Abstract
Previous research demonstrated that the sympathoadrenal catecholamine norepinephrine could promote the growth of Bordetella bronchiseptica in iron-restricted medium containing serum. In this study, norepinephrine was demonstrated to stimulate growth of this organism in the presence of partially iron-saturated transferrin but not lactoferrin. Although norepinephrine is known to induce transcription of the Bordetella bfeA enterobactin catechol xenosiderophore receptor gene, neither a bfeA mutant nor a bfeR regulator mutant was defective in growth responsiveness to norepinephrine. However, growth of a tonB mutant strain was not enhanced by norepinephrine, indicating that the response to this catecholamine was the result of high-affinity outer membrane transport. The B. bronchiseptica genome encodes a total of 19 known and predicted iron transport receptor genes, none of which, when mutated individually, were found to confer a defect in norepinephrine-mediated growth stimulation in the presence of transferrin. Labeling experiments demonstrated a TonB-dependent increase in cell-associated iron levels when bacteria grown in the presence of 55Fe-transferrin were exposed to norepinephrine. In addition, TonB was required for maximum levels of cell-associated norepinephrine. Together, these results demonstrate that norepinephrine facilitates B. bronchiseptica iron acquisition from the iron carrier protein transferrin and this process may represent a mechanism by which some bacterial pathogens obtain this essential nutrient in the host environment.
The acquisition of essential nutrients, such as iron, in the host environment is key to successful infection by bacterial pathogens. However, much of the extracellular iron in the host is bound by high-affinity iron-chelating glycoproteins belonging to the transferrin family. Transferrin and lactoferrin are two members of this family that are predominantly found in serum and mucosal secretions, respectively (26, 34, 47). These proteins play an important role in iron homeostasis and sequestration, but several bacterial species are able to exploit them as sources of nutritional iron. Bacterial utilization of these host iron-binding proteins can occur via dedicated surface receptors, as exemplified by the TbpAB and LbpAB transporter complexes of Neisseria species (15). Siderophores produced and excreted by many microbes can also contribute to iron acquisition by stripping the iron from transferrins and delivering it to microbial recipients through ferric siderophore transport machinery (29).
The respiratory pathogens Bordetella bronchiseptica and Bordetella pertussis have multiple characterized mechanisms of iron acquisition, including transport of heme-iron (55), biosynthesis and utilization of the alcaligin siderophore (10, 27), and uptake of catechol xenosiderophores, such as enterobactin (4, 8). In addition, there are other putative outer membrane iron transporters encoded in the Bordetella genome for which the ligand is unknown (41). Bordetella species can obtain the iron from transferrin (transferrin-iron) and lactoferrin (45); however, neither the B. bronchiseptica nor B. pertussis genome appears to code for TbpAB or LbpAB receptor homologs. Two low-molecular-mass Bordetella proteins that bound transferrin and lactoferrin were previously isolated, but the identity and function of these proteins remain unknown (35). Even though both transferrin and lactoferrin associate tightly with the B. pertussis cell surface (45), direct contact is reportedly not essential for internalization of the iron (25, 35). It is therefore likely that Bordetella iron acquisition from transferrin and lactoferrin is mediated by siderophores or other iron-chelating molecules.
The catecholamines epinephrine, norepinephrine, and dopamine are widely distributed throughout plant and animal species. In humans, epinephrine is synthesized in the adrenal medulla and released into the bloodstream as a result of impulses from the central nervous system. Norepinephrine is synthesized and stored primarily in peripheral sympathetic nerve endings where it exerts local physiologic effects. Dopamine is a neurotransmitter of the central nervous system but is also present in sympathetic nerves and the adrenal medulla (58). All three molecules are effectors of the mammalian sympathetic nervous system, and there is increasing evidence that these catecholamines are also perceived by bacterial pathogens (48, 52). One bacterial response that has been attributed to catecholamines is the ability to stimulate growth in the presence of serum, transferrin, or lactoferrin (13, 21, 31). Freestone et al. originally identified the growth-promoting serum component as transferrin and although the mechanism remains unclear, it was found that norepinephrine liberates iron from transferrin and lactoferrin, making it available for growth of Escherichia coli (23). Complexes of norepinephrine with ferric transferrin or ferric lactoferrin were demonstrated, but a stable ferric norepinephrine complex was not observed. However, it is known that catecholamines are capable of binding iron, and norepinephrine-iron complexes have been analyzed in other neurochemical studies (40, 51).
In iron-starved Bordetella cells, enterobactin induces transcription of its cognate receptor gene bfeA in a process that is dependent on the BfeR AraC-type positive regulator (3). Analysis of other catechol compounds demonstrated that dopamine, norepinephrine, and epinephrine also induced transcription of bfeA, and norepinephrine was additionally capable of stimulating Bordetella growth in iron-limiting medium containing serum (4). The localization of norepinephrine in peripheral tissues suggests that bacterial inhabitants of the respiratory tract, such as Bordetella species, are more likely to encounter this catecholamine during infection than epinephrine or dopamine. Based on our experimental observations and evidence from the literature that transferrin and lactoferrin potentiate norepinephrine-mediated growth stimulation of bacteria (23), we sought to further characterize the Bordetella growth response to norepinephrine.
MATERIALS AND METHODS
Bacterial strains and plasmids.
B. bronchiseptica strains B013N (5) and RB50 (17) were used as wild-type strains for this study. The BRM18 ΔfauA (10), BRM24 ΔbfeR (3), BRM26 ΔalcA (9), and BRM31 tonB::pSSt2 (4) mutants derived from B. bronchiseptica strain B013N have been described previously. E. coli DH5α (Invitrogen, Carlsbad, CA) was used as the host strain for routine cloning purposes and was the plasmid donor in triparental matings. DNA mobilization functions for conjugations using the DH5α donor strain were provided by plasmid pRK2013 (20) or were chromosomally encoded by strain S17-1 (50). Plasmid pGEM3Z (Promega, Madison, WI) was used as a general cloning vector in E. coli. The tonB+ and exbBD+ plasmid pBB41 (3) was constructed by subcloning a DNA fragment containing the TonB system genes of B. bronchiseptica from previously described plasmid pRKTon (39) to broad-host-range plasmid pBBR1MCS-1 (30). Suicide plasmids pSS1129 (54) and its derivative pEG7 (18) were used in the construction of B. bronchiseptica mutants by allelic exchange or plasmid integration.
