Abstract
The entry of methanol into the methylotrophic pathway of methanogenesis is mediated by the concerted effort of two methyltransferases, namely, methyltransferase 1 (MT1) and methyltransferase 2 (MT2). The mtaA1, mtaA2, and mtbA genes of Methanosarcina acetivorans C2A encode putative methanol- or methylamine-specific MT2 enzymes. To address the in vivo roles of these genes in growth and methanogenesis from known substrates, we constructed and characterized mutants with deletions of each of these genes. The mtaA1 gene is required for growth on methanol, whereas mtaA2 was dispensable. However, the mtaA2 mutant had a reduced rate of methane production from methanol. Surprisingly, deletion of mtaA1 in combination with deletions of the genes encoding three methanol-specific MT1 isozymes led to lack of growth on acetate, suggesting that MT1 and MT2 enzymes might play an important role during growth on this substrate. The mtbA gene was required for dimethylamine and monomethylamine (MMA) utilization and was important, but not required, for trimethylamine utilization. Analysis of reporter gene fusions revealed that both mtaA1 and mtbA were expressed on all methanogenic substrates tested. However, mtaA1 expression was induced on methanol, while mtbA expression was down-regulated on MMA and acetate. mtaA2 was expressed at very low levels on all substrates. The mtaA1 transcript had a large 5′ untranslated region (UTR) (275 bp), while the 5′ UTR of the mtbA transcript was only 28 bp long.
Methanogenesis, the biological formation of methane (CH4), is carried out by a unique group of microorganisms from the domain Archaea known as methanogens. These organisms convert a limited number of small carbon-containing compounds to CH4, conserving energy for growth in the process. The substrates used by methanogens include H2-CO2, acetate, and a variety of one-carbon compounds (C1 compounds) that are disproportionated into CO2 and CH4 via the methylotrophic methanogenic pathways (11, 41). Methylotrophic methanogens are found exclusively among members of the Methanosarcinales, including the three sequenced species Methanosarcina barkeri, Methanosarcina mazei, and Methanosarcina acetivorans. Although Methanosphaera species (members of the Methanobacteriales) are also able to utilize methanol, they do so via a distinct methanogenic pathway that requires hydrogen as a cosubstrate (47).
Detailed biochemical characterization of methylotrophic methanogenesis has demonstrated that the methyl group from C1 compounds enters the methanogenic pathway at the level of methyl-coenzyme M (CH3-CoM) (reviewed in reference 23). This is mediated by the concerted action of two methyltransferases called methyltransferase 1 (MT1) and MT2. MT1 consists of two protein components; the first component is a methyltransferase [encoded by the genes mtaB for methanol, mttB for trimethylamine [TMA], mtbB for dimethylamine [DMA], and mtmB for monomethylamine [MMA]) that catalyzes the transfer of the methyl group from the methylated substrate to a second protein component, a cognate corrinoid protein (encoded by the genes mtaC for methanol, mttC for TMA, mtbC for DMA, and mtmC for MMA). The methylated corrinoid protein then becomes the substrate for the MT2 methyltransferase, which transfers the methyl group to CoM.
A variety of in vitro biochemical studies in M. barkeri have shown that the MT1 enzyme systems are exquisitely specific with respect to their substrates. Thus, discrete MT1 enzymes for the activation of methanol, MMA, DMA, and TMA have been purified and biochemically characterized (7, 14, 15, 43). This substrate specificity is reflected in the amino acid sequences of the MT1 proteins. Although the corrinoid proteins are similar, there is no significant homology between the methyltransferase proteins for any of the MT1 enzymes. Interestingly, however, there are multiple, highly homologous MT1 enzymes for each of the known C1 substrates in Methanosarcina spp. Thus, there are three methanol-specific (MtaCB1, -2, and -3), two TMA-specific (MttCB1 and -2), three DMA-specific (MtbCB1, -2, and -3), and two MMA-specific (MtmCB1 and -2) MT1 isozymes (10, 17, 26).
In M. barkeri Fusaro two different MT2 isozymes have been described, one that predominates in methanol-grown cells (MT2-M) and another that predominates in acetate-grown cells (MT2-A); however, both proteins are present in methanol- and acetate-grown cells (19). Later, MT2-M was renamed MtaA while MT2-A was renamed MtbA in this organism (21). These MT2 isozymes are also substrate specific but not to the same degree as the MT1 components. Accordingly, MtaA is capable of transferring the methyl group from the methanol-specific corrinoid protein (MtaC) to CoM in vitro, whereas MtbA catalyzes the analogous transfer from the MMA-, DMA-, and TMA-specific corrinoid proteins in vitro. Interestingly, biochemical studies demonstrate that MtaA can also act as the MT2 enzyme for TMA, but not for DMA and MMA, in M. barkeri (6, 14-16, 45).
