Abstract
Diverse organisms time their cellular activities to occur at distinct phases of Earth's solar day, not through the direct regulation of these processes by light and darkness but rather through the use of an internal biological (circadian) clock that is synchronized with the external cycle. Input pathways serve as mechanisms to transduce external cues to a circadian oscillator to maintain synchrony between this internal oscillation and the environment. The circadian input pathway in the cyanobacterium Synechococcus elongatus PCC 7942 requires the kinase CikA. A cikA null mutant exhibits a short circadian period, the inability to reset its clock in response to pulses of darkness, and a defect in cell division. Although CikA is copurified with the Kai proteins that constitute the circadian central oscillator, no direct interaction between CikA and either KaiA, KaiB, or KaiC has been demonstrated. Here, we identify four proteins that may help connect CikA with the oscillator. Phenotypic analyses of null and overexpression alleles demonstrate that these proteins are involved in at least one of the functions—circadian period regulation, phase resetting, and cell division—attributed to CikA. Predictions based on sequence similarity suggest that these proteins function through protein phosphorylation, iron-sulfur cluster biosynthesis, and redox regulation. Collectively, these results suggest a model for circadian input that incorporates proteins that link the circadian clock, metabolism, and cell division.
Recurrent environmental fluctuations have an impact on cellular activities in diverse organisms by acting both as direct regulatory stimuli and as modulators of an endogenous biological clock (4). For example, light is an acute regulator of many genes and is also a powerful cue for resetting the circadian clock, a genetically defined endogenous timing system that controls the expression of target genes. This internal timing mechanism is synchronized with the environment much like a mechanical clock is set to local time.
The prokaryotic model system for studying biological rhythms is the unicellular cyanobacterium Synechococcus elongatus PCC 7942. The protein products of three genes, kaiA, kaiB, and kaiC, constitute the cyanobacterial central oscillator that generates the endogenous circadian rhythm (19). This Kai-based oscillator can be reconstituted in vitro using only the three recombinant Kai proteins and ATP, producing a rhythm in KaiC phosphorylation with a temperature-compensated, nearly wild-type (WT) period (37). However, in vivo the Kai proteins associate dynamically with other components in a large heteromultimeric complex, termed a periodosome (13), during the circadian cycle (24). Rhythmic gene expression is controlled by this oscillator through the coordination of a rhythmic compaction of the bacterial nucleoid (42) and the action of an oscillator-associated two-component signal transduction pathway (23, 45).
In order for rhythmic processes to be advantageous to the organism, their relative phasing must be appropriately referenced to the day-night cycle. The input pathway of a clock system is responsible for transducing external cues to the central oscillator to maintain this synchrony. Three proteins, Pex (period extender), LdpA (light-dependent period protein), and CikA (circadian input kinase), affect the ability of S. elongatus to respond to external stimuli. Pex delays the internal oscillation to synchronize the endogenous clock with the external light-dark cycle (44). Bound iron-sulfur clusters allow LdpA to sense the redox state of the cell, which in cyanobacteria reflects the flux of light that drives photosynthesis, and adjust the period length (20, 25). A cikA null mutation has pleiotropic effects. In addition to a clock-resetting defect, strains that lack cikA display a shortened circadian period of the gene expression rhythm (41) and elongated cells (32), phenotypes that suggest a central role for CikA in tying the circadian clock to both environmental sensing and the cell division machinery.
CikA contains a central histidine protein kinase (HPK) domain that autophosphorylates, which is an essential activity for CikA function (35). Flanking GAF and pseudoreceiver (PsR) domains activate and repress, respectively, the level of CikA kinase activity (35). The C-terminal PsR domain localizes CikA to the cell pole through predicted interactions with unidentified proteins (49) and directly binds quinones, which suggests sensitivity to changes in the cellular redox state (12, 21).
Both LdpA and CikA are copurified with a complex that also contains the KaiA and KaiC circadian oscillator proteins, indicating the physical colocalization of the oscillator and environmental input divisions of the circadian clock (20). The interaction between CikA and the Kai proteins is likely indirect (21) via partner proteins that are needed to adjust the period or phase of the rhythm to match that of the environmental day. To find these critical intermediate clock components, we used a yeast two-hybrid system to identify direct partners of CikA. Four candidates that consistently passed the stringent selective requirements of the system by their interaction with CikA in Saccharomyces cerevisiae also showed genetic evidence of a relationship to the circadian clock. Phenotypic analyses of null and overexpression alleles of each of the corresponding genes supported roles for their protein products in the circadian clock and provided links to the three functions—cell division, phase resetting, and circadian period regulation—in which CikA is involved. Together, they outline a circadian input pathway that is closely tied to cellular metabolism and the cell cycle.
MATERIALS AND METHODS
Yeast two-hybrid assay.
Expression vectors for CikA variant proteins fused to the GAL4 DNA-binding domain were created by amplifying segments of cikA with primers that incorporated NdeI and SalI sites and ligating the digested PCR products to NdeI/SalI-digested pGBKT7 (Clontech). The full-length cikA sequence encodes 754 amino acids; bait constructs encoded the following CikA residues: CikA-GHR, amino acids 87 to 754 (the GAF, HPK, and PsR domains); CikA-HR, amino acids 322 to 754 (the HPK and PsR domains); and CikA-R, amino acids 611 to 754 (the PsR domain). The prey library contained 1- to 3-kb fragments of Sau3AI-digested S. elongatus genomic DNA, from a strain with the kai locus deleted, ligated to the BamHI site of the prey vector, pGADT7 (Clontech). Bait plasmids were introduced into yeast strain AH109 by small-scale transformation; CikA bait strains were then transformed with the prey library by using a large-scale transformation protocol (Clontech). Doubly transformed cells (107 independent clones) were plated onto minimal synthetic dropout medium (which lacks Trp, Leu, His, and adenine) at 30°C; 25 of 247 initial transformants survived a second selection. Twelve transformants that carried insertions of fewer than 30 nucleotides, six that lacked motifs or orthologs listed in GenBank, and one with a nucleotide sequence whose protein product could not be overexpressed in either Escherichia coli or cyanobacterial cells were not further analyzed; six are described here. Positive (murine p53 prey and simian virus 40 large T antigen bait) and negative (human lamin C prey and T antigen bait) controls were provided by Clontech. Strains that carried CikA bait plasmids and an empty prey vector or an empty bait vector and previously identified prey plasmids did not survive when plated onto minimal synthetic dropout medium. Prey plasmids extracted from yeast strains were used to transform DH10B E. coli cells. The resulting clones were sequenced and used in BLAST analysis (2). Open reading frames (ORFs) are numbered according to the annotation of the complete S. elongatus genome (version 21jun05; http://genome.ornl.gov/microbial/syn_PCC7942/21jun05/syn_PCC7942.html).
Bacterial strains, growth conditions, and DNA manipulations.