Culture conditions.
Luria-Bertani broth and agar (46) were used for routine cultivation of E. coli, and B. bronchiseptica strains were grown on Luria-Bertani agar or blood agar (Becton, Dickinson and Co., Franklin Lakes, NJ). Stainer-Scholte (SS) medium (53), modified as described previously (49), was used as a chemically defined medium for the cultivation of B. bronchiseptica strains in growth stimulation assays. SS basal medium was rendered iron depleted by treatment with Chelex 100 (Bio-Rad, Richmond, CA) and iron replete by the addition of 36 μM FeSO4 (5). Enterobactin was purified based on previously published methods (3, 38) and added to Bordetella cultures at a final concentration of 1 μM. Stock solutions of norepinephrine bitartrate salt (Sigma-Aldrich, St. Louis, MO) were made fresh and added to SS cultures at a concentration of 50 μM. Purified, partially iron-saturated human transferrin and lactoferrin were purchased from Sigma-Aldrich and used to supplement cultures at 200 μg/ml. Media were supplemented with the following concentrations of antibiotics as appropriate: ampicillin, 100 μM; chloramphenicol, 30 μM; gentamicin, 10 μM; and nalidixic acid, 35 μM.
General genetic methods.
Standard genetic methods (46) were used to construct recombinant plasmids, and plasmid DNA was introduced into E. coli and B. bronchiseptica cells by conjugation (11) or electroporation. The B. bronchiseptica RB50 genomic DNA sequence was obtained from the Wellcome Trust Sanger Institute (http://www.sanger.ac.uk). Identification of putative TonB-dependent receptor genes was accomplished using the Integrated Microbial Genomics website (32). Analysis of DNA sequences was aided by the use of the Lasergene program suite (DNASTAR, Madison, WI). Oligonucleotide primers were purchased from Integrated DNA Technologies (Coralville, IA). DNA sequences that were determined for this laboratory were provided by the BioMedical Genomics Center at the University of Minnesota.
Construction of TonB-dependent receptor mutants.
An in-frame 582-bp deletion in a cloned copy of the B. pertussis bfeA gene was generated by StuI and PmlI restriction digestion and religation. The ΔbfeA allele was subcloned to allelic exchange vector pEG7 along with the sacBR-containing BamHI fragment from pEG18.3 (2) and delivered to B. bronchiseptica B013N via conjugation. Following allelic exchange, mutant BRM33 was isolated and confirmed to harbor the ΔbfeA mutation by PCR.
Overlap extension PCR was used to engineer a 2,289-bp in-frame deletion in the bhuR coding region. Primer pair ΔbhuR1 (5′-GAATTCTGCGCCAGGACGATGCCGAC-3′) and ΔbhuR2 (5′-CAGATCCACCACGCCGTAGCCCGTACCGGCCAGTGCGCATGC-3′) and primer pair ΔbhuR3 (5′-GCATGCGCACTGGCCGGTACGGGCTACGGCGTGGTGGATCTG-3′) and ΔbhuR4 (5′-CCCCAAGCTTCACCTTGTGCACCGCCACGCC-3′) were used to PCR amplify 660-bp and 683-bp fragments, respectively, from B. pertussis genomic DNA template. Both products were combined in a second PCR using primers ΔbhuR1 and ΔbhuR4 to create the full-length 1.3-kb ΔbhuR allele, which was ligated to allelic exchange vector pSS1129 along with the sacBR-containing BamHI fragment from pEG18.3. The construct was delivered to B. bronchiseptica B013N, and following allelic exchange, mutant BRM34 was isolated and confirmed to harbor the ΔbhuR mutation by PCR.
Other TonB-dependent receptor mutants analyzed in this study, with the exception of B. bronchiseptica BRM18 (ΔfauA) (10), were constructed by plasmid integration. Primer pairs listed in Table 1 were used to PCR amplify internal DNA fragments from target genes, and products were ligated to vector pGEM3Z. Bordetella insert DNA was subcloned to the pSS1129 or pEG7 suicide plasmid and introduced into the B. bronchiseptica B013N or RB50 parent strain by conjugal transfer. Plasmid integrants were selected on the basis of gentamicin resistance, and the integration site was confirmed by PCR for each of the strains constructed by this method.
TABLE 1.
B. bronchiseptica TonB-dependent receptor genes
| Locus tag | Gene name and/or reference | Primer sequencea (5′→3′) |
|---|---|---|
| BB0078 | bfrC (6) | GGCCGAATTCGCCGGTGCGCAACGACAATGAC |
| GGCCAAGCTTCGCGGCCGCCACGAACT | ||
| BB0189 | This study | GCGCGGATCCTGCCCGGCCACAACCACGAGT |
| CGCGGAATTCTGCGGCGCGAAGTCCCAGATG | ||
| BB0832 | 41 | GCGCGGATCCCGTAGCGCCGGCCGAACAGGT |
| GCGCGAATTCCGCCGCGGCTACGACAACTACA | ||
| BB1294 | bfrG (41) | GGCCGAATTCGCCGACCGCGATTTCCAGA |
| GGCCAAGCTTCGGCGCGGCGTGTTGACC | ||
| BB1785 | bfrI (41) | GGCCGAATTCCGGCGGCGTGGTCAACCTCGTCAG |
| GGCCAAGCTTGGTGGCGTTGTCGGTTTCCTTGTC | ||
| BB1846 | bfrB (6) | GGCCGAATTCGTATTTCATCCGCGGCTTCATCCT |
| GGCCAAGCTTCGGCGGCGGGCGAGTTGTGG | ||
| BB1905 | 41 | CCGGGGATCCGCCGCGCTCGTCCGTCTATGTGTC |
| GATCCGTGGCGTCCGTGAACTG | ||
| BB1942 | bfeA (8) | NAb |
| BB2179 | This study | CGCGGAATTCACACATAATCGGCCCGTTCCACA |
| GCGCGGATCCCCCGGTCAGCCATGCCTCCTA | ||
| BB3658 | bfrH (41) | GGCCGAATTCCCGGCGGGTCAACATGGATTTTTC |
| GGCCAAGCTTCCCGGCGGCTGGTATTTCACG | ||
| BB3825 | bfrE (41) | GGCCAAGCTTGCGGCGGGCAGCATCTTCGTC |
| GGCCAAGCTTTTGTCTTCGCGGCTGGTGCTGTAG | ||
| BB3826 | bfrD (42) | GGCCGAATTCGTCAGCCGCCGCAACTTCTACG |
| GGCCAAGCTTCGGCGACTTCGATGCTGGTGTT | ||
| BB3900 | fauA (10) | NA |
| BB4122 | bfrF (41) | GGCCGAATTCCAGCGCGATGTCCGAACCCTACG |
| GGCCAAGCTTGCGGCCGCTGAGCACCACCAC | ||
| BB4457 | This study | CGCGGAATTCCTCAACGGCCAGCGCATCAACAA |
| GCGCGGATCCGCCGGTGGGGGTGCTCATCTG | ||
| BB4655 | bhuR (55) | NA |
| BB4744 | bfrZ (44) | GCGCGAATTCCGGGCGGCATCGTCAACTACACCT |
| CTTGCTGCGGCCCTGCGAGAACG | ||
| BB4761 | bfrA (7) | GCGCGAATTCGCGCGGCCCTATGTCCACCCTG |
| GCGCGGATCCCTCCACTTGCCGCCGAACTGCTC | ||
| BB4956 | hemC (41) | GCGCGAATTCGGCACCCGCTTCGCATACTCG |
| GCGCGGATCCCGCGCCGCCTTCCAGACCAC |
Primer sequences for PCR amplification of internal gene fragments used in the construction of insertion mutations.