Regulation of the mtaA and mtbA genes in M. barkeri is consistent with their biochemical function, i.e., that MtaA is the methanol-specific MT2 while MtbA is the methylamine-specific MT2. Qualitative expression levels determined using Northern blot analysis revealed that the mtaA transcript predominates in methanol-grown cells, whereas transcription of mtbA is most abundant during growth on TMA and H2-CO2. Nevertheless, these mRNA studies and the biochemical studies described above indicate that both genes are expressed on multiple substrates (19, 21). Thus, it seems quite possible that these proteins might play as-yet-unknown metabolic roles during growth on these substrates. Interestingly, two mtaA genes, designated mtaA1 and mtaA2, are present in each of the sequenced Methanosarcina genomes. Akin to the methanol-specific mtaCB1, mtaCB2, and mtaCB3 operons, these two genes might be differentially regulated and/or encode isozymes with different functions (4). Importantly, it should be noted that there are numerous other MT2 proteins encoded in the Methanosarcina genomes. For example, M. acetivorans has 10 MT2 homologs in addition to the mtaA1, mtaA2, and mtbA genes (17). Whether these play a role in methanol or methylamine metabolism or in the metabolism of other substrates has yet to be experimentally addressed. Thus, numerous questions regarding the in vivo function of MT2 enzymes remain to be answered.
Here, we report the first use of genetic methods to understand the in vivo role of the mtaA and mtbA genes of M. acetivorans C2A. Our data reveal a novel and broader role for these enzymes than previously suspected.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Standard conditions were used for growth of Escherichia coli strains (44). DH5α λ pir (30) was used as the host for all pir-dependent replicons. DH10B (Stratagene, La Jolla, CA) was used for all other plasmid replicons. M. acetivorans C2A (DSM 2834) (37) was from laboratory stocks. Methanosarcina was grown in single-cell morphology (38) at 37°C in high-salt (HS) broth medium containing either 125 mM methanol, 50 mM trimethylamine, 50 mM dimethylamine, 50 mM monomethylamine, or 120 mM acetate. Growth of M. acetivorans on medium solidified with 1.5% agar was as described previously (2). All plating manipulations were carried out under strictly anaerobic conditions in an anaerobic glove box. Solid-medium plates were incubated in an intrachamber anaerobic incubator as described previously (29). Puromycin (CalBiochem, San Diego, CA) was added from sterile, anaerobic stocks at a final concentration of 2 μg/ml for selection of Methanosarcina strains carrying the puromycin transacetylase gene cassette (pac) (18, 28). The purine analog 8-aza-2,6-diaminopurine (Sigma, St. Louis, MO) was added from sterile, anaerobic stocks at a final concentration of 20 μg/ml for selection against the hpt gene.
DNA methods.
Standard methods were used throughout for isolation and manipulation of plasmid DNA from E. coli (1). Genomic DNA from M. acetivorans was isolated as described previously (32). DNA hybridizations were performed using the DIG System (Roche, Mannheim, Germany). MagnaGraph Nylon transfer membranes were from Micron Separations Inc. The DNA sequence was determined from double-stranded templates at the W. M. Keck Center for Comparative and Functional Genomics, University of Illinois.
Transformation.
E. coli strains were transformed by electroporation using an E. coli Gene Pulser (Bio-Rad, Hercules, CA) as recommended. Liposome-mediated transformation was used for Methanosarcina species as described previously (28).
Plasmid constructions.
Standard methods were used for construction of all plasmids. The plasmid constructions and primers used are described in Tables 1 and 2, respectively. pJK41 and all derivatives are nonreplicating in Methanosarcina.
TABLE 1.
Plasmids used in the study
| Plasmid | Description and/or construction | Reference or source |
|---|---|---|
| pSL1180 | Apr cloning vector; pUC replicon | 5 |
| pJK3 | pac cassette source | 28 |
| pJK41 | Apr Pmr cloning vector; R6K replicon | 27 |
| pMP44 | Vector used to construct up- and down-region cassettes to delete genes from the M. acetivorans C2A chromosome using the markerless exchange method | 33 |
| pMP47 | HindIII/KpnI-digested up-mtaA1 PCR product (with primers 5-upmtaA and 3-upmtaA) cloned into HindIII/KpnI-digested pSL1180 | This study |
| pMP48 | KpnI/BstBI-digested dn-mtaA1