMNs after phagocytosis.
MATERIALS AND METHODS
Strains and growth conditions.
Reference strains COL, MW2, N315, USA300, MRSA252, and MSSA476 were provided by NARSA (i.e., the Network on Antimicrobial Resistance in Staphylococcus aureus). Other strains and plasmids are listed in Table 1. S. aureus strains were grown in CYPG (Casamino Acids [10 g/liter], yeast extract [10 g/liter], NaCl [5 g/liter], 20% glucose, 1.5 M phosphoglycerate) (31) supplemented with the appropriate antibiotics for strains carrying resistance genes (kanamycin at 50 μg/ml, erythromycin at 10 μg/ml, and tetracycline at 5 μg/ml).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| One Shot, TOP10 | Competent E. coli for plasmid transformation | Invitrogen |
| CYL316 | RN4220, pYL112Δ19, L54 int gene, r− | 27 |
| RN6390 | Derivate of NCTC 8325-4 | 36 |
| RN6112 | RN6390 agr::Tn551 | Richard Novick |
| 8325 (ΔsigB) | ΔrsbVW-sigB::erm | 24 |
| RN4220 | Restriction-deficient S. aureus strain | 23 |
| RN4220-29 | RN4220 Δsae::kan | Unpublished data |
| Newman | Wild type | 7 |
| Newman-29 | Newman, Δsae::kan | This study |
| Newman-agr | Newman, agr::Tn551 | This study |
| ISP479R | ISP479R, derivative of strain 8325-4, in which the mutation in rsbU was repaired to gain full sigma B activity | 40 |
| ISP479R-29 | Δsae::kan | This study |
| ISP479R-agr | agr::Tn551 | This study |
| ISP479R-sigB | IS479R, ΔrsbVW-sigB::erm | This study |
| UAMS-1 | Osteomyelitis isolate | 9 |
| UAMS-1-agr | agr::Tn551 | This study |
| UAMS-1-sigB | UAMS-1, ΔrsbVW-sigB::erm | This study |
| Plasmids | ||
| pDG783 | Vector containing kanamycin resistance cassette | 18 |
| pKO010 | Cloning vector with spoVG-lacZ | 34 |
| pCL84 | Single-copy integration vector, att-site for chromosomal integration in geh | 27 |
| pCG6 | pCL84 with integrated spoVG-lacZ gene cassette from pKO010 | This study |
| pCG7 | pCG6 with sae P1 (nt 1 to 400) | This study |
| pCG8 | pCG6 with sae P2 + short (nt 496 to 1141) | This study |
| pCG38 | pCG6 with sae P2 + middle (nt 496 to 1258) | This study |
| pCG39 | pCG6 with sae P2 + long (nt 496 to 1269) | This study |
| pCG9 | pCG6 with sae P3 + short (nt 1127 to 1529) | This study |
| pCG36 | pCG6 with sae P3 + middle (nt 974 to 1529) | This study |
| pCG15 | pCG6 with sae P3 + long (nt 496 to 1529) | This study |
| pCWSAE28 | pCL84 with saePQRS operon (long, nt 1 to 3515) | This study |
| pCWSAE47 | pCL84 with partial sae operon including P3 (short, nt 1066 to 3515) | This study |
| pCWSAE48 | pCL84 with partial sae operon including P2 and P3 (middle, nt 724 to 3515) | This study |
For promoter assays and RNA analysis, bacteria from an overnight culture were diluted to an initial optical density at 600 nm (OD600) of 0.05 in fresh medium without antibiotics and grown with shaking (222 rpm) at 37°C to the mid-exponential growth phase (OD600 = 0.5). The influence of agr and SigB on P1 activity was determined in bacteria grown to the post-exponential growth phase (OD600 = 6). The influence of pH, high NaCl concentration, and cell wall antibiotics was evaluated after 1 h of induction. Therefore, bacteria were grown to the mid-exponential growth phase (OD600 = 0.5) in CYPG and centrifuged (5 min, 5,000 rpm). Pellets were resuspended in CYPG adjusted to pH 7.4 or 5.5 with or without the addition of either 1 M NaCl, H2O2 (0.5 to 20 mM, AppliChem, Darmstadt, Germany), or the cell-wall-active antibiotics vancomycin (5 to 20 μg/ml; Sigma-Aldrich, Munich, Germany) or bacitracin (20 to 40 μg/ml; Sigma-Aldrich). Bacteria were further incubated for 1 h with shaking (222 rpm) at 37°C, the OD600 was determined, and equal bacterial numbers (corresponding to a 2-ml culture of OD600 = 1) were harvested.
For induction experiments with antimicrobial peptides, bacteria were grown in a modified Luria-Bertani broth (half yeast extract [2.5g/liter] and half tryptone [5 g/liter], no NaCl) as described elsewhere (37), a protocol that was shown not to inactivate α-defensins. The overnight cultures were diluted to an initial OD600 of 0.05 in fresh LB medium without antibiotics and grown with shaking (222 rpm) at 37°C to the mid-exponential growth phase (OD600 = 0.5). Then, 2-ml portions of cultures were supplemented with different concentrations of human neutrophil α-defensins (HNP1 to HNP3; 0.5 to 15 μg/ml), synthetic HNP2 (2 to 15 μg/ml; Peptanova, Sandhausen, Germany), or LL37 (1 to 10 μg/ml) and incubated for 2 h with shaking (222 rpm) at 37°C, and an equal number of bacteria was harvested, corresponding to a 1-ml culture with an OD600 of 1. For induction experiments using daptomycin (Cubicin Novartis, Nürnberg, Germany), the modified LB medium was supplemented with 50 mg of CaCl2/liter. α-Defensins and LL37 were purified from human peripheral blood neutrophils as described previously (37).
Determination of MICs.
Bacteria from the exponential growth phase (100 μl at an OD600 of 0.5) were diluted in 1 ml of phosphate-buffered saline. In 96-well microtiter plates 10 μl of bacterial suspension was mixed with 80 μl of medium, and increasing amounts of H2O2 (0.01 to 20 mM), LL37 (25 to 400 μg/ml), and HNP1 to HNP3 (50 to 400 μg/ml) were added. CYPG medium was used for determination of the H2O2 MICs, and modified LB medium was used for determination of the defensin MICs. Plates were incubated at 37°C, and the turbidity was determined after 24 and 48 h. The bactericidal activity was determined by plating of the dilutions for which no growth was detected.
Construction of agr, sigB, and sae mutant strains.