NA, not applicable.
Growth assays.
For growth curves, B. bronchiseptica BRM26 seed cultures were grown in iron-replete SS medium for 24 h and then washed with SS basal medium to remove excess iron. Bacteria were subcultured at an initial density of ∼100 CFU/ml to iron-replete or iron-depleted SS medium containing partially iron-saturated transferrin or lactoferrin. Iron-depleted cultures were further supplemented with norepinephrine, enterobactin, or both compounds as indicated. Growth of BRM26 was monitored by optical density using a Klett-Summerson colorimeter fitted with a no. 54 filter. The reported growth curves are representative of the trends from at least two experiments. The growth curves are graphed on an arithmetic scale rather than the standard logarithmic scale due to limited sensitivity of the Klett meter at low optical densities.
For growth yield experiments, B. bronchiseptica strains B013N and RB50 or their mutant derivatives were grown as seed cultures in iron-replete SS medium. The cells were harvested, washed, and subcultured at an initial density of ∼1 × 106 CFU/ml to iron-replete medium or iron-depleted medium containing either transferrin or transferrin and norepinephrine. For growth assays that involved strain BRM31, all cultures were inoculated at a 1:50 dilution from washed iron-replete seed growth. Growth yield was measured densitometrically after 40 h at a wavelength of 600 nm, and the values reported are the means of triplicate cultures ± 1 standard deviation.
55Fe and [3H]norepinephrine binding assays.
Preparation of radiolabeled transferrin was based on a previously published method (23). Apotransferrin (Sigma-Aldrich) solutions were prepared in 0.1 M sodium citrate and 0.1 M sodium bicarbonate buffer. A mixture of FeCl3 and 55FeCl3 (PerkinElmer, Boston, MA) was added to the transferrin solution to achieve an iron saturation level of 30% and an activity of 5 μCi/mg protein. Ferric transferrin complexes were allowed to form at 37°C for 5 h followed by separation of unbound iron using Sephadex G25 gel filtration.
B. bronchiseptica strains were grown in iron-replete SS medium for 24 h followed by growth for 16 h in iron-depleted SS medium. Bacteria were harvested by centrifugation and subcultured to parallel iron-depleted SS cultures containing 200 μg/ml 55Fe-transferrin at an initial optical density (600 nm) of 0.2. One set of parallel cultures was additionally supplemented with either 50 μM norepinephrine or 50 μM norepinephrine and 36 μM FeSO4. Triplicate aliquots of bacteria were harvested by centrifugation after growing for 24 h and washed with a 0.9% NaCl solution containing 5 μM FeCl3. Cell-associated radioactivity (cpm) was determined from 0.5 ml of the washed bacterial suspension using a scintillation counter. Reported values were normalized to the optical density of the bacterial suspension and are the means from triplicate determinations ± 1 standard deviation. The results are representative of two experiments.
Cell-associated norepinephrine levels were determined using bacterial cultures prepared and inoculated as described above for the iron transport assay. Experimental cultures consisted of iron-depleted SS medium containing 200 μg/ml partially iron-saturated transferrin supplemented with either 50 μM norepinephrine or 50 μM norepinephrine and 36 μM FeSO4. Each culture additionally contained 1.67 μCi/ml of [3H]norepinephrine (GE Healthcare, Buckinghamshire, England). Cell-associated radioactivity was determined as described above for 55Fe. The means of triplicate determinations ± 1 standard deviation are reported and representative of two experiments.
RESULTS
Iron acquisition using siderophores and norepinephrine.
Norepinephrine was previously shown to stimulate growth of B. bronchiseptica in iron-depleted SS medium containing serum (4). Since it was reasoned that transferrin was the likely serum iron source responsible for this growth enhancement, the ability of B. bronchiseptica to obtain iron from transferrin via norepinephrine was examined. B. bronchiseptica alcA mutant strain BRM26 is unable to synthesize alcaligin and therefore allowed for the analysis of norepinephrine- and transferrin-mediated growth stimulation without the influence of the native siderophore on iron acquisition. In iron-replete medium at a low inoculum of ∼100 CFU/ml, strain BRM26 exhibited an extended lag phase but was able to achieve significantly elevated growth levels over the course of the experiment (Fig. 1A). In the absence of any inorganic iron source or transferrin, strain BRM26 showed a similarly long lag phase but then relatively poor growth, regardless of the presence of norepinephrine or enterobactin. These results indicate that norepinephrine itself is not a growth-stimulating nutrient and that an iron source must be available in the culture system. Addition of norepinephrine to BRM26 cultures in iron-depleted medium containing partially iron-saturated transferrin resulted in a reduced lag phase and an increase in growth yield compared to parallel cultures that contained transferrin alone (Fig. 1B). These results are similar to those from our previous B. bronchiseptica growth studies using serum (4) and demonstrate that transferrin can substitute for serum in norepinephrine-mediated growth stimulation assays. Remarkably, norepinephrine alone was capable of stimulating the growth of B. bronchiseptica in the presence of transferrin; no siderophore was required to achieve this effect. This norepinephrine feeding effect was also observed using the RB50 strain of B. bronchiseptica (data not shown). Norepinephrine-mediated growth of E. coli has been reported to involve the native enterobactin siderophore (13, 21), and since Bordetella species use enterobactin as a xenosiderophore, its influence on growth stimulation by norepinephrine was investigated. In the presence of transferrin, enterobactin facilitated robust growth compared to growth of unsupplemented cultures, and only a modest increase in growth yield was observed when cultures were additionally supplemented with norepinephrine (Fig. 1B).