Plasmids and S. elongatus PCC 7942 strains are described in Tables 1 and 2, respectively. Independent null alleles of each of the four identified genes were created by the insertion of an antibiotic resistance cassette into the coding region of each gene. A Mu transposon (18) inserted into the nhtA gene gave rise to the 1A8-L4 clone. The kanamycin resistance (Kmr)-Ω cassette from pHP45Ω-Km (11) was used to individually inactivate prkE, ircA, and cdpA. Plasmids that contained genes interrupted by antibiotic resistance cassettes were used to transform cyanobacterial reporter strains; mutant allele replacement at the native locus was verified by PCR (5, 14). Overexpression clones were constructed by amplifying the ORF of each gene of interest from S. elongatus genomic DNA by PCR with primers that incorporated restriction enzyme sequences that were compatible for cloning into the neutral site 1 (NS1) vector pAM2991, as well as the codons to encode six adjacent amino- or carboxy-terminal histidine residues (an N-terminal six-His tag or a C-terminal six-His tag, respectively) for immunoblot detection. The primers for nhtA, ircA, and cdpA encode an N-terminal six-His tag; an additional ircA construct encoding a C-terminal six-His tag was made. The primers for prkE encode a C-terminal six-His tag. Each gene was overexpressed by adding IPTG (isopropyl-β-d-thiogalactopyranoside) at a 1 mM final concentration to cyanobacterial cell cultures to induce transcription from the Ptrc promoter present in pAM2991. Recombinant overexpression constructs were integrated at NS1 of the S. elongatus chromosome (5). All cyanobacterial strains were grown in BG-11 medium as described previously (1, 29).
TABLE 1.
Plasmids used in this study
| Plasmid | Characteristic(s) | Antibiotic resistance profilea | Source or reference |
|---|---|---|---|
| 1A8-L4 | Source of Mu insertion in nhtA | Apr Cmr Kmr | Lab collection |
| pBluescript | E. coli cloning vector | Apr | Strategene |
| pGADT7 | Prey vector for yeast two-hybrid assay | Apr | Clontech |
| pGBKT7 | Bait vector for yeast two-hybrid assay | Kmr | Clontech |
| pHP45Ω-Km | Source of Kmr-Ω cassette | Apr Kmr | 11 |
| pAM2152 | Gmr cassette in cikA | Apr Gmr | 41 |
| pAM2956 | Kmr-Ω cassette from pHP45Ω-Km in pBluescript at EcoRI site | Apr Kmr | Lab collection |
| pAM2957 | Kmr-Ω cassette from pHP45Ω-Km in pBluescript at BamHI site | Apr Kmr | Lab collection |
| pAM2991 | NS1 cloning vector; carries IPTG-inducible promoter | Spr Smr | 20 |
| pAM3032 | pGADT7-nhtA | Apr | This study |
| pAM3034 | pGADT7-prkE | Apr | This study |
| pAM3036 | pGADT7-ircA | Apr | This study |
| pAM3037 | pGADT7-cdpA | Apr | This study |
| pAM3038 | pGBKT7 with CikA-GHR construct | Kmr | This study |
| pAM3041 | pGBKT7 with CikA-R construct | Kmr | This study |
| pAM3044 | Kmr-Ω cassette in HindIII site of ircA in pAM3036 | Apr Kmr | This study |
| pAM3047 | pGADT7-kaiA | Apr | This study |
| pAM3048 | pGADT7-kaiB | Apr | This study |
| pAM3050 | pGADT7-kaiC | Apr | This study |
| pAM3055 | Kmr-Ω cassette in ApaI/XbaI sites of cdpA in pAM3037 | Apr Kmr | This study |
| pAM3292 | prkE overexpression construct in pAM2991; expresses C-terminal six-His tag | Spr Smr | This study |
| pAM3307 | nhtA overexpression construct in pAM2991; expresses N-terminal six-His tag | Spr Smr | This study |
| pAM3330 | Kmr-Ω cassette in HindIII/XbaI sites of prkE subcloned in pBluescript | Apr Kmr | This study |
| pAM3627 | ircA overexpression construct in pAM2991; expresses N-terminal six-His tag | Spr Smr | This study |
| pAM3697 | cdpA overexpression construct in pAM2991; expresses N-terminal six-His tag | Spr Smr | This study |
| pAM3864 | pGBKT7 with CikA-HR construct | Kmr | This study |
Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Gmr, gentamicin resistance; Kmr, kanamycin resistance. Spr, spectinomycin resistance; Smr, streptomycin resistance.
TABLE 2.
Cyanobacterial reporter strains used in this study
| S. elongatus strain | Genetic background | Plasmida | Reporter (integration site)b | Antibiotic resistance profilec | Source or reference |
|---|---|---|---|---|---|
| AMC462 | WT | None | PkaiBC::luxAB (NS1) | Spr Smr Cmr | 36 |
| AMC564 | cikA null | None | PkaiBC::luxAB (NS1) | Spr Smr Cmr Gmr | 41 |
| AMC669 | WT | None | PpsbAI::luxAB (NS2.1) | Cmr | 36 |
| AMC1004 | WT | None | PkaiBC::luxAB (NS2.1) | Cmr Kmr | 49 |
| AMC1005 | cikA null | None | PkaiBC::luxAB (NS2.1) | Cmr Kmr Gmr | 49 |
| AMC1300 | WT | None | PkaiBC::luxAB (NS1) | Spr Smr Kmr | Lab collection |
| AMC1343 | WT | pAM3330 | PkaiBC::luxAB (NS1) | Spr Smr Cmr Kmr | This study |
| AMC1434 | WT | pAM3627 | PpsbAI::luxAB (NS2.1) | Spr Smr Cmr | This study |
| AMC1460 | WT | pAM3292 | PpsbAI::luxAB (NS2.1) | Spr Smr Cmr | This study |
| AMC1465 | WT | pAM3307 | PkaiBC::luxAB (NS2.1) | Spr Smr Cmr Kmr | This study |
| AMC1472 | cikA null | pAM3307 | PkaiBC::luxAB (NS2.1) | Spr Smr Cmr; Kmr Gmr | This study |
| AMC1473 | cikA null | pAM3330 | PkaiBC::luxAB (NS1) | Spr Smr Cmr Kmr Gmr | This study |
| AMC1475 | cikA null | None | PpsbAI::luxAB (NS2.1) | Cmr Gmr | Lab collection |
| AMC1476 | WT | 1A8-L4 | PkaiBC::luxAB (NS1) | Spr Smr Cmr Kmr | This study |
| AMC1478 | WT | pAM3697 | PpsbAI::luxAB (NS2.1) | Spr Smr Cmr | This study |
| AMC1482 | cikA null | pAM3697 | PpsbAI::luxAB (NS2.1) | Spr Smr Cmr Gmr | This study |
| AMC1527 | cikA null | pAM3627 | PpsbAI::luxAB (NS2.1) | Spr Smr Cmr Gmr | This study |
| AMC1683 | WT | pAM3627 | PpsbAI::luxAB (NS2.1) | Spr Smr Cmr Gmr | This study |
All overexpression constructs were integrated at NS1 following homologous recombination with the indicated plasmid, which does not replicate or persist in the cyanobacterial cells.