The sae locus was replaced by a kanamycin-resistant cassette using the temperature-sensitive shuttle vector pMAD (unpublished data). Briefly, two fragments flanking the sae locus and the kanamycin-resistant cassette derived from plasmid pCG783 were amplified and annealed by overlapping PCR. The amplicon was cloned into the EcoRI/SalI restriction sites of pMAD to gain pCWSAE29. pCWSAE29 was used to mutagenize strain RN4220 as described previously (2). The obtained sae gene replacement mutant strain (RN4220-29) was verified by PCR. In the mutant the whole sae operon (nucleotides [nt] 310 to 3279, based on GenBank accession no. AJ556795) was replaced by the kanamycin-resistant cassette.
sae, sigB, and agr mutants of different S. aureus strains were obtained by transduction using lysates of strains RN4220-29, 8325 (ΔsigB) (24) and RN6112, respectively. Transductants were verified by PCR and pulsed-field gel electrophoresis. All of the agr mutants were negative for delta-hemolysin; all of the sigB mutants were nonpigmented.
Chromosomal integration of sae into geh.
The cloned and sequenced 3.5-kb sae operon of strain ISP479C(pCWSAE7) (39) was subcloned into the single EcoRI site of pCL84, resulting in pCWSAE28. Shorter sae fragments were generated using oligonucleotides sae-724U and sae-1066U, together with sae-3515L, to generate sae fragments encompassing nt 724 to 3515 or nt 1066 to 3515, respectively (Fig. 1A). The fragments were cloned into the integration vector pCL84 (27), resulting in pCWSAE47 and pCWSAE48. Plasmids were integrated into geh of strain CYL316 by protoplast transformation and tetracycline selection. All integrants were lipase negative on Baird-Parker plates supplemented with egg yolk. The integrated plasmids were then transduced into the sae mutant Newman-29 using Φ11 lysates.
FIG. 1.
(A) Genetic organization of the sae operon. Indicated are the four transcriptional units (T1 to T4) and the fragments used for complementation of the sae mutation. Nucleotide numbers correspond to the sae sequence (GenBank accession no. AJ556795). In addition, the probes used for the detection of saeP (T1 and T4) and saeRS (T1, T2, and T3) in Northern blot analysis are indicated. (B) Northern blot analysis of RNA from the sae mutant strain Newman-29 complemented with pCWSAE28 (long), pCWSAE48 (middle), and pCWSAE47 (short) and hybridized with the saeRS-specific probe. (C) Indication of fragments upstream of saeRS used for promoter fusion assays (for details, see Table 1).
Construction of chromosomally integrated promoter-lacZ fusions.
The spoVG/lacZ gene cassette from the vector pKO010 (34) was purified after BamHI digestion and cloned into the BamHI restriction site of pCL84 (27), producing the vector pCG6. This vector integrates into the lipase gene (geh) of S. aureus due to a cloned phage attachment site. Putative sae promoter fragments were amplified with the appropriate oligonucleotides with additional EcoRI restriction sites (Table 2) using high-fidelity polymerase (Phusion HF polymerase; Finnzymes, Espoo, Finland). Amplicons were ligated into the EcoRI-digested integration vector pCG6 in front of the promoterless spoVG/lacZ gene. Plasmids were verified by restriction digestion, and the correct orientation of the inserts was verified by PCR using the oligonucleotide lacZL together with the respective sae-specific upstream oligonucleotides. Plasmids were introduced into S. aureus strain CYL316 by protoplast transformation and tetracycline selection. All integrants were lipase negative on Baird-Parker plates. The integrated plasmids were subsequently transduced into different S. aureus strains by using Φ11 phage lysates of the CYL316 derivatives. All transductants were verified by PCR as described above, and the agr activities were monitored by cross-striking of the strains with RN4220 on sheep blood agar plates as described previously (1).
TABLE 2.
Oligonucleotides
| Description | Primera | Sequence |
|---|---|---|
| Oligonucleotides used for promoter constructs | ||
| P3 sae promoter with a short upstream region | saeP3U | GTCGACGAATTCTCGTGTGGGTTCAGGTATTGTTATG |
| saeP3L | GTCGACGAATTCACCTCTGTTCTTACGACC | |
| P3 sae promoter with a middle upstream region | sae974U | CCGGCGAATTCCTTAAGCAACCCATGAGC |
| saeP3L | GTCGACGAATTCACCTCTGTTCTTACGACC | |
| P3 sae promoter with a long upstream region | saeP2U | GTCGACGAATTCACTGTTGAAGGTAAAGCTG |
| saeP3L | GTCGACGAATTCACCTCTGTTCTTACGACC | |
| P2 sae promoter with a short downstream region | saeP2U | GTCGACGAATTCACTGTTGAAGGTAAAGCTG |
| saeP2L | GTCGACGAATTCCCTGAACCCACACGAATGATAAATG | |
| P2 sae promoter with a middle downstream region | saeP2U | GTCGACGAATTCACTGTTGAAGGTAAAGCTG |
| sae1258L | CCGGCGAATTCGCGAAAAACCACTTATAC | |
| P2 sae promoter with a long downstream region | saeP2U | GTCGACGAATTCACTGTTGAAGGTAAAGCTG |
| sae1269 | CCGGCGAATTCCAACTATATTTGCGAAAA | |
| P1 sae promoter | saeP1U | GTCGACGAATTCTTATTGTGGCAAAAGGTT |
| saeP1L | GTCGACGAATTCTACCTTGATCTTGTGAAT | |
| lacZ control | lacZL | CGGATTGACCGTAATGGGATAGG |
| Oligonucleotides used for sae cloning in pCL84 | ||
| sae complemention with a short fragment | sae-1066U | CAATAAATAGAAAGAATGTGA |
| sae-3515L | ATTATTAGGCGGCATACAG | |
| sae complemention with a middle fragment | sae-724U | ATCTTCGGCGGCGCTAAA |
| sae-3515L | ATTATTAGGCGGCATACAG | |
| Full-length sae complemention | sae-1U | TTATTGTGGCAAAAGGTTT |
| sae-3515L | ATTATTAGGCGGCATACAG |
U, upper primer; L, lower primer.
RNA isolation, Northern blot hybridization, and real-time RT-PCR.