FIG. 1.
Analysis of Bordetella iron acquisition via norepinephrine and siderophores. B. bronchiseptica ΔalcA strain BRM26 was cultured in iron-depleted SS medium (A), iron-depleted medium containing 200 μg/ml transferrin (B), or iron-depleted medium containing 200 μg/ml lactoferrin (C). Bacteria were inoculated at an initial density of ∼100 CFU/ml, and growth was measured densitometrically. Parallel cultures were additionally supplemented as follows: 36 μM FeSO4 (diamonds), 50 μM norepinephrine and 1 μM enterobactin (circles), 1 μM enterobactin (triangles), 50 μM norepinephrine (squares), and no supplementation (asterisks).
When lactoferrin was used as the iron source, growth of B. bronchiseptica BRM26 was inhibited and addition of norepinephrine failed to stimulate growth (Fig. 1C). The siderophores enterobactin (Fig. 1C) and alcaligin (data not shown) relieved the bacteriostatic effects of lactoferrin. As observed with transferrin, norepinephrine only moderately enhanced the growth of lactoferrin-containing BRM26 cultures supplemented with enterobactin or alcaligin. The bacteriostatic effect of lactoferrin on B. bronchiseptica resulted from iron sequestration, since addition of excess FeSO4 relieved the growth inhibition. Although the iron-binding affinity of lactoferrin is higher than that of transferrin (1), these results indicate that both glycoproteins can be utilized as iron sources by B. bronchiseptica. However, only transferrin was effectively used as a substrate for norepinephrine-mediated growth stimulation.
Roles of the enterobactin and alcaligin receptors in the norepinephrine response.
Since enterobactin and norepinephrine can induce transcription of the bfeA ferric enterobactin receptor gene by a BfeR-dependent mechanism (3, 4), it was hypothesized that norepinephrine-mediated acquisition of transferrin-iron would also require the BfeA receptor. When iron-depleted and transferrin-supplemented cultures of ΔbfeA mutant strain BRM33 were provided with norepinephrine, an approximately twofold increase in growth yield was observed over that of cultures lacking norepinephrine (Fig. 2A). Since a similar norepinephrine-mediated increase in growth was observed for the wild-type strain B013N, this suggested that if BfeA were a ferric norepinephrine receptor, it is not the sole receptor. The levels of norepinephrine-stimulated growth for both strains were nearly equivalent to the growth observed in medium containing 36 μM iron. The ΔbfeR mutant BRM24 also exhibited wild-type levels of norepinephrine-stimulated growth (Fig. 2B), indicating that BfeR-dependent induction of bfeA transcription by norepinephrine is not required for this growth response.
FIG. 2.
Growth stimulation by norepinephrine is independent of the BfeA enterobactin receptor and BfeR regulator proteins. (A) B. bronchiseptica strains B013N (bfeA+) and BRM33 (ΔbfeA) and (B) strains B013N (bfeR+) and BRM24 (ΔbfeR) were used. B. bronchiseptica cells were grown in iron-replete medium (solid bars) or iron-depleted medium supplemented with either 200 μg/ml transferrin (shaded bars) or 200 μg/ml transferrin and 50 μM norepinephrine (open bars). Growth yields were measured densitometrically after 40 h of incubation. Values represent means ± 1 standard deviation (error bars) from triplicate cultures.
The presence of the alcaligin siderophore did not influence the ability of norepinephrine to promote the growth of B. bronchiseptica, since an alcaligin mutant (Fig. 1) as well as alcaligin proficient strains (Fig. 2) responded similarly to the catecholamine. Furthermore, a ΔfauA alcaligin receptor mutant strain also exhibited a similar growth response to norepinephrine compared to the wild-type, bfeA, and bfeR mutant strains (data not shown). Together, these results do not suggest a role for either alcaligin or the alcaligin receptor in transferrin-iron acquisition via norepinephrine.
TonB-dependent norepinephrine utilization.
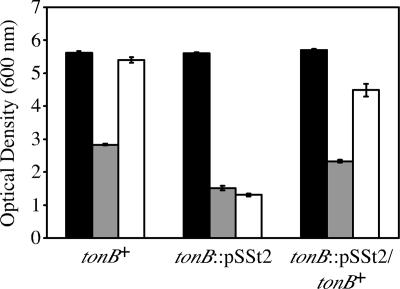
To determine whether B. bronchiseptica iron acquisition by norepinephrine required a TonB-dependent transport system, the growth responsiveness of tonB mutant strain BRM31 was tested. The growth levels of strain BRM31 in iron-depleted SS medium containing transferrin were similarly low both in the presence and absence of norepinephrine (Fig. 3). The requirement for TonB was confirmed by genetic complementation in trans using tonB+ exbBD+ plasmid pBB41, restoring the ability of norepinephrine to stimulate BRM31 growth to near wild-type levels. The tonB mutation had no effect on growth of B. bronchiseptica iron-replete control cultures. The growth yield of the tonB strain was decreased in iron-restricted medium lacking norepinephrine compared to those of the wild-type and complemented mutant strains. This result likely reflects the contribution of alcaligin-mediated acquisition of transferrin-iron to the growth of B. bronchiseptica, since ferric alcaligin transport is TonB dependent (39).
FIG. 3.