Reporter constructs were integrated at NS1 or NS2 following homologous recombination. All reporter strains also contained PpsbAI::luxCDE in the NS (either 1 or 2) not occupied by the luxAB reporter. AMC669 and its derivatives contained both PpsbAI::luxAB and PpsbAI::luxCDE in NS2.1.
Antibiotics were added to cyanobacterial culture medium at the following concentrations (in micrograms per milliliter): chloramphenicol, 7.5; gentamicin, 1.5; kanamycin, 5.0; spectinomycin and streptomycin, 2.0 each. Spr, Smr, Cmr, Gmr, and Kmr indicate spectinomycin, streptomycin, chloramphenicol, gentamicin, and kanamycin resistance, respectively.
Whole-cell extract preparation and immunoblot analysis.
Whole-cell protein extracts from cyanobacterial strains that harbored overexpression constructs were prepared as described elsewhere (6) from 10 to 25 ml of an S. elongatus culture, grown under constant light (LL), with an optical density at 750 nm of 0.4 (or higher). Equal amounts of soluble protein (20 to 30 μg) were separated on sodium dodecyl sulfate-12.5% polyacrylamide gel electrophoresis (SDS-12.5% PAGE) gels and transferred onto a 0.45-μm-pore-size Protran nitrocellulose membrane by semidry blot transfer per the instructions of the manufacturer (Bio-Rad). His-tagged proteins were detected using a penta-His antibody according to the directions of the manufacturer (Qiagen) and a peroxidase-conjugated goat anti-mouse immunoglobulin G antibody (Jackson ImmunoResearch). The signal was visualized with the SuperSignal West Pico chemiluminescent substrate detection system (Pierce) and exposed to X-ray film. Prior to induction, the His-tagged PrkE and CdpA proteins could not be detected and NHT-1 produced only a very weak signal on an anti-His immunoblot. After induction, protein levels increased substantially: a gross approximation of induction, given the low basal levels, is 30-fold.
To verify the overexpression of IrcA, disrupted whole-cell cyanobacterial samples were prepared as described previously (22). Very little N-terminally six-His-tagged IrcA was detected in cyanobacterial samples, but the overexpression of C-terminally six-His-tagged IrcA was verified. The circadian effect and heme binding of the N-terminally six-His-tagged variant are described herein; both IrcA constructs provided identical results.
TMBZ staining.
WT (AMC669) and IrcA-overexpressing (AMC1434) cells were grown for 24 h in the presence of 1 mM IPTG. Whole-cell cyanobacterial extracts were prepared as described previously (22) using 5 ml of a culture with an optical density at 750 nm of 0.48. Soluble protein was extracted from cyanobacterial cells as described elsewhere (6). Whole-cell samples or 75-μg soluble protein samples were separated on SDS-12.5% PAGE gels. Peroxidase activity was measured by incubating gels in TMBZ (3,3′,5,5′-tetramethylbenzidine) solution for 1 h as described previously (16). Stain was removed with 70 mM Na2SO3, and the gels were washed three times with 30% isopropanol and stained with Coomassie brilliant blue.
Measurement and analysis of in vivo bioluminescence.
The automated measurement of bioluminescence from S. elongatus reporter strains was performed using a Packard TopCount luminometer (Perkin-Elmer) as described previously (3, 29). Data for inoculated 96-well plates subjected to two synchronizing cycles of 12 h of light and 12 h of darkness and 7 to 12 days of LL were recorded. Because light intensity has a small effect on the circadian period (7, 25), results from samples that received equivalent levels of illumination and were grown in parallel under identical conditions were calculated. For overexpression experiments, a 1 mM final concentration of IPTG was added to WT and cikA mutant strains that did not harbor overexpression constructs as negative controls; the addition of IPTG to these strains did not change the period or phase of the rhythm of bioluminescence. Period values for each strain, with or without the overexpression construct, were determined using at least two cycles before the addition of IPTG and at least three cycles after the addition of IPTG.
All TopCount data were graphed using the import and analysis Excel interface (44; S. A. Kay laboratory, The Scripps Research Institute, La Jolla, CA). Circadian periods and standard deviations were calculated using the Biological Rhythms Analysis software system (available from A. Millar and P. Brown, University of Edinburgh, Edinburgh, United Kingdom).
Phase resetting was assayed by removing an inoculated 96-well plate from the TopCount stacker and placing it in the dark for 5 h at 30°C; an empty black plate was put in its place. Plates were removed at a time point 8 h after they were placed in LL conditions following too clock-synchronizing cycles of 12 h of light and 12 h of darkness (LL8, a point in mid-subjective day equivalent to midday in a diurnal cycle). Phase shifts were determined by comparing the phases of peak bioluminescence from samples that had been subjected to the dark pulse to those from identical plates that remained in LL.
Light microscopy.
Bright-field images were captured through an Olympus IX-70 inverted microscope with a Hamamatsu Orca-ER camera using a 100× oil immersion lens objective. Images were processed with Adobe Photoshop 7.0 to increase the resolution and improve the contrast.
RESULTS
Identification of potential input pathway components.
The Matchmaker 3 Gal4 two-hybrid system (Clontech) was used to identify proteins from S. elongatus that interact with the product of a bait construct (CikA-HR) that expresses the HPK and PsR domains of CikA as a protein fusion to the GAL4 DNA-binding domain (Fig. 1A). The prey library expresses S. elongatus genomic fragments as fusion proteins with the GAL4 activation domain. The interaction between the CikA bait protein and a prey protein activates the transcription of selectable nutritional markers and reporter genes that provide color screens.
FIG. 1.
Identification of proteins found in a yeast two-hybrid assay with CikA bait. (A) Representation of CikA bait constructs. Each carries the GAL4 DNA-binding domain (BD) fused to the GAF, HPK, and/or PsR domain of CikA. (B) Each doubly transformed yeast clone containing one prey construct and one bait construct was streaked onto medium deficient in Trp and Leu to select for the plasmids (SD2DO) or Trp, Leu, His, and adenine (SD4DO) to select for interaction between the prey and bait constructs. Panels 1, p53 and T antigen positive control; panels 2, human lamin C and T antigen negative control; panels 3 to 7, the indicated CikA variant bait construct with the following prey proteins or construct: 3, NHT-1; 4, PrkE; 5, IrcA; 6, CdpA; and 7, empty prey vector. (C) Functional domains of each identified protein. Solid lines and black shapes depict the portion of the protein present in the prey. Dotted lines and white shapes show the remainder of the full-length protein. Proteins and domains are as follows: NHT-1, class V aminotransferase; PrkE, serine/threonine protein kinase; IrcA, potential transmembrane/export signal (TM/ES) and cytochrome c-like (Cyt) domains; and CdpA, no predicted domains.