RNA isolation and Northern blot analysis were performed as described previously (14). Briefly, bacteria were lysed in 1 ml of TRIzol reagent (Invitrogen/Life Technologies, Karlsruhe, Germany) with 0.5-ml zirconia-silica beads (0.1 mm in diameter) in a high-speed homogenizer (Savant Instruments, Farmingdale, NY). RNA was isolated as described in the instructions provided by the manufacturer of TRIzol. Digoxigenin-labeled probes for the detection of specific transcripts were generated by using the digoxigenin labeling PCR kit according to the manufacturer's instructions (Roche Biochemicals, Mannheim, Germany). The oligonucleotides used for probe generation were as described previously (39). Quantification of transcripts coding for saeP, saeR, gyr, agr, and hla was performed as described by LightCycler reverse transcription-PCR (RT-PCR) (15).
Promoter activity assay.
For the different promoter activity assays the bacteria were grown in the corresponding media and harvested at the indicated time points with equal numbers of bacteria. Bacteria were centrifuged and resuspended in 1 ml of 0.1 M sodium phosphate buffer (pH 7.4) and lysed with 0.5-ml zirconia-silica beads (0.1 mm in diameter) in a high-speed homogenizer (Savant Instruments) for 10 s at a speed of 6,500 rpm. Aliquots of the supernatants were frozen until use. β-Galactosidase activity was measured by using the FluoReporter galactosidase quantitation kit (Invitrogen). The emerging fluorescence emission was measured in a 96-well microtiter plate (Nunc, Roskilde, Denmark) with a luminescence spectrometer (LS50B; Perkin-Elmer, Waltham, MA). The promoter activities are expressed as ng of β-galactosidase/ml with reference to a standard curve generated by purified β-galactosidase (Sigma-Aldrich) according to the manufacturer's instructions for the FluoReporter galactosidase quantitation kit. All values are means of at least two independently grown bacterial cultures. Significance was calculated by using a Student t test.
RESULTS
Delineation of P1 and P3 promoter activities of the sae upstream region.
We had previously mapped three transcriptional start sites upstream of saeRS, indicating the three distinct promoter elements P1, P2, and P3 (39) (Fig. 1). First, we analyzed the activity of the proposed promoters in strain Newman and in strain ISP479R (40), a SigB-proficient derivative of strain 8325-4. Different fragments within the upstream region were amplified and cloned into pCG6, an integration vector that contains lacZ with a ribosomal binding site of B. subtilis (Fig. 1C). The plasmids were integrated into the chromosomes of different S. aureus strains, and promoter activities were assessed by using a β-galactosidase assay.
A fragment containing P1 showed the highest activity in both strains analyzed (Fig. 2). However, the P1 activity in strain Newman was much higher than in strain ISP479R (166 versus 4.7 ng of β-galactosidase) (Fig. 2). This is consistent with previous results from Northern blot analysis showing that the sae transcripts T1, T2, and T4 are highly expressed in strain Newman compared to other strains (39). The hyperactivation of sae in strain Newman is due to a point mutation within the first membrane-spanning domain of the HK, SaeS. This is supported by our finding that cloning of the sae operon from strain Newman and expression in other sae mutant strains also leads to constitutive sae overexpression (M. Mainiero et al., unpublished data). This mutation most probably results in constitutive sae activation.
FIG. 2.
Promoter fusion assay using strain ISP479R and the sae mutant ISP479R-29 (A) or Newman and the sae mutant Newman-29 (B). Promoter fragments are indicated as in Fig. 1C. Bacteria were grown to mid-exponential growth phase, equal numbers of bacteria were lysed, and promoter activities are expressed as equivalents of β-galactosidase (in nanograms). Standard deviations are derived from at least two independent cultures.
Thus far, it is not clear whether the transcriptional start site of T2 is due to a distinct promoter activity or to processing of the T1 transcript. In order to further delineate the activity of a putative P2 promoter, different fragments surrounding the T2 transcriptional start site were analyzed (see Fig. 1C). No β-galactosidase activity was detectable. Also, extending the region surrounding the putative P2 to include downstream sequences but excluding P3 did not result in any activity. For further analysis, we cloned saeRS fragments containing different upstream regions into the single-copy integration vector pCL84 and complemented the sae mutant strain Newman-29, in which the whole sae locus encompassing saePQRS was replaced by a kanamycin-resistant cassette. Northern blot analysis revealed that full-length complementation resulted in the expected expression of the three distinct sae transcripts (T1 to T3) (Fig. 1B). However, complementation with saeRS containing the putative P2 promoter (middle fragment, see Fig. 1A) resulted in the expression of only the short T3 transcript, a finding comparable to a construct containing only P3 (short fragment). The results strongly indicate that the T2 transcript is due to RNA processing rather than initiation from an additional internal promoter.
Finally, we analyzed the activity of the putative P3 promoter. A fragment containing a short P3 fragment (pCG9) only was hardly active (Fig. 2). However, P3 activity could clearly be enhanced in constructs with extended upstream regions, indicating the presence of a potential binding site for cis-acting enhancer molecules.
Autoregulation of P1 and P3 promoters.
Bacterial regulatory systems are often characterized by positive feedback activation. Thus, we analyzed the autoregulation of P1 and P3 promoters by the sae operon (Fig. 2). Comparison of the sae-positive wild-type strains with their isogenic sae gene replacement mutants revealed that P1 is strongly autoregulated: no β-galactosidase activity was detectable in the sae mutants. In contrast, the P3 activity was increased in the sae mutants compared to the wild-type strains. Overall, the results indicate that upon signaling sae transcription is mainly driven by the strongly autoactivated P1 promoter.
Influence of agr and sigB on sae promoter activities.