TonB-dependent growth stimulation by norepinephrine. B. bronchiseptica strains B013N (tonB+), BRM31(pBBR1MCS-1) (tonB::pSSt2), and BRM31(pBB41) (tonB::pSSt2/tonB+) were used. B. bronchiseptica cells were grown in iron-replete medium (solid bars) or iron-depleted medium supplemented with either 200 μg/ml transferrin (shaded bars) or 200 μg/ml transferrin and 50 μM norepinephrine (open bars). Growth yields were measured densitometrically after 40 h of incubation. Values represent means ± 1 standard deviation (error bars) from triplicate cultures.
Mutational analysis of putative TonB-dependent receptor genes.
The fact that norepinephrine utilization was TonB dependent suggested the requirement for a TonB-dependent outer membrane receptor. There are 16 annotated TonB-dependent outer membrane receptor genes in the published genome sequence of B. bronchiseptica strain RB50 (Table 1) (41). Three of these receptors, BfeA (enterobactin) (8), BhuR (heme) (55), and FauA (alcaligin) (10) are well characterized, while the ligands for the remaining 13 receptor proteins have not been identified. In the present study, three additional putative TonB-dependent receptors (BB0189, BB2179, and BB4457) were identified in the B. bronchiseptica genome sequence, based on the presence of a TonB-dependent receptor motif (Pfam 00593) and the receptor plug domain (Pfam 07715). Each of the 17 receptor genes not yet analyzed in this study was disrupted, in either the B013N or RB50 parental strain background, and the resulting mutants were tested for enhanced growth in response to norepinephrine in the ferric transferrin utilization assay. No single TonB-dependent receptor mutant was found to be defective in norepinephrine-mediated growth stimulation compared with its parental control strain, nor was there an observable growth stimulation difference between the two parent strains (data not shown). The growth phenotype of each mutant in the iron acquisition assay was similar to that of the ΔbfeA and ΔfauA mutants. These data suggest that more than one TonB-dependent receptor is capable of facilitating transport of iron from transferrin via norepinephrine.
Association of norepinephrine and iron with Bordetella cells.
If norepinephrine mediates transport of transferrin-iron for B. bronchiseptica cells, then cellular localization of iron should increase in a norepinephrine-dependent manner. Apotransferrin was loaded with a mixture of FeCl3 and 55FeCl3 and used as the bacterial iron source in binding experiments. Wild-type B. bronchiseptica provided with norepinephrine exhibited a significantly higher level of cell-associated 55Fe label than cultures that lacked the catecholamine, suggesting that norepinephrine-mediated growth stimulation is the result of iron transport (Fig. 4A). The nearly twofold increase in cell association of iron correlates with the levels of growth stimulation observed in previous experiments (Fig. 2 and 3). When FeSO4 was added to norepinephrine-containing cultures at a concentration calculated to exceed the iron-binding capacity of transferrin, cell association of 55Fe decreased dramatically. This result suggests that expression of norepinephrine-mediated iron transport functions is repressible when iron is abundant (perhaps at the transcriptional level by the Fur repressor), similar to the regulation of other Bordetella TonB-dependent iron transport systems (3, 10, 27, 55). Importantly, the tonB mutant strain exhibited low levels of bound iron in both the presence and absence of norepinephrine, further reinforcing the conclusion that the TonB system is required for norepinephrine-mediated iron acquisition. Genetic complementation in trans using the tonB+ exbBD+ plasmid restored the ability of norepinephrine to mediate acquisition of transferrin-iron.
FIG. 4.
TonB-dependent cell association of 55Fe and [3H]norepinephrine. (A) B. bronchiseptica strains B013N (tonB+), BRM31(pBBR1MCS- 1) (tonB::pSSt2), and BRM31(pBB41) (tonB::pSSt2/tonB+) were used. Bacteria were grown in iron-depleted medium containing 200 μg/ml 55Fe-transferrin (solid bars), 200 μg/ml 55Fe-transferrin plus 50 μM norepinephrine (shaded bars), or 200 μg/ml 55Fe-transferrin plus 50 μM norepinephrine plus 36 μM FeSO4 (open bars). (B) B. bronchiseptica strains B013N (solid bars), BRM31(pBBR1MCS-1) (shaded bars), and BRM31(pBB41) (open bars) were used. Iron-depleted basal medium containing [3H]norepinephrine was supplemented with either 200 μg/ml transferrin plus 50 μM norepinephrine (FeTf + NE) or 200 μM transferrin plus 50 μg/ml norepinephrine plus 36 μM FeSO4 (FeTF + NE + FeSO4). Cell-associated levels of 55Fe or [3H]norepinephrine were determined by scintillation counting and normalized based on the optical density at 600 nm (OD600) after 24 h of incubation. Values represent means ± 1 standard deviation (error bars) from triplicate determinations.
Since norepinephrine facilitated bacterial association with iron in a TonB-dependent manner, we examined whether norepinephrine itself became cell associated during the iron acquisition process. In iron-depleted conditions in the presence of transferrin, the amount of [3H]norepinephrine bound to the tonB mutant cells was significantly lower than that associated with the wild-type strain or the genetically complemented tonB mutant strain (Fig. 4B). However, in the presence of excess iron, cell-associated [3H]norepinephrine levels were similar among all three strains, indicating that the TonB system is not important for the association of norepinephrine with iron-replete bacteria. The reason underlying the increased binding of [3H]norepinephrine to the tonB mutant under iron-replete versus iron-depleted conditions is presently unknown. In sum, these binding studies indicate that both norepinephrine and the iron from transferrin localize to B. bronchiseptica cells in a tonB-dependent manner that is consistent with a receptor-driven transport process.
DISCUSSION
Examples of bacterial responses to sympathoadrenal catecholamines, such as norepinephrine, have been reported in the scientific literature. Epinephrine and norepinephrine induce production of the tick colonization factor OspA from the Lyme disease pathogen Borrelia burgdorferi (48). Both compounds also activate, via the QseC histidine kinase, the autoinducer-3 quorum-sensing system of enterohemorrhagic E. coli, which is responsible for the controlled expression of several virulence genes (14). Other bacterial responses to catecholamines are seemingly related to iron transport, such as the induced production of the E. coli enterobactin receptor protein FepA by norepinephrine (13) and induced transcription of the B. bronchiseptica bfeA enterobactin receptor gene by epinephrine, norepinephrine, and dopamine (4). The growth of several gram-negative and gram-positive bacterial species in iron-restrictive culture conditions is stimulated by catecholamines (19, 22, 28, 31, 37). With this report, we have established that B. bronchiseptica utilizes norepinephrine as an iron source in the presence of transferrin and that utilization of norepinephrine appears to require high-affinity TonB-dependent transport.