Six clones, representing four genes, expressed proteins that consistently supported growth in the presence of CikA-HR in yeast on selective medium (Fig. 1B) and produced a dark blue color in colony lift filter β-galactosidase assays (data not shown). These genes were predicted to encode a class V aminotransferase (orf2160), a serine/threonine protein kinase (orf0600), a protein with an N-terminal transmembrane span (as predicted by TMpred [17]) or a hydrophobic export signal (as predicted by SignalP [10]) and a C-terminal conserved heme-binding sequence (orf2387), and a protein with no identifiable features (orf1604). We designated these previously undescribed genes based on their functional domains and the corresponding phenotypic characteristics described below, as follows: aminotransferase-1 gene nhtA; phase-resetting kinase gene prkE; input-related cytochrome gene ircA; and cell division and phase gene cdpA, respectively. Figure 1C shows the predicted domains of each protein product. PrkE and CdpA were each identified from two independent prey clones.
Prey proteins were retested with bait constructs that encoded different domains of CikA (Fig. 1A) to better delineate the interactions. All four prey proteins gave positive results with either CikA-GHR (GAF, HPK, and PsR) or the original CikA-HR bait construct (Fig. 1B); only IrcA and CdpA interacted with the product of a PsR-only bait construct (CikA-R) (Fig. 1B). In order to test the prediction that these proteins are part of CikA-related signaling pathways, both null and overexpression mutants were tested for circadian phenotypes. Our results showed that each of the four proteins described here—NHT-1, PrkE, IrcA, and CdpA—plays a role in the S. elongatus circadian clock and helps to define the multiple pathways that CikA integrates within the cell.
The inactivation of prkE alters phase resetting.
Null alleles of each newly identified gene were generated by interrupting each ORF with an antibiotic resistance cassette (Tables 1 and 2). These insertional mutations were used to transform cyanobacterial PkaiBC::luxAB luciferase reporter strains, in which the inserted alleles replaced the WT alleles by recombination (5). PCR analysis of genomic DNA from putative null strains showed that both inactivated and WT copies of the genes ircA and cdpA were present (data not shown). In S. elongatus, which carries multiple copies of its chromosome (33), the failure of a selected null allele to completely segregate is evidence of an essential gene (15). In each case, the downstream gene was on the opposite strand, which suggests that the deleterious effect upon the inactivation of either ircA or cdpA was due to the loss of the targeted gene and was not the result of a polar effect. The other two null alleles, those for nhtA and prkE, fully segregated. No differences in the periods, phases, or amplitudes of the rhythms of bioluminescence between the WT and either of the mutants with fully segregated alleles under free-running (LL) conditions were detected (data not shown).
CikA plays multiple roles in S. elongatus cells, and interacting proteins may affect aspects that would not be evident under free-running conditions. A predicted role for the identified proteins involves input pathways of the circadian clock; the inactivation of the corresponding genes would affect the ability to detect changes in the external environment, as previously reported (43). Thus, the mutant strains with fully segregated alleles (nhtA and prkE) were tested for the ability to recognize exposure to darkness and reset their rhythms. A cikA null strain exhibits very little resetting in response to 5-h dark pulses throughout the circadian cycle (41). The response of WT cells to darkness is the greatest and most reproducible at LL8. Therefore, WT and mutant strains were tested in response to dark pulses at LL8.
When WT cells were subjected to the dark pulse, the phase of bioluminescence expression from the PkaiBC::luxAB reporter consistently advanced, such that the peak of bioluminescence from these cells occurred about 6 h earlier than that from cells maintained in LL (Fig. 2A). The nhtA null strain showed WT resetting (data not shown); the prkE null mutant, however, showed a different magnitude of phase shifting from that of the WT. As shown in Fig. 2B, the prkE mutant reset its rhythm unpredictably, even in independent samples of the same clone, with phase advances ranging from 6 to 11 h. With each experimental trial, the prkE mutant consistently shifted differently from the WT, although the magnitude and direction of the phase shift varied among trials; at times, wells inoculated with samples from the same liquid culture of a prkE mutant strain exhibited both advances and delays even when all other experimental parameters were identical (see Fig. S1 in the supplemental material). In contrast, the direction and magnitude of resetting in the WT were predictable and reproducible.
FIG. 2.
Proper phase resetting requires the prkE gene. Bioluminescence from a PkaiBC::luxAB reporter in a WT background (A), a prkE inactivation mutant (B), a cikA null mutant (C), and a cikA prkE double mutant (D) is expressed as counts per second. Open symbols represent results for cells subjected to LL with no dark pulse; closed symbols represent results for cells subjected to a 5-h dark pulse at LL8, which is depicted as a black box on the x axis. Two independent traces for dark-pulsed strains are shown to demonstrate the reproducible phase resetting of the WT (A) and the lack of phase resetting in the absence of cikA, either in a single mutant (C) or in the cikA prkE double mutant (D). Three independent traces for the prkE mutant are shown to demonstrate the unpredictable magnitude of its phase shift (B). Arrows indicate the phase of peak expression after the dark pulse. A more extreme example of erratic resetting in the prkE mutant is show in Fig. S1 in the supplemental material.
Because CikA is necessary for the circadian system to reset in response to environmental cues, the ability of a strain that lacked cikA and either nhtA or prkE to respond to dark pulses was also tested. Consistent with previously published data (12), the cikA null strain was unresponsive to the dark pulse at LL8 and did not change its pattern of rhythmicity (Fig. 2C). Each of the double mutants resembled the cikA mutant strain, with no noticeable phase resetting (Fig. 2D and data not shown). The cikA prkE double mutant displayed a cikA null, nonresetting phenotype throughout the circadian cycle (data not shown). Thus, CikA is required for phase resetting with or without PrkE, and PrkE likely lies upstream of CikA in the input pathway.
The overexpression of NHT-1, IrcA, or CdpA affects circadian properties.
The overexpression of many cyanobacterial clock proteins, including KaiA, KaiC, and CikA, causes arrhythmia (19, 49), presumably by competing for binding of clock components and disrupting the flow of communication. In particular, the circadian phenotype when CikA is overexpressed (arrhythmic) is more severe than that when CikA is absent (rhythms are dampened but still present) (49). The overexpression of other clock-related proteins alters the circadian period but the rhythm remains robust (36). We tested whether the overexpression of each identified protein would help to elucidate the roles of the proteins in the clock where the null alleles did not, either because the mutant alleles were unable to segregate completely or because other proteins in the cell have similar functions. Each of the four identified ORFs was cloned downstream of an IPTG-inducible promoter, and each construct was integrated into the S. elongatus chromosomes of reporter strains. Despite the striking phenotype of the prkE null mutant, the overexpression of prkE did not cause any significant change to the period or phase of bioluminescence in LL (data not shown).