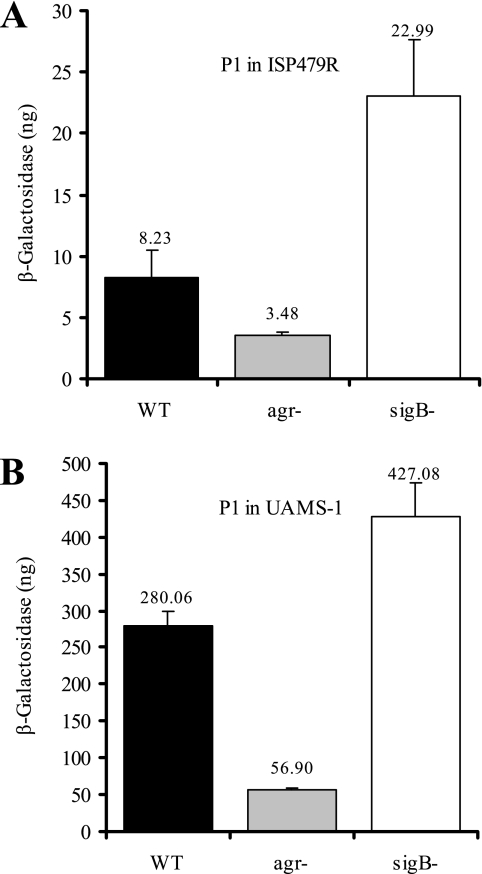
Previously, we found by Northern blot analysis that in strain Newman neither SigB (15) nor agr (data not shown) had a significant impact on the expression of any of the sae transcripts. However, results from Novick and Jiang (33) using derivatives of strain 8325-4 indicate that SigB inhibits and that agr activates transcription of the sae operon. The proposed constitutively active sae phenotype of strain Newman may account for its insensitivity toward regulatory signals such as agr and SigB. Thus, in further experiments in which we wanted to analyze different signals leading to saeRS activation, we focused on strains ISP479R and UAMS-1. The interaction with agr and SigB was analyzed by comparison of P1 promoter activities in wild types and their mutants. We analyzed bacteria grown to the postexponential phase (Fig. 3), since agr and SigB activity is maximal in the later phases of the growth cycle. Notably, activity of the P1 promoter was >30-fold higher in strain UAMS-1 than in strain ISP479R. Nevertheless, in both strains P1 activity is decreased in the agr mutant but increased in the sigB mutants compared to the wild-type bacteria. Thus, with the exception of strain Newman, agr leads to sae activation, and SigB leads to sae repression in two unrelated prototypic strains.
FIG. 3.
Promoter fusion assay using strains ISP479R (A) and UAMS-1 (B) and their agr and sigB mutants with cloned P1 promoter fragments. Bacteria were grown to the post-exponential growth phase, equal numbers of bacteria were lysed, and promoter activities are expressed as equivalents of β-galactosidase (in nanograms). Standard deviations are derived from at least two independent cultures.
Influence on P1 promoter activity by pH, NaCl concentration, and cell-wall-active antibiotics.
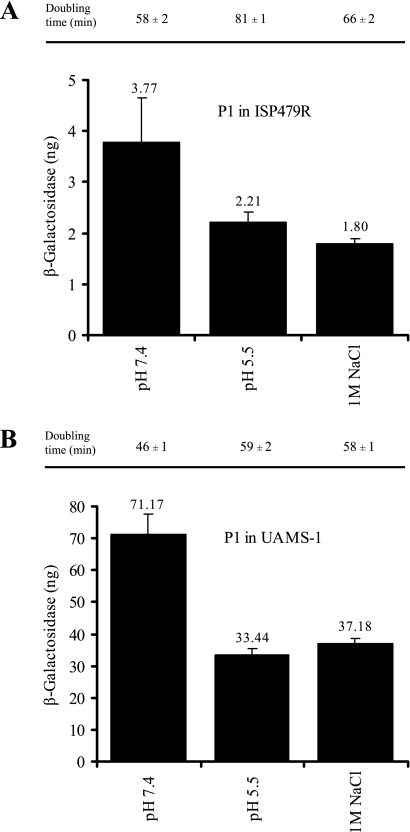
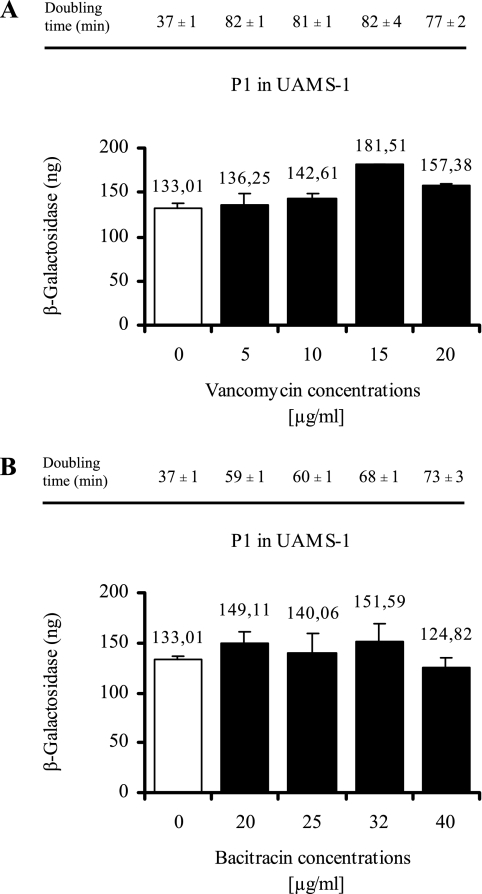
Thus far, the signals leading to saeRS activation are not well defined. Since P1 is strongly autoactivated, we assumed that the P1 activity correlates to the signal-specific activation of the HK SaeS. We applied different external signals to exponentially growing bacterial cultures and measured P1 promoter activity after 1 h of induction. First, we analyzed conditions that were already described in the literature as influencing sae expression. We were able to confirm that lowering the pH or adding 1 M NaCl leads to repression of P1 (33) (Fig. 4). Next, we analyzed increasing concentrations of cell-wall-active antibiotics (5 to 20 μg of vancomycin/ml and 20 to 40 μg of bacitracin/ml) on P1 activity (Fig. 5). Only for vancomycin, at concentrations of >10 μg/ml, was a slight but significant (P < 0.05) increase in P1 promoter activity observed. However, at subinhibitory concentrations no effect was detectable. In summary, previous results from the literature concerning sae signaling could be confirmed with regard to the repression at low pH and high NaCl concentrations (33) and partly with regard to those concerning activation by vancomycin (25).
FIG. 4.
Promoter fusion assay using strains ISP479R (A) and UAMS-1 (B) with cloned P1 promoter fragment. Bacteria were grown to mid-exponential growth phase, washed, and reincubated for 1 h with medium adjusted to pH 7.4 or 5.5 or in medium supplemented with 1 M NaCl (pH 7.4). Equal numbers of bacteria were lysed, and promoter activities are expressed as equivalents of β-galactosidase (in nanograms). Standard deviations are derived from at least two independent cultures. Bacterial doubling times were assessed during the different incubation conditions based on OD600 measurement and are indicated above the columns.
FIG. 5.
Promoter fusion assay using strain UAMS-1 with cloned P1 promoter fragment. Bacteria were grown to mid-exponential growth phase, washed, and reincubated for 1 h with (5 to 20 μg of vancomycin/ml [A], 20 to 40 μg of bacitracin/ml [B]). Equal numbers of bacteria were lysed, and promoter activities are expressed as equivalents of β-galactosidase (in nanograms). Standard deviations are derived from at least two independent cultures. Bacterial doubling times were assessed during the different incubation conditions based on OD600 measurement and are indicated above the columns.