The mechanism of iron acquisition via norepinephrine remains unknown. However, the catechol structure of norepinephrine suggests that it may be able to form a bidentate interaction with ferric iron. Bordetella cells can utilize norepinephrine for iron retrieval from transferrin in the absence of a siderophore, suggesting that a TonB-dependent receptor recognizes and transports ferric norepinephrine. Early work demonstrated that transferrin bound tightly to Bordetella cell surfaces to the degree that it copurified with outer membrane fractions (45). Therefore, it is unlikely that norepinephrine-mediated iron chelation from transferrin would need to occur at a distance. Rather, hypothesized ternary interactions between transferrin, iron, and norepinephrine (23) in close association with the bacterial surface may be sufficient for disruption of ferric transferrin complexes and bacterial assimilation of the liberated iron. In contrast, norepinephrine-mediated growth stimulation of E. coli apparently requires enterobactin (13, 21), and for Salmonella enterica, it requires either the enterobactin precursor 2,3-dihydroxybenzoic acid or the enterobactin or salmochelin monomeric breakdown products 2,3-dihydroxybenzoylserine and SX, respectively (36). The ability to use norepinephrine for iron retrieval has important implications in pathogenesis, especially since pretreatment with norepinephrine significantly enhanced the colonization and dissemination of S. enterica in infected mice (36, 55).
Bordetella iron sources, such as enterobactin (3), alcaligin (12), and heme-iron (55, 56) induce transcription of their cognate transport genes. Since norepinephrine is a transcriptional inducer of the bfeA receptor gene required for utilization of enterobactin and other catechol siderophores (3), we hypothesized that BfeA also facilitates iron transport via norepinephrine. However, no apparent defect in iron acquisition by norepinephrine was detected in two different bfeA mutant strains (Fig. 2A and data not shown). The transcriptional response to norepinephrine may be due simply to its structural similarity to enterobactin, a hypothesis that is supported by the finding that the presence of a catechol group is a key structural element of inducer recognition by BfeR (4). The requirement for TonB in norepinephrine-mediated growth strongly suggests the existence of at least one outer membrane receptor for ferric norepinephrine. Of the 16 annotated TonB-dependent receptors in the published B. bronchiseptica genome (41) and the three predicted TonB-dependent receptors identified in this work, no single receptor mutant exhibited a norepinephrine utilization defect. We hypothesize that Bordetella norepinephrine utilization likely involves multiple TonB-dependent transporters, one of which could still be BfeA. A recent study of E. coli reported that each of the three known catechol siderophore receptor genes (fepA, iroN, and cir) was involved in growth stimulation by norepinephrine (57).
B. bronchiseptica inhabits the upper respiratory mucosa of the host, and it is well-known that the lactoferrin in that environment sequesters extracellular iron from invading microbial pathogens (33, 34). We have demonstrated that both alcaligin and enterobactin were able to stimulate Bordetella growth in iron-depleted medium containing lactoferrin. In the absence of siderophores, lactoferrin was bacteriostatic, emphasizing the importance of siderophore-mediated iron acquisition to Bordetella cells colonizing that anatomical site. Although norepinephrine did not stimulate B. bronchiseptica growth in the presence of lactoferrin, others have shown that both transferrin and lactoferrin can serve as substrates for E. coli iron acquisition via norepinephrine (23).
Although transferrin is primarily located in serum (1) and B. bronchiseptica is generally considered a noninvasive mucosal pathogen (24), Bordetella cells can acquire transferrin-bound iron by way of siderophores or norepinephrine. Another pathogen that inhabits a mucosal surface, Neisseria gonorrhoeae, was unable to initiate urethritis in human volunteers without a functional transferrin-iron uptake system, suggesting that sufficient transferrin is naturally present to support infection (16). Plasma exudation through intact respiratory epithelium occurs in response to the presence of various stimuli, including microbial pathogens (43), and may result in the presence of transferrin on respiratory surfaces. Therefore, Bordetella species may encounter transferrin during the course of infection. Norepinephrine-mediated transport of transferrin-associated iron may represent an important mechanism by which bacteria are able to obtain an essential nutrient in the host environment.
Acknowledgments
We acknowledge Ryan Suhadolc and Carin Vanderpool for construction of the BRM33 and BRM34 mutant strains and Pam McKelvey for her contributions to the assembly of the TonB-dependent receptor mutant collection. We also thank Timothy Brickman for helpful discussions.
Support for this study was provided by Public Health Service grant AI-31088 from the National Institute of Allergy and Infectious Diseases. M.T.A. was supported by a Public Health Service grant T32 AI-07421 from the National Institute for Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 4 April 2008.