The nhtA overexpression construct caused a lengthening of the circadian period upon induction by IPTG (Fig. 3). Excess NHT-1 in a WT background caused the period of oscillation to increase by approximately 1 h (Fig. 3A). In the cikA null background, the overexpression of nhtA increased the period length by nearly 2 h (Fig. 3B), producing the same period seen when NHT-1 protein was provided in excess in the WT strain (Fig. 3C). These results suggest that NHT-1 exerts its effect downstream of CikA or participates in a pathway parallel to that of CikA to the central oscillator.
FIG. 3.
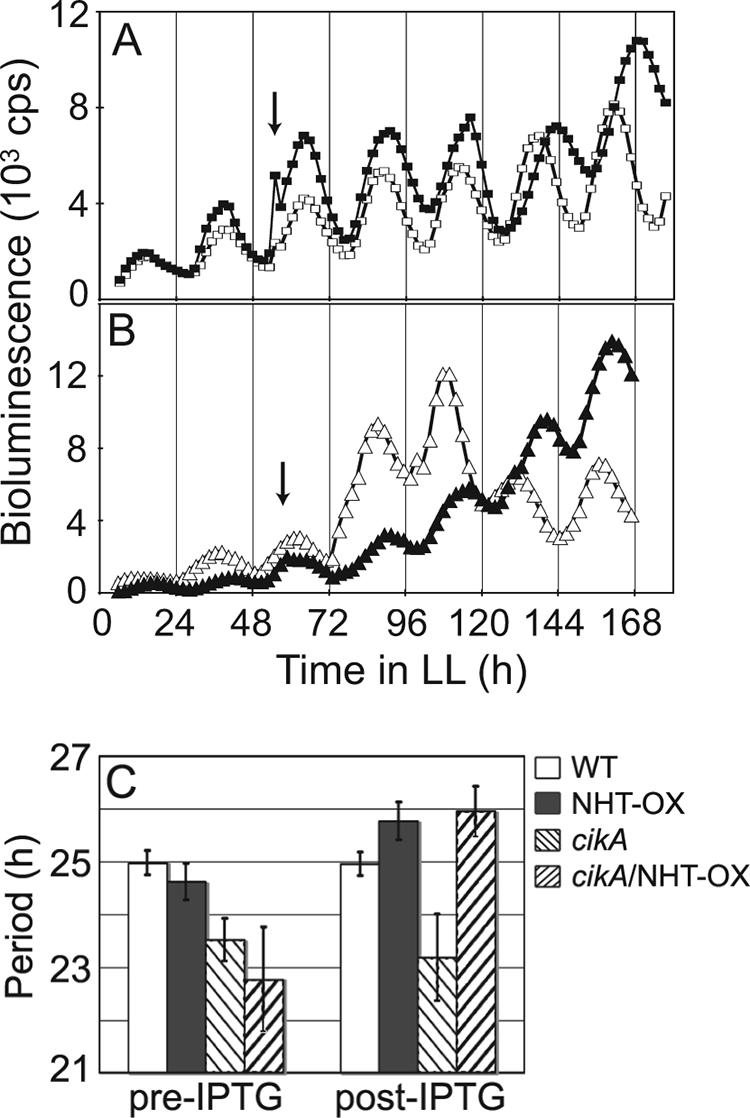
The overexpression of NHT-1 lengthens the circadian period independently of CikA. Bioluminescence from a PkaiBC::luxAB reporter is expressed as counts per second. Arrows show the time point at which 1 mM IPTG was added. (A) Open squares, WT; closed squares, strain overexpressing NHT-1 in a WT background. (B) Open triangles, cikA null mutant; closed triangles, cikA null mutant overexpressing NHT-1. (C) Quantitative comparison of mean period lengths pre-IPTG addition versus post-IPTG addition for the WT (24.98 ± 0.23 h versus 24.95 ± 0.22 h; n = 4), a strain overexpressing NHT-1 in a WT background (NHT-OX; 24.62 ± 0.34 h versus 25.77 ± 0.35 h; n = 7), a cikA null mutant (23.52 ± 0.40 h versus 23.18 ± 0.82 h; n = 5), and a cikA null mutant overexpressing NHT-1 (cikA/NHT-OX; 22.77 ± 0.99 h versus 25.95 ± 0.47 h; n = 3).
In contrast to the overexpression of NHT-1, which changed the period, the induction of ircA or cdpA did not alter the period of the circadian rhythm but instead caused a stable change in the phase of peak expression from a PpsbAI::luxAB reporter (Fig. 4A and C); the peak of the rhythm occurred 8 or 6 h, respectively, later than that of the WT rhythm. The overexpression of either IrcA or CdpA also altered the timing of peak bioluminescence in the absence of CikA, without changing the characteristically short period of a cikA null mutant (Fig. 4B and D). These data suggest that IrcA and CdpA act in input pathways parallel to that of CikA.
FIG. 4.
The overexpression of either IrcA or CdpA delays the phase of circadian gene expression. Bioluminescence from a PpsbAI::luxAB reporter is expressed as counts per second. (A) Open circles, WT; closed circles, strain overexpressing IrcA in a WT background. (B) Open diamonds, cikA null mutant; closed diamonds, cikA null mutant overexpressing IrcA. (C) Open squares, WT; closed squares, strain overexpressing CdpA in a WT background. (D) Open triangles, cikA null mutant; closed triangles, cikA null mutant overexpressing CdpA. Arrows show the time point at which 1 mM IPTG was added.
IrcA and CdpA.
The amino acid sequence of IrcA predicts the presence of a cytochrome c domain with a conserved heme-binding sequence (Fig. 1C). We tested whether IrcA possesses heme-dependent peroxidase activity by incubating SDS-PAGE gels in a solution of TMBZ and hydrogen peroxide. Extracts from cyanobacterial cells that overexpressed the IrcA protein showed a blue band upon SDS-PAGE after TMBZ staining, indicative of a covalently bound heme (Fig. 5). Interestingly, the IrcA protein overexpressed in E. coli did not bind heme (data not shown) despite its elevated abundance as determined by Coomassie brilliant blue staining; IrcA found in the soluble fractions of cyanobacterial extracts, presumably prior to association with the membrane, also did not have a covalently bound heme (Fig. 5). These results suggest that the local environment is important for IrcA redox sensitivity and is specific to the cyanobacterial cell.
FIG. 5.

IrcA covalently binds a heme. Whole-cell and soluble protein extracts from WT (AMC669) and IrcA-overexpressing (AMC1434) cells were separated by electrophoresis, and peroxidase activity was measured by incubating gels in TMBZ solution. A blue, TMBZ-positive band is present at the predicted size (76 kDa) for IrcA in the whole-cell extract (indicated by an arrowhead) but absent in the soluble extract. The soluble extract contains an elevated abundance of IrcA as determined by Coomassie brilliant blue (CBB) staining (indicated by an arrowhead).