Activation of P1 promoter activity by H2O2.
Recently, it was shown that in community-associated methicillin-resistant S. aureus (ca-MRSA) strains phagocytosis leads to the upregulation of several virulence factors (41), many of which are strongly sae regulated. We speculated that H2O2 may trigger sae activation. We could show that 5 to 20 mM H2O2 resulted in a significant activation of the P1 promoter in strains ISP479R and UAMS-1 (Fig. 6). These concentrations were above the MIC of 1.5 mM. However, under the assay conditions the bacteria were still able to grow at concentrations up to 15 mM, although with slower growth rates than those of the control without H2O2. Lowering the H2O2 concentration below 2 mM did not result in a significant activation of P1. We also analyzed whether H2O2 can activate P1 under low pH in which P1 is usually repressed. At pH 5.5, a 1.5-fold increase in P1 activation by H2O2 could be observed.
FIG. 6.
Promoter fusion assay using strains ISP479R (A) and UAMS-1 (B) with cloned P1 promoter fragment. Bacteria were grown to mid-exponential growth phase (OD600 = 0.5), followed by incubation with increasing concentrations of H2O2 for 1 h. Equal numbers of bacteria were lysed, and promoter activities are expressed as equivalents of β-galactosidase (in nanograms). Standard deviations are derived from at least two independent cultures. Bacterial doubling times were assessed during the different incubation conditions based on OD600 measurement and are indicated above the columns. At 20 mM H2O2, no growth was detectable.
Activation of P1 promoter by human α-defensin.
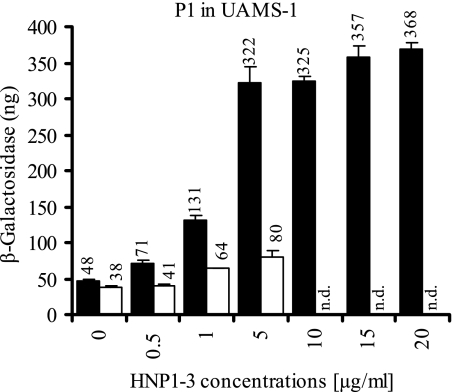
We assumed that PMN-derived antimicrobial peptides may also play a role in sae signaling. Indeed, the addition of α-defensins purified from PMN granulae exhibited a strong dose-dependent activation of the P1 promoter in strain UAMS-1 (Fig. 7). The concentrations used in this assay are subinhibitory for bacterial growth: no inhibitory effect on the growth rate was observed with up to 20 μg of α-defensins/ml. The sae sensitivity was also detectable at pH 5.5, where the addition of α-defensin (5 μg/ml) still resulted in a twofold increase in P1 promoter activity. We could confirm that synthetic α-defensin HNP2 also resulted in a strong P1 activation, indicating that the observed effect is not due to potential impurities in the α-defensin preparation. We next analyzed whether other antimicrobial peptides can also activate P1. However, neither the antimicrobial peptide LL37 (1 to 10 μg/ml) nor the membrane-active antibiotic daptomycin (0.01 to 10 μg/ml) activated P1, emphasizing a strong specificity of the sae system toward sensing of α-defensins.
FIG. 7.
Promoter fusion assay using strain UAMS-1 with cloned P1 promoter fragment. Bacteria were grown to mid-exponential growth phase in modified LB medium adjusted to pH 7.4 (▪) or pH 5.5 (□), followed by incubation with increasing concentrations of α-defensins (HNP1 to HNP3) for 2 h. Equal numbers of bacteria were lysed, and promoter activities are expressed as equivalents of β-galactosidase (in nanograms). Standard deviations are derived from at least two independent cultures. n.d., not determined.
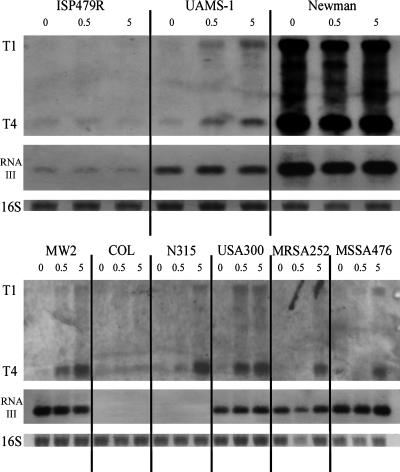
Interestingly, the strong activation of P1 by α-defensins was not seen in strain ISP479R. To further follow strain-specific differences, we extended the analysis to a set of genome-sequenced S. aureus strains. There was no difference in the MIC or minimal bactericidal activity (both 200 μg of HNP1 to HNP3/ml) between the strains analyzed (ISP479R, UAMS-1, and USA300). Strains were incubated with two subinhibitory concentrations (0.5 and 5 μg/ml) of α-defensins for 2 h, and the induction of the saeP-specific transcripts (T1 and T4) was analyzed by Northern blot analysis (Fig. 8) and qRT-PCR (Fig. 9). saeP-specific transcript levels strongly correlated with the P1 promoter activities determined in strains ISP479R and UAMS-1. Most of the additional strains (MW2, N315, USA300, MRSA252, and MSSA476) analyzed showed a strongly α-defensin-responsive sae phenotype. Other than the constitutively sae-activated strain Newman, only strain ISP479R and strain COL turned out to be insensitive with respect to sae-dependent α-defensin sensing. Of note, strain USA300 showed a strong induction already with 0.5 μg of α-defensins/ml. The degree of induction was also assessed quantitatively by RT-PCR, revealing a 2.5- to 9-fold increase of saeP-encoded transcripts after the addition of 0.5 μg of α-defensins/ml and a 9- to 17-fold increase after addition of 5 μg of α-defensins/ml.
FIG. 8.
Northern blot analysis of 2 μg of total RNA from different S. aureus strains. Bacteria were grown to mid-exponential growth phase in modified LB medium adjusted to pH 7.4, followed by incubation with 0, 0.5, or 5 μg of α-defensins (HNP1 to HNP3)/ml for 2 h. Blots were hybridized with probes specific for saeP and RNAIII (agr operon). For descriptions of sae transcripts T1 and T4 and probes, see Fig. 1A. In the lower lanes the 16S rRNA detected in the ethidium bromide-stained gels are indicated as loading controls.
FIG. 9.