REFERENCES
- 1.Aisen, P., and A. Leibman. 1972. Lactoferrin and transferrin: a comparative study. Biochim. Biophys. Acta 257314-323. [DOI] [PubMed] [Google Scholar]
- 2.Akerley, B. J., P. A. Cotter, and J. F. Miller. 1995. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell 80611-620. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, M. T., and S. K. Armstrong. 2004. The BfeR regulator mediates enterobactin-inducible expression of Bordetella enterobactin utilization genes. J. Bacteriol. 1867302-7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, M. T., and S. K. Armstrong. 2006. The Bordetella bfe system: growth and transcriptional response to siderophores, catechols, and neuroendocrine catecholamines. J. Bacteriol. 1885731-5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong, S. K., and M. O. Clements. 1993. Isolation and characterization of Bordetella bronchiseptica mutants deficient in siderophore activity. J. Bacteriol. 1751144-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beall, B. 1998. Two iron-regulated putative ferric siderophore receptor genes in Bordetella bronchiseptica and Bordetella pertussis. Res. Microbiol. 149189-201. [DOI] [PubMed] [Google Scholar]
- 7.Beall, B., and T. Hoenes. 1997. An iron-regulated outer-membrane protein specific to Bordetella bronchiseptica and homologous to ferric siderophore receptors. Microbiology 143135-145. [DOI] [PubMed] [Google Scholar]
- 8.Beall, B., and G. N. Sanden. 1995. A Bordetella pertussis fepA homologue required for utilization of exogenous ferric enterobactin. Microbiology 1413193-3205. [DOI] [PubMed] [Google Scholar]
- 9.Brickman, T. J., and S. K. Armstrong. 2005. Bordetella AlcS transporter functions in alcaligin siderophore export and is central to inducer sensing in positive regulation of alcaligin system gene expression. J. Bacteriol. 1873650-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brickman, T. J., and S. K. Armstrong. 1999. Essential role of the iron-regulated outer membrane receptor FauA in alcaligin siderophore-mediated iron uptake in Bordetella species. J. Bacteriol. 1815958-5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brickman, T. J., and S. K. Armstrong. 1996. The ornithine decarboxylase gene odc is required for alcaligin siderophore biosynthesis in Bordetella spp.: putrescine is a precursor of alcaligin. J. Bacteriol. 17854-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brickman, T. J., H. Y. Kang, and S. K. Armstrong. 2001. Transcriptional activation of Bordetella alcaligin siderophore genes requires the AlcR regulator with alcaligin as inducer. J. Bacteriol. 183483-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton, C. L., S. R. Chhabra, S. Swift, T. J. Baldwin, H. Withers, S. J. Hill, and P. Williams. 2002. The growth response of Escherichia coli to neurotransmitters and related catecholamine drugs requires a functional enterobactin biosynthesis and uptake system. Infect. Immun. 705913-5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke, M. B., D. T. Hughes, C. Zhu, E. C. Boedeker, and V. Sperandio. 2006. The QseC sensor kinase: a bacterial adrenergic receptor. Proc. Natl. Acad. Sci. USA 10310420-10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelissen, C. N. 2003. Transferrin-iron uptake by Gram-negative bacteria. Front. Biosci. 8d836-d847. [DOI] [PubMed] [Google Scholar]
- 16.Cornelissen, C. N., M. Kelley, M. M. Hobbs, J. E. Anderson, J. G. Cannon, M. S. Cohen, and P. F. Sparling. 1998. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol. Microbiol. 27611-616. [DOI] [PubMed] [Google Scholar]
- 17.Cotter, P. A., and J. F. Miller. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 623381-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotter, P. A., and J. F. Miller. 1997. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol. Microbiol. 24671-685. [DOI] [PubMed] [Google Scholar]
- 19.Coulanges, V., P. Andre, and D. J. Vidon. 1998. Effect of siderophores, catecholamines, and catechol compounds on Listeria spp. Growth in iron-complexed medium. Biochem. Biophys. Res. Commun. 249526-530. [DOI] [PubMed] [Google Scholar]
- 20.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 761648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freestone, P. P., R. D. Haigh, P. H. Williams, and M. Lyte. 2003. Involvement of enterobactin in norepinephrine-mediated iron supply from transferrin to enterohaemorrhagic Escherichia coli. FEMS Microbiol. Lett. 22239-43. [DOI] [PubMed] [Google Scholar]
- 22.Freestone, P. P., R. D. Haigh, P. H. Williams, and M. Lyte. 1999. Stimulation of bacterial growth by heat-stable, norepinephrine-induced autoinducers. FEMS Microbiol. Lett. 17253-60. [DOI] [PubMed] [Google Scholar]
- 23.Freestone, P. P., M. Lyte, C. P. Neal, A. F. Maggs, R. D. Haigh, and P. H. Williams. 2000. The mammalian neuroendocrine hormone norepinephrine supplies iron for bacterial growth in the presence of transferrin or lactoferrin. J. Bacteriol. 1826091-6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodnow, R. A. 1980. Biology of Bordetella bronchiseptica. Microbiol. Rev. 44722-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorringe, A. R., G. Woods, and A. Robinson. 1990. Growth and siderophore production by Bordetella pertussis under iron-restricted conditions. FEMS Microbiol. Lett. 54101-105. [DOI] [PubMed] [Google Scholar]
- 26.Johanson, B. 1960. Isolation of an iron-containing red protein from human milk. Acta Chem. Scand. 14510-512. [Google Scholar]
- 27.Kang, H. Y., T. J. Brickman, F. C. Beaumont, and S. K. Armstrong. 1996. Identification and characterization of iron-regulated Bordetella pertussis alcaligin siderophore biosynthesis genes. J. Bacteriol. 1784877-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinney, K. S., C. E. Austin, D. S. Morton, and G. Sonnenfeld. 2000. Norepinephrine as a growth stimulating factor in bacteria—mechanistic studies. Life Sci. 673075-3085. [DOI] [PubMed] [Google Scholar]
- 29.Konopka, K., A. Bindereif, and J. B. Neilands. 1982. Aerobactin-mediated utilization of transferrin iron. Biochemistry 216503-6508. [DOI] [PubMed] [Google Scholar]
- 30.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop II, and K. M. Peterson. 1994. pBBR1MCS: a broad-host-range cloning vector. BioTechniques 16800-802. [PubMed] [Google Scholar]
- 31.Lyte, M., and S. Ernst. 1992. Catecholamine induced growth of Gram negative bacteria. Life Sci. 50203-212. [DOI] [PubMed] [Google Scholar]
- 32.Markowitz, V. M., F. Korzeniewski, K. Palaniappan, E. Szeto, G. Werner, A. Padki, X. Zhao, I. Dubchak, P. Hugenholtz, I. Anderson, A. Lykidis, K. Mavromatis, N. Ivanova, and N. C. Kyrpides. 2006. The integrated microbial genomes (IMG) system. Nucleic Acids Res. 34D344-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masson, P. L., J. F. Heremans, J. J. Prignot, and G. Wauters. 1966. Immunohistochemical localization and bacteriostatic properties of an iron-binding protein from bronchial mucus. Thorax 21538-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masson, P. L., J. F. Heremans, and C. H. Dive. 1966. An iron-binding protein common to many external secretions. Clin. Chim. Acta 14735-739. [Google Scholar]
- 35.Menozzi, F. D., C. Gantiez, and C. Locht. 1991. Identification and purification of transferrin- and lactoferrin-binding proteins of Bordetella pertussis and Bordetella bronchiseptica. Infect. Immun. 593982-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Methner, U., W. Rabsch, R. Reissbrodt, and P. H. Williams. Effect of norepinephrine on colonisation and systemic spread of Salmonella enterica in infected animals: role of catecholate siderophore precursors and degradation products. Int. J. Med. Microbiol., in press. [DOI] [PubMed]
- 37.Neal, C. P., P. P. Freestone, A. F. Maggs, R. D. Haigh, P. H. Williams, and M. Lyte. 2001. Catecholamine inotropes as growth factors for Staphylococcus epidermidis and other coagulase-negative staphylococci. FEMS Microbiol. Lett. 194163-169. [DOI] [PubMed] [Google Scholar]
- 38.Neilands, J. B., and K. Nakamura. 1991. Detection, determination, isolation, characterization and regulation of microbial iron chelates, p. 1-14. In G. Winkelmann (ed.), Handbook of microbiol iron chelates. CRC Press, Inc., Boca Raton, FL.