The CdpA protein has no recognizable motifs in its amino acid sequence, and the inability to inactivate cdpA suggests that the gene is essential for viability. Because CdpA interacts with CikA in the yeast system, and cikA mutants display a defect in cell division such that the lengths of the cells are more than twice that of the WT cell (32), the cell morphologies of strains that overexpressed CdpA were examined. The addition of IPTG to WT (Fig. 6A) or cikA null (Fig. 6D) cells did not affect cell size compared to the sizes of cells that did not receive the inducer (data not shown). In the absence of the inducer, the cdpA transgene was expressed at a low basal level due to a leaky promoter; populations of cikA mutant cells that carried the inducible cdpA construct displayed a range of cell lengths and included more normal-sized cells than were seen among populations of cells with the cikA mutation alone (Fig. 6E). When CdpA was expressed at high levels in the cikA null strain, the cell division defect was suppressed and cells exhibited WT lengths (Fig. 6F). After the induction of cdpA in the WT background, nearly every cell was in the process of cell division (Fig. 6C). Thus, CdpA appears to act in the regulation of cell division and, when produced at high levels, can suppress the negative effects exhibited in the absence of CikA. The overexpression of NHT-1 or PrkE, confirmed by immunoblot analysis, did not affect cell morphology (data not shown). The induction of IrcA by IPTG resulted in the immediate cessation of cell division, even though cells were viable, as was evident by circadian monitoring (Fig. 4A and B). Cell morphologies showed defects in thylakoid arrangement, consistent with a role for IrcA in metabolic regulation (see Fig. S2 in the supplemental material).
FIG. 6.
The overexpression of CdpA suppresses the cell division defect of a cikA mutant. Strains with WT (A to C) and cikA null (D to F) backgrounds with and without an ectopically expressed IPTG-inducible cdpA allele were analyzed by bright-field microscopy. The addition of 0.5 mM IPTG to WT (A) and cikA mutant (D) cells had no effect when no cdpA transgene was present; no-IPTG samples were indistinguishable from the transgene-lacking samples to which IPTG was added and are not shown. The cikA null mutation produced elongated cells compared to those of the WT. (E) In the absence of an inducer, the cdpA transgene was expressed at a low basal level; more normal-sized cells are evident in panel E than in panel D. Twenty hours after IPTG induction, the length of cells in the cikA mutant population overexpressing CdpA was nearly normal (F), and in the sample of WT cells overexpressing CdpA, almost all cells were undergoing division (C).
DISCUSSION
Cells that lack CikA display three main phenotypes: an altered circadian period, the inability to reset the phases of rhythms in response to external stimuli, and an elongated shape. Additionally, CikA binds a redox cofactor. Four proteins identified as interacting with CikA by using a yeast two-hybrid assay each affect at least one function in which CikA is involved. We predict that these proteins act as bridges that connect CikA to the periodosome (either directly or indirectly), as CikA can be copurified with the Kai complex (21), LdpA (20), and an output pathway component, SasA (20), yet CikA bait variants cotransformed with individual Kai prey constructs (see Fig. S3 in the supplemental material) or an LdpA prey construct (20) are negative in the yeast two-hybrid assay. This screen did not identify a putative cognate response regulator for CikA, which is a canonical HPK and is predicted to have such a partner (30). Although some cognate HPK-response regulator pairs have been detected previously using the yeast two-hybrid system (26, 39), many interactions are transient and would not provide sufficient interaction to overcome the nutritional selection of the system.
Three of the CikA-interacting proteins—NHT-1, IrcA, and CdpA—substantially changed the properties of the circadian rhythm of gene expression in LL when they were produced in excess. The overexpression of NHT-1 increased the period to the same length in either a WT or a cikA mutant background (Fig. 3), which is consistent with a role downstream of CikA in the circadian system. The NHT-1 sequence is highly conserved among 40 cyanobacterial sequences available in GenBank as of February 2008 and has extensive similarity to class V aminotransferases (see Fig. S4 in the supplemental material). The NifS protein also belongs to this class and is involved in the synthesis of iron-sulfur clusters (48). The presence of such clusters is important to the function of LdpA, which is part of a complex that also includes CikA, in sensing changes in the redox state (i.e., light intensity) to adjust the period length of the S. elongatus circadian rhythm. A possible role for NHT-1 is in the assembly of the iron-sulfur cofactor for LdpA, which is also nearly ubiquitous among cyanobacteria (8).
CikA directly binds a quinone, and the degradation of CikA in vivo is accelerated by the binding of a quinone analog (12, 21), suggesting that the resetting of the phase of the cyanobacterial circadian rhythm is tightly linked to cellular metabolism. The IrcA protein covalently binds a heme and has peroxidase activity (Fig. 5), which would allow it to sense and control the redox state of the cell. Although yeast two-hybrid assay results consistently supported the interaction of IrcA with CikA, topological predictions (10, 17) are consistent with a topology in which the heme-binding domain of IrcA is in the periplasm of the cell. This topology would also be consistent with the maturation of c-type cytochromes in the periplasm of bacteria, such as E. coli, in which the process has been studied; however, cyanobacteria use different maturation proteins (46). If most or all of the mature protein is localized in the periplasm, then interaction with CikA would likely occur with the precursor before it is exported. Alternatively, the proteins do not genuinely interact in the cyanobacterium even though both have roles in the circadian clock and bind redox-active cofactors (21).
Despite the central role of CikA in resetting the Kai-based clock, which is ubiquitous among cyanobacteria (28), only S. elongatus strains PCC 7942 and PCC 6301 (syc0882), among 40 cyanobacterial strains with available genome sequences (as listed in GenBank as of February 2008), encode full-length CikA protein sequences, with the 150-amino-acid N-terminal sequence distinguishing CikA from many other related cyanobacterial histidine kinases. Sequences similar to that encoding the full-length IrcA protein are found in only S. elongatus PCC 6301 (syc1718_d) and Synechocystis sp. strain PCC 6803 (sll1359). The presence of clear CikA and IrcA homologs in only these strains suggests that the cyanobacteria, known to be diverse and deeply branching, are likely to have different input pathways for their circadian systems. Likewise, some cyanobacterial species possess more than one copy of the kai genes or lack the kaiA gene entirely, such that multiple variations on the basic oscillator mechanism are likely to exist (9).
The CdpA protein plays a role not only in the regulation of the circadian phase but also in another cellular process, cell division, in which CikA is involved. A link between cell division and the clock has been demonstrated previously (33, 34). The biological clock gates cytokinesis, such that there are times within the circadian cycle that division is inhibited. This interval in S. elongatus extends from late day into early night (33). During the window in which cell division is blocked, the Kai proteins and SasA are beginning their formation of the periodosome, a large multimeric complex that assembles and disassembles over the course of a day; the temporal information that this complex maintains in the mother cell is passed on to the daughter cells with a heritable and precise period and phase (31). Attempting to split up this complex during the inhibition phase of the circadian cycle, when the complex is vulnerable to perturbations, may lead to a disruption of the timing mechanism within the cell. Also during this cytokinesis-gated time, the chromosome is undergoing topological changes from a decondensed nucleoid into a tightly condensed state, and it is likely detrimental to the cells to attempt to divide when the chromosome is in this condensed state (42).