Quantification of saeP-specific mRNA in different S. aureus strains by using LightCycler RT-PCR. The same RNA was used as for the Northern blot analysis shown in Fig. 7. Specific saeP transcripts were quantified with reference to the constitutively expressed gene gyr. Changes in saeP expression levels are indicated as factor differences of the expression from a given strain grown with 0.5 μg (□) or 5 μg (▪) of HNP1 to HNP3/ml divided by the expression from the strain grown without α-defensins (HNP1 to HNP3).
To verify that the α-defensins specifically induce the sae system, the expression of RNAIII from the agr operon was analyzed in parallel (Fig. 8). Two of the strains (strain COL and strain N315) analyzed did not produce detectable amounts of RNAIII. In none of the strains was RNAIII expression influenced by α-defensins. Thus, a global effect on gene expression can be excluded.
DISCUSSION
The central role of the sae regulatory system in virulence gene regulation has been demonstrated in several studies (11, 13, 15, 16, 29, 33, 38, 39). However, the mechanisms leading to sae activation are only partially understood. Here we could show using promoter fusions that the main P1 promoter of the sae locus is repressed by low pH and high NaCl levels, confirming previous results obtained by Northern blot analysis (33). In addition, the P1 promoter could be activated by the addition of H2O2 and by α-defensins. Judging from the genetic structure, the sensing is most probably mediated by the HK SaeS. The N-terminal sensory module of SaeS is composed of only two membrane-spanning stretches separated by a short extracellular loop of 13 amino acids. Signaling toward the cytoplasmic kinase domain is probably mediated by a conserved HAMP domain. This organization indicates that SaeS is a typical member of the intramembrane-sensing HKs recently described in firmicutes (30). Because of the absence of any obvious extracellular or intracellular sensory domains in this class of HKs, it is thought that the sensing occurs via the transmembrane helices within the membrane. The importance of signal perception by the transmembrane helices is supported by the constitutively active sae in strain Newman, which could be traced to a single amino acid exchange in the first of the two transmembrane loops of SaeS (39; unpublished data). According to the constitutively active phenotype, sae transcription in strain Newman is independent of agr and/or SigB and insensitive toward signals such as H2O2 or α-defensins. It is also possible that SaeS can communicate with other membrane proteins such SaeQ to become activated. Although there is no direct evidence for this assumption, the similarly organized LiaS-like HK from B. subtilis has been shown to interact with the topologically linked membrane protein LiaF (22). LiaS-like HKs were shown to be involved in the sensing of perturbations in the bacterial envelope by cell-wall-active antibiotics, as well as by the antimicrobial peptide LL37 (for a review, see reference 30). However, neither the antimicrobial peptides LL37 nor daptomycin led to sae activation, indicating a high specificity of the sae system toward α-defensins. The molecular link between the different signals interfering with SaeS may be alterations within the membrane rather than cell wall perturbations.
Four distinct transcripts are expressed within the sae operon, of which the T2 transcript initiating between saeQ and saeP seems to be the most abundant. The results from promoter fusion assays and Northern blot analysis using cloned sae fragments void of P1 strongly indicate that the T2 transcript is most probably generated by specific endoribonucleolytic processing of the T1 transcript. Half-life determinations using rifampin indicated that the processed T2 transcript is stabilized compared to T1 (data not shown). Thus, the processing may contribute to a prolonged synthesis of the sae gene products once the P1 promoter has been activated. The enzyme responsible for this specific RNA cleavage is not known because, in general, information on endoribonucleases in gram-positive bacteria is limited. For S. aureus it was recently shown that the double-stranded specific RNase III determines the mRNA stability of the spa mRNA (coding for protein A), and the enzyme may also be involved in mRNA processing of the sae transcripts (21).
The transcripts T1 and T3 are initiated from the two distinct promoter elements P1 and P3. P3 is autorepressed, and its activity depends on an extensive upstream region, indicating a potential operator domain. SaeRS expressed from P3 may enable the bacteria to sense an appropriate signal, which in turn leads to upregulation on the transcriptional level due to the strongly autoactivated P1 promoter. The P1 activation probably leads to expression of SaeP and SaeQ, which may be linked to the regulatory function of SaeS and SaeR.
For a detailed analysis of the sae-specific promoter activities, most of the experiments were performed with strains ISP479R and UAMS-1. ISP479R is a SigB-proficient derivative of the well-characterized 8325-4 (clonal complex 8, multilocus sequence typing database [http://www.mlst.net/]). Strain UAMS-1 is a clinical isolate belonging to the widely disseminated strain from clonal complex 30 and was shown to exert a different regulatory pattern with respect to agr and sarA regulation (5). The low sae activity observed in strain ISP479R is partly due to the inhibitory effect of SigB, since the SigB-deficient parental strain ISP479C showed higher levels of sae transcription than did strain ISP479R (but still below that of strain UAMS-1 [data not shown]). The high sae expression found in strain UAMS-1 is in good agreement with previous results from Cassat et al. (5) and was even further increased in the sigB mutant (P < 0.05 [wild type versus sigB mutant]). SigB may act indirectly on sae expression due to SigB-dependent inhibition of the agr system (20). The agr-dependent P1 activation is consistent with previous results from others (12, 33) emphasizing that within the regulatory cascade of virulence gene regulation, the sae system is placed as a central downstream regulator.
ca-MRSA strains such as MW2 and USA300 are known to account for more severe disease outcomes (44) and to be more resistant toward PMN phagocytosis than hospital-acquired MRSA strains (41). Resistance to phagocytosis was linked to an upregulation of extracellular toxins, most of which are regulated by sae. We hypothesized that sae may play a major role in virulence gene activation upon PMN interaction. Within the PMNs sae can be activated first by the initial rise in the pH due to the generation of reduced oxygen species (45), the production of H2O2, and most prominently by antimicrobial peptides (HNP1 to HNP3). Sae activation will enable the bacteria to synthesize a plethora of toxic enzymes such as alpha-hemolysin or leukocidins, which may eventually lead to PMN destruction. The sae response to α-defensins was seen in concentrations that are far below the growth inhibitory effect of these peptides and below those found within PMNs. Thus, one may also assume that extracellular α-defensins released from PMNs result in sae activation in the blood or at the side of infection prior to phagocytosis. The plasma levels of α-defensins detected in infected patients (35) are well within a range in which sae activation can occur.