- 39.Nicholson, M. L., and B. Beall. 1999. Disruption of tonB in Bordetella bronchiseptica and Bordetella pertussis prevents utilization of ferric siderophores, haemin and haemoglobin as iron sources. Microbiology 1452453-2461. [DOI] [PubMed] [Google Scholar]
- 40.Paris, I., P. Martinez-Alvarado, C. Perez-Pastene, M. N. Vieira, C. Olea-Azar, R. Raisman-Vozari, S. Cardenas, R. Graumann, P. Caviedes, and J. Segura-Aguilar. 2005. Monoamine transporter inhibitors and norepinephrine reduce dopamine-dependent iron toxicity in cells derived from the substantia nigra. J. Neurochem. 921021-1032. [DOI] [PubMed] [Google Scholar]
- 41.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, A. M. Cerdeno-Tarraga, L. Temple, K. James, B. Harris, M. A. Quail, M. Achtman, R. Atkin, S. Baker, D. Basham, N. Bason, I. Cherevach, T. Chillingworth, M. Collins, A. Cronin, P. Davis, J. Doggett, T. Feltwell, A. Goble, N. Hamlin, H. Hauser, S. Holroyd, K. Jagels, S. Leather, S. Moule, H. Norberczak, S. O'Neil, D. Ormond, C. Price, E. Rabbinowitsch, S. Rutter, M. Sanders, D. Saunders, K. Seeger, S. Sharp, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, L. Unwin, S. Whitehead, B. G. Barrell, and D. J. Maskell. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 3532-40. [DOI] [PubMed] [Google Scholar]
- 42.Passerini de Rossi, B. N., L. E. Friedman, C. B. Belzoni, S. Savino, B. Arico, R. Rappuoli, V. Masignani, and M. A. Franco. 2003. Vir90, a virulence-activated gene coding for a Bordetella pertussis iron-regulated outer membrane protein. Res. Microbiol. 154443-450. [DOI] [PubMed] [Google Scholar]
- 43.Persson, C. G. A., I. Erjefalt, U. Alkner, C. Baumgarten, L. Greiff, B. Gustafsson, A. Luts, U. Pipkorn, F. Sundler, C. Svensson, and P. Wollmer. 1991. Plasma exudation as a first line respiratory mucosal defence. Clin. Exp. Allergy 2117-24. [DOI] [PubMed] [Google Scholar]
- 44.Pradel, E., and C. Locht. 2001. Expression of the putative siderophore receptor gene bfrZ is controlled by the extracytoplasmic-function sigma factor BupI in Bordetella bronchiseptica. J. Bacteriol. 1832910-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Redhead, K., T. Hill, and H. Chart. 1987. Interaction of lactoferrin and transferrins with the outer membrane of Bordetella pertussis. J. Gen. Microbiol. 133891-898. [DOI] [PubMed] [Google Scholar]
- 46.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 47.Schade, A. L., and L. Caroline. 1946. An iron-binding component in human blood plasma. Science 104340-341. [DOI] [PubMed] [Google Scholar]
- 48.Scheckelhoff, M. R., S. R. Telford, M. Wesley, and L. T. Hu. 2007. Borrelia burgdorferi intercepts host hormonal signals to regulate expression of outer surface protein A. Proc. Natl. Acad. Sci. USA 1047247-7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider, D. R., and C. D. Parker. 1982. Effect of pyridines on phenotypic properties of Bordetella pertussis. Infect. Immun. 38548-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simon, R., U. Priefer, and A. Puhler. 1982. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology 1784-791. [Google Scholar]
- 51.Siraki, A. G., J. Smythies, and P. J. O'Brien. 2000. Superoxide radical scavenging and attenuation of hypoxia-reoxygenation injury by neurotransmitter ferric complexes in isolated rat hepatocytes. Neurosci. Lett. 29637-40. [DOI] [PubMed] [Google Scholar]
- 52.Sperandio, V., A. G. Torres, B. Jarvis, J. P. Nataro, and J. B. Kaper. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. USA 1008951-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stainer, D. W., and M. J. Scholte. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 63211-220. [DOI] [PubMed] [Google Scholar]
- 54.Stibitz, S. 1994. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol. 235458-465. [DOI] [PubMed] [Google Scholar]
- 55.Vanderpool, C. K., and S. K. Armstrong. 2001. The Bordetella bhu locus is required for heme iron utilization. J. Bacteriol. 1834278-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanderpool, C. K., and S. K. Armstrong. 2003. Heme-responsive transcriptional activation of Bordetella bhu genes. J. Bacteriol. 185909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams, P. H., W. Rabsch, U. Methner, W. Voigt, H. Tschape, and R. Reissbrodt. 2006. Catecholate receptor proteins in Salmonella enterica: role in virulence and implications for vaccine development. Vaccine 243840-3844. [DOI] [PubMed] [Google Scholar]
- 58.Young, J. B., and L. Landsberg. 1998. Catecholamines and the adrenal medulla, p. 665-728. In J. D. Wilson, D. W. Foster, H. M. Kronenberg, and P. R. Larsen (ed.), Williams textbook of endocrinology, 9th ed. W. B. Saunders Co., Philadelphia, PA.