The unprecedented, erratic phase resetting of the prkE null mutant clearly places PrkE in the input pathway of the cyanobacterial clock. Even among samples that came from the same original liquid culture, the individual wells of microtiter plates exhibited unpredictable resetting in response to a 5-h dark pulse (Fig. 2; also see Fig. S1 in the supplemental material). Individual WT cells, monitored for circadian periods and phases in microcolonies, inherit circadian properties through cell divisions without apparent coupling between cells (31). Thus, it is unclear how the entire population of prkE mutant cells within a sample well could reset in unison while the resetting times of clonal siblings on the same plate could be quite different. The disrupted resetting in the prkE null mutant required CikA, because when CikA and PrkE were both absent, the cells could not reset the phase of their rhythm at all, a phenotype typical of cikA null strains.
Based on sequence similarity, the function predicted for PrkE is the phosphorylation of other proteins at serine and/or threonine residues. Among the many possible substrates of PrkE is KaiC, which has been shown previously to autophosphorylate on two or three residues in vitro (38, 40). When any of these phosphorylation sites are eliminated, the rhythms of bioluminescence are lost in vivo (47). Although this phosphorylation is due to autokinase activity, it is possible that in vivo the autophosphorylation event is required before the additional phosphorylation of KaiC by other proteins can occur. A mutation identified in kaiC (pr1) abolishes the ability of the strain to reset in response to dark pulses (27). This mutation also severely decreases the rhythm in the levels of phosphorylated KaiC over the circadian cycle (27). In the pr1 mutant, KaiC exists in both the phosphorylated and unphosphorylated forms in approximately equal amounts in LL for at least 2 days. In the absence of PrkE, the phosphorylation status of KaiC may be compromised, which may lead to the inability of the oscillator to accurately reflect changes in the environment.
Overall, we have identified four additional proteins involved in the S. elongatus circadian system. These proteins predict a circadian input pathway that is tightly linked to fundamental cellular processes, including metabolism and cell division.
Supplementary Material
Acknowledgments
We thank R. A. Mella for assistance with microscopy procedures T. M. Bricker for advice on TMBZ staining, Y. Chen, C. K. Holtman, H. Guo, and N. B. Ivleva for plasmids, and J. L. Ditty and L. Z. Bartoszek for careful reading of the manuscript. L. Harris-Haller and the Gene Technology Laboratory staff (Institute of Developmental and Molecular Biology, Texas A&M University) provided sequence support services.
This research was supported by a grant from the National Institutes of Health (R01 GM62419) to S.S.G.
Footnotes
Published ahead of print on 14 March 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Allen, M. M. 1968. Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 41-4. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215403-410. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, C. R., N. F. Tsinoremas, J. Shelton, N. V. Lebedeva, J. Yarrow, H. Min, and S. S. Golden. 2000. Application of bioluminescence to the study of circadian rhythms in cyanobacteria. Methods Enzymol. 305527-542. [DOI] [PubMed] [Google Scholar]
- 4.Bell-Pedersen, D., V. M. Cassone, D. J. Earnest, S. S. Golden, P. E. Hardin, T. L. Thomas, and M. J. Zoran. 2005. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat. Rev. Genet. 6544-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clerico, E. M., J. L. Ditty, and S. S. Golden. 2007. Specialized techniques for site-directed mutagenesis in cyanobacteria, p. 155-172. In E. Rosato (ed.), Methods in molecular biology. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 6.Ditty, J. L., S. R. Canales, B. E. Anderson, S. B. Williams, and S. S. Golden. 2005. Stability of the Synechococcus elongatus PCC 7942 circadian clock under directed anti-phase expression of the kai genes. Microbiology 1512605-2613. [DOI] [PubMed] [Google Scholar]
- 7.Ditty, J. L., S. B. Williams, and S. S. Golden. 2003. A cyanobacterial circadian timing mechanism. Annu. Rev. Genet. 37513-543. [DOI] [PubMed] [Google Scholar]
- 8.Dvornyk, V. 2005. Molecular evolution of ldpA, a gene mediating the circadian input signal in cyanobacteria. J. Mol. Evol. 60105-112. [DOI] [PubMed] [Google Scholar]
- 9.Dvornyk, V., O. Vinogradova, and E. Nevo. 2003. Origin and evolution of circadian clock genes in prokaryotes. Proc. Natl. Acad. Sci. USA 1002495-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emanuelsson, O., S. Brunak, G. von Heijne, and H. Nielsen. 2007. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc. 2953-971. [DOI] [PubMed] [Google Scholar]
- 11.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene 52147-154. [DOI] [PubMed] [Google Scholar]
- 12.Gao, T., X. Zhang, N. B. Ivleva, S. S. Golden, and A. LiWang. 2007. NMR structure of the pseudo-receiver domain of CikA. Protein Sci. 16465-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golden, S. S. 2004. Meshing the gears of the cyanobacterial circadian clock. Proc. Natl. Acad. Sci. USA 10113697-13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golden, S. S. 1988. Mutagenesis of cyanobacteria by classical and gene-transfer-based methods. Methods Enzymol. 167714-727. [DOI] [PubMed] [Google Scholar]
- 15.Golden, S. S., J. Brusslan, and R. Haselkorn. 1987. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 153215-231. [DOI] [PubMed] [Google Scholar]
- 16.Guikema, J. A., and L. A. Sherman. 1980. Electrophoretic profiles of cyanobacterial membrane polypeptides showing heme-dependent peroxidase activity. Biochim. Biophys. Acta 637189-201. [Google Scholar]
- 17.Hofmann, K., and W. Stoffel. 1993. TMbase: a database of membrane spanning protein segments. Biol. Chem. 374166. [Google Scholar]
- 18.Holtman, C. K., Y. Chen, P. Sandoval, A. Gonzales, M. S. Nalty, T. L. Thomas, P. Youderian, and S. S. Golden. 2005. High-throughput functional analysis of the Synechococcus elongatus PCC 7942 genome. DNA Res. 12103-115. [DOI] [PubMed] [Google Scholar]
- 19.Ishiura, M., S. Kutsuna, S. Aoki, H. Iwasaki, C. R. Andersson, A. Tanabe, S. S. Golden, C. H. Johnson, and T. Kondo. 1998. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science 2811519-1523. [DOI] [PubMed] [Google Scholar]