The regulatory properties of α-defensins seem to be very specific toward sae activation. Neither of the two-component regulatory systems agr (Fig. 8) or graRS (aps) (data not shown) was influenced by α-defensins (HNP1 to HNP3). Interestingly, other antimicrobial peptides were shown to activate the graRS (aps) system, leading to a better protection of the bacteria from these peptides (19, 28). In contrast, activation of the sae system by α-defensins probably does not render the bacteria more antimicrobial peptide resistant because there is no indication that sae is involved in the regulation of structural compounds of the cell envelope (29, 38). Instead, sae specifically activates virulence genes that may be important for the bacteria to evade the hostile environment, e.g., after PMN phagocytosis.
One intriguing finding of the present study was the prominent strain-specific differences in sae activation. The unusually high activity seen in strain Newman can be traced to a single amino acid exchange in the first transmembrane loop of the HK SaeS (39). However, sequence analysis of the sae operon from other strains did not reveal any link to the different responsiveness of the strains toward α-defensins. In most strains, including strain USA300, sae could be activated by α-defensins. In the 8325 derivative (ISP479R) and in strain COL the basal low level of P1 activity could not be enhanced by α-defensins. Strains 8325, COL, and USA300 are evolutionarily related (clonal complex 8) and show 100% sequence identity within the sae locus. Thus, the molecular basis for strain-dependent differences must be due to either differences in other regulatory elements interacting with sae or in different properties of the cell envelope. Interestingly, the α-defensin-responsive ca-MRSA strains USA300 and MW2 were shown to be more resistant to phagocytosis than the nonresponsive strain COL (41). More recently, this property could be linked to the expression of phenol-soluble modulins (42). Whether the sae responsiveness causes the synthesis of these compounds, the observed phagocytosis resistance, or even differences in strain virulence will be analyzed in future studies.
Acknowledgments
Purified preparations of the α-defensins HNP1 to HNP3 and LL37 were kindly provided by Hubert Kalbacher. We are grateful to Inigo Lasa for the donation of strain ISP479R, to Knut Ohlsen for providing the vector pKO010, and to NARSA for providing reference strains. We thank Vittoria Bisanzio for excellent technical assistance.
The study was supported by grants to C.W. from the Deutsche Forschungsgemeinschaft.
Footnotes
Published ahead of print on 14 March 2008.
REFERENCES
- 1.Adhikari, R. P., S. Arvidson, and R. P. Novick. 2007. A nonsense mutation in agrA accounts for the defect in agr expression and the avirulence of Staphylococcus aureus 8325-4 traP::kan. Infect. Immun. 754534-4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnaud, M., A. Chastanet, and M. Debarbouille. 2004. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl. Environ. Microbiol. 706887-6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benton, B. M., J. P. Zhang, S. Bond, C. Pope, T. Christian, L. Lee, K. M. Winterberg, M. B. Schmid, and J. M. Buysse. 2004. Large-scale identification of genes required for full virulence of Staphylococcus aureus. J. Bacteriol. 1868478-8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronner, S., H. Monteil, and G. Prevost. 2004. Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol. Rev. 28183-200. [DOI] [PubMed] [Google Scholar]
- 5.Cassat, J., P. M. Dunman, E. Murphy, S. J. Projan, K. E. Beenken, K. J. Palm, S. J. Yang, K. C. Rice, K. W. Bayles, and M. S. Smeltzer. 2006. Transcriptional profiling of a Staphylococcus aureus clinical isolate and its isogenic agr and sarA mutants reveals global differences in comparison to the laboratory strain RN6390. Microbiology 1523075-3090. [DOI] [PubMed] [Google Scholar]
- 6.Cheung, A. L., A. S. Bayer, G. Zhang, H. Gresham, and Y. Q. Xiong. 2004. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 401-9. [DOI] [PubMed] [Google Scholar]
- 7.Duthie, E. S., and L. L. Lorenz. 1952. Staphylococcal coagulase: mode of action and antigenicity. J. Gen. Microbiol. 695-107. [DOI] [PubMed] [Google Scholar]
- 8.Foster, T. J. 2005. Immune evasion by staphylococci. Nat. Rev. Microbiol. 3948-958. [DOI] [PubMed] [Google Scholar]
- 9.Gillaspy, A. F., S. G. Hickmon, R. A. Skinner, J. R. Thomas, C. L. Nelson, and M. S. Smeltzer. 1995. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect. Immun. 633373-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giraudo, A. T., A. Calzolari, A. A. Cataldi, C. Bogni, and R. Nagel. 1999. The sae locus of Staphylococcus aureus encodes a two-component regulatory system. FEMS Microbiol. Lett. 17715-22. [DOI] [PubMed] [Google Scholar]
- 11.Giraudo, A. T., A. L. Cheung, and R. Nagel. 1997. The sae locus of Staphylococcus aureus controls exoprotein synthesis at the transcriptional level. Arch. Microbiol. 16853-58. [DOI] [PubMed] [Google Scholar]
- 12.Giraudo, A. T., C. Mansilla, C. Ana, C. Raspanti, and R. Nagel. 2003. Studies on the expression of regulatory locus sae in Staphylococcus aureus. Curr. Microbiol. 46246-250. [DOI] [PubMed] [Google Scholar]
- 13.Giraudo, A. T., H. Rampone, A. Calzolari, and R. Nagel. 1996. Phenotypic characterization and virulence of a sae− agr− mutant of Staphylococcus aureus. Can. J. Microbiol. 42120-123. [DOI] [PubMed] [Google Scholar]
- 14.Goerke, C., S. Campana, M. G. Bayer, G. Döring, K. Botzenhart, and C. Wolz. 2000. Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect. Immun. 681304-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goerke, C., U. Fluckiger, A. Steinhuber, V. Bisanzio, M. Ulrich, M. Bischoff, J. M. Patti, and C. Wolz. 2005. The role of Staphylococcus aureus global regulators sae and sigB in virulence gene expression during device-related infection. Infect. Immun. 733415-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goerke, C., U. Fluckiger, A. Steinhuber, W. Zimmerli, and C. Wolz. 2001. Impact of the regulatory loci agr, sarA, and sae of Staphylococcus aureus on the induction of α-toxin during device-related infection resolved by direct quantitative transcript analysis. Mol. Microbiol. 401439-1448. [DOI] [PubMed] [Google Scholar]
- 17.Goerke, C., and C. Wolz. 2004. Regulatory and genomic plasticity of Staphylococcus aureus during persistent colonization and infection. Int. J. Med. Microbiol. 294195-202. [DOI] [PubMed] [Google Scholar]
- 18.Guerout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167335-336. [DOI] [PubMed] [Google Scholar]
- 19.Herbert, S., A. Bera, C. Nerz, D. Kraus, A. Peschel, C. Goerke, M. Meehl, A. Cheung, and F. Gotz. 2007. Molecular basis of resistance to muramidase and cationic antimicrobial peptide activity of lysozyme in staphylococci. PLoS Pathog. 3e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 1845457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huntzinger, E., S. Boisset, C. Saveanu, Y. Benito, T. Geissmann, A. Namane, G. Lina, J. Etienne, B. Ehresmann, C. Ehresmann, A. Jacquier, F. Vandenesch, and P. Romby. 2005. Staphylococcus aureus RNAIII and the endoribonuclease III coordinately regulate spa gene expression. EMBO J. 24824-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan, S., A. Junker, J. D. Helmann, and T. Mascher. 2006. Regulation of LiaRS-dependent gene expression in Bacillus subtilis: identification of inhibitor proteins, regulator binding sites, and target genes of a conserved cell envelope stress-sensing two-component system. J. Bacteriol. 1885153-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreiswirth, B. N., S. Lofdahl, M. J. Betley, M. O'Reilly, P. M. Schlievert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305709-712. [DOI] [PubMed] [Google Scholar]
- 24.Kullik, I., P. Giachino, and T. Fuchs. 1998. Deletion of the alternative sigma factor σB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 1804814-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuroda, H., M. Kuroda, L. Cui, and K. Hiramatsu. 2007. Subinhibitory concentrations of beta-lactam induce haemolytic activity in Staphylococcus aureus through the SaeRS two-component system. FEMS Microbiol. Lett. 26898-105. [DOI] [PubMed] [Google Scholar]
- 26.Kuroda, M., H. Kuroda, T. Oshima, F. Takeuchi, H. Mori, and K. Hiramatsu. 2003. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol. Microbiol. 49807-821. [DOI] [PubMed] [Google Scholar]
- 27.Lee, C. Y., S. L. Buranen, and Z. H. Ye. 1991. Construction of single-copy integration vectors for Staphylococcus aureus. Gene 103101-105. [DOI] [PubMed] [Google Scholar]
- 28.Li, M., D. J. Cha, Y. Lai, A. E. Villaruz, D. E. Sturdevant, and M. Otto. 2007. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 661136-1147. [DOI] [PubMed] [Google Scholar]
- 29.Liang, X., C. Yu, J. Sun, H. Liu, C. Landwehr, D. Holmes, and Y. Ji. 2006. Inactivation of a two-component signal transduction system, SaeRS, eliminates adherence and attenuates virulence of Staphylococcus aureus. Infect. Immun. 744655-4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mascher, T. 2006. Intramembrane-sensing histidine kinases: a new family of cell envelope stress sensors in Firmicutes bacteria. FEMS Microbiol. Lett. 264133-144. [DOI] [PubMed] [Google Scholar]
- 31.Novick, R. P. 1991. Genetic systems in staphylococci. Methods Enzymol. 204587-636. [DOI] [PubMed] [Google Scholar]
- 32.Novick, R. P. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 481429-1449. [DOI] [PubMed] [Google Scholar]
- 33.Novick, R. P., and D. Jiang. 2003. The staphylococcal saeRS system coordinates environmental signals with agr quorum sensing. Microbiology 1492709-2717. [DOI] [PubMed] [Google Scholar]
- 34.Ohlsen, K., K. P. Koller, and J. Hacker. 1997. Analysis of expression of the alpha-toxin gene (hla) of Staphylococcus aureus by using a chromosomally encoded hla::lacZ gene fusion. Infect. Immun. 653606-3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panyutich, A. V., E. A. Panyutich, V. A. Krapivin, E. A. Baturevich, and T. Ganz. 1993. Plasma defensin concentrations are elevated in patients with septicemia or bacterial meningitis. J. Lab. Clin. Med. 122202-207. [PubMed] [Google Scholar]
- 36.Peng, H. L., R. P. Novick, B. N. Kreiswirth, J. Kornblum, and P. Schlievert. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 1704365-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peschel, A., M. Otto, R. W. Jack, H. Kalbacher, G. Jung, and F. Gotz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 2748405-8410. [DOI] [PubMed] [Google Scholar]
- 38.Rogasch, K., V. Ruhmling, J. Pane-Farre, D. Hoper, C. Weinberg, S. Fuchs, M. Schmudde, B. M. Broker, C. Wolz, M. Hecker, and S. Engelmann. 2006. Influence of the two-component system SaeRS on global gene expression in two different Staphylococcus aureus strains. J. Bacteriol. 1887742-7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinhuber, A., C. Goerke, M. G. Bayer, G. Döring, and C. Wolz. 2003. Molecular architecture of the regulatory locus sae of Staphylococcus aureus and its impact on the expression of virulence factors. J. Bacteriol. 1856278-6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toledo-Arana, A., N. Merino, M. Vergara-Irigaray, M. Debarbouille, J. R. Penades, and I. Lasa. 2005. Staphylococcus aureus develops an alternative, ica-independent biofilm in the absence of the arlRS two-component system. J. Bacteriol. 1875318-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voyich, J. M., K. R. Braughton, D. E. Sturdevant, A. R. Whitney, B. Said-Salim, S. F. Porcella, R. D. Long, D. W. Dorward, D. J. Gardner, B. N. Kreiswirth, J. M. Musser, and F. R. DeLeo. 2005. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 1753907-3919. [DOI] [PubMed] [Google Scholar]
- 42.Wang, R., K. R. Braughton, D. Kretschmer, T. H. Bach, S. Y. Queck, M. Li, A. D. Kennedy, D. W. Dorward, S. J. Klebanoff, A. Peschel, F. R. DeLeo, and M. Otto. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 131510-1514. [DOI] [PubMed] [Google Scholar]
- 43.Xiong, Y. Q., J. Willard, M. R. Yeaman, A. L. Cheung, and A. S. Bayer. 2006. Regulation of Staphylococcus aureus alpha-toxin gene (hla) expression by agr, sarA, and sae in vitro and in experimental infective endocarditis. J. Infect. Dis. 1941267-1275. [DOI] [PubMed] [Google Scholar]
- 44.Zetola, N., J. S. Francis, E. L. Nuermberger, and W. R. Bishai. 2005. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect. Dis. 5275-286. [DOI] [PubMed] [Google Scholar]
- 45.Zusman, T., O. Gal-Mor, and G. Segal. 2002. Characterization of a Legionella pneumophila relA insertion mutant and toles of RelA and RpoS in virulence gene expression. J. Bacteriol. 18467-75. [DOI] [PMC free article] [PubMed] [Google Scholar]