- 20.Ivleva, N. B., M. R. Bramlett, P. A. Lindahl, and S. S. Golden. 2005. LdpA: a component of the circadian clock senses redox state of the cell. EMBO J. 241202-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivleva, N. B., T. Gao, A. C. LiWang, and S. S. Golden. 2006. Quinone sensing by the circadian input kinase of the cyanobacterial circadian clock. Proc. Natl. Acad. Sci. USA 10317468-17473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivleva, N. B., and S. S. Golden. 2007. Protein extraction, fractionation, and purification from cyanobacteria, p. 365-373. In E. Rosato (ed.), Methods in molecular biology. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 23.Iwasaki, H., S. B. Williams, Y. Kitayama, M. Ishiura, S. S. Golden, and T. Kondo. 2000. A KaiC-interacting sensory histidine kinase, SasA, necessary to sustain robust circadian oscillation in cyanobacteria. Cell 101223-233. [DOI] [PubMed] [Google Scholar]
- 24.Kageyama, H., T. Kondo, and H. Iwasaki. 2002. Circadian formation of clock protein complexes by KaiA, KaiB, KaiC and SasA in cyanobacteria. J. Biol. Chem. 2782388-2395. [DOI] [PubMed] [Google Scholar]
- 25.Katayama, M., T. Kondo, J. Xiong, and S. S. Golden. 2003. ldpA encodes an iron-sulfur protein involved in light-dependent modulation of the circadian period in the cyanobacterium Synechococcus elongatus PCC 7942. J. Bacteriol. 1851415-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirby, J. R., and D. R. Zusman. 2003. Chemosensory regulation of developmental gene expression in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 1002008-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiyohara, Y. B., M. Katayama, and T. Kondo. 2005. A novel mutation in kaiC affects resetting of the cyanobacterial circadian clock. J. Bacteriol. 1872559-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorne, J., J. Scheffer, A. Lee, M. Painter, and V. P. Miao. 2000. Genes controlling circadian rhythm are widely distributed in cyanobacteria. FEMS Microbiol. Lett. 189129-133. [DOI] [PubMed] [Google Scholar]
- 29.Mackey, S. R., J. L. Ditty, E. M. Clerico, and S. S. Golden. 2007. Detection of rhythmic bioluminescence from luciferase reporters in cyanobacteria, p. 115-129. In E. Rosato (ed.), Methods in molecular biology. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 30.Mackey, S. R., and S. S. Golden. 2007. Winding up the cyanobacterial circadian clock. Trends Microbiol. 15381-388. [DOI] [PubMed] [Google Scholar]
- 31.Mihalcescu, I., W. Hsing, and S. Leibler. 2004. Resilient circadian oscillator revealed in individual cyanobacteria. Nature 43081-85. [DOI] [PubMed] [Google Scholar]
- 32.Miyagishima, S. Y., C. P. Wolk, and K. W. Osteryoung. 2005. Identification of cyanobacterial cell division genes by comparative and mutational analyses. Mol. Microbiol. 56126-143. [DOI] [PubMed] [Google Scholar]
- 33.Mori, T., B. Binder, and C. H. Johnson. 1996. Circadian gating of cell division in cyanobacteria growing with average doubling times of less than 24 hours. Proc. Natl. Acad. Sci. USA 9310183-10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori, T., and C. H. Johnson. 2000. Circadian control of cell division in unicellular organisms. Prog. Cell Cycle Res. 4185-192. [DOI] [PubMed] [Google Scholar]
- 35.Mutsuda, M., K. P. Michel, X. Zhang, B. L. Montgomery, and S. S. Golden. 2003. Biochemical properties of CikA, an unusual phytochrome-like histidine protein kinase that resets the circadian clock in Synechococcus elongatus PCC 7942. J. Biol. Chem. 27819102-19110. [DOI] [PubMed] [Google Scholar]
- 36.Nair, U., J. L. Ditty, H. Min, and S. S. Golden. 2002. Roles for sigma factors in global circadian regulation of the cyanobacterial genome. J. Bacteriol. 1843530-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakajima, M., K. Imai, H. Ito, T. Nishiwaki, Y. Murayama, H. Iwasaki, T. Oyama, and T. Kondo. 2005. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science 308414-415. [DOI] [PubMed] [Google Scholar]
- 38.Nishiwaki, T., H. Iwasaki, M. Ishiura, and T. Kondo. 2000. Nucleotide binding and autophosphorylation of the clock protein KaiC as a circadian timing process of cyanobacteria. Proc. Natl. Acad. Sci. USA 97495-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohta, N., and A. Newton. 2003. The core dimerization domains of histidine kinases contain recognition specificity for the cognate response regulator. J. Bacteriol. 1854424-4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pattanayek, R., J. Wang, T. Mori, Y. Xu, C. H. Johnson, and M. Egli. 2004. Visualizing a circadian clock protein: crystal structure of KaiC and functional insights. Mol. Cell 15375-388. [DOI] [PubMed] [Google Scholar]
- 41.Schmitz, O., M. Katayama, S. B. Williams, T. Kondo, and S. S. Golden. 2000. CikA, a bacteriophytochrome that resets the cyanobacterial circadian clock. Science 289765-768. [DOI] [PubMed] [Google Scholar]
- 42.Smith, R. M., and S. B. Williams. 2006. Circadian rhythms in gene transcription imparted by chromosome compaction in the cyanobacterium Synechococcus elongatus. Proc. Natl. Acad. Sci. USA 1038564-8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanewsky, R., M. Kaneko, P. Emery, B. Beretta, K. Wager-Smith, S. A. Kay, M. Rosbash, and J. C. Hall. 1998. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95681-692. [DOI] [PubMed] [Google Scholar]
- 44.Takai, N., S. Ikeuchi, K. Manabe, and S. Kutsuna. 2006. Expression of the circadian clock-related gene pex in cyanobacteria increases in darkness and is required to delay the clock. J. Biol. Rhythms 21235-244. [DOI] [PubMed] [Google Scholar]
- 45.Takai, N., M. Nakajima, T. Oyama, R. Kito, C. Sugita, M. Sugita, T. Kondo, and H. Iwasaki. 2006. A KaiC-associating SasA-RpaA two-component regulatory system as a major circadian timing mediator in cyanobacteria. Proc. Natl. Acad. Sci. USA 10312109-12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thony-Meyer, L. 2002. Cytochrome c maturation: a complex pathway for a simple task? Biochem. Soc. Trans. 30633-638. [DOI] [PubMed] [Google Scholar]
- 47.Xu, Y., T. Mori, R. Pattanayek, S. Pattanayek, M. Egli, and C. H. Johnson. 2004. Identification of key phosphorylation sites in the circadian clock protein KaiC by crystallographic and mutagenetic analyses. Proc. Natl. Acad. Sci. USA 10113933-13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuvaniyama, P., J. N. Agar, V. L. Cash, M. K. Johnson, and D. R. Dean. 2000. NifS-directed assembly of a transient [2Fe-2S] cluster within the NifU protein. Proc. Natl. Acad. Sci. USA 97599-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, X., G. Dong, and S. S. Golden. 2006. The pseudo-receiver domain of CikA regulates cyanobacterial circadian input. Mol. Microbiol. 60658-668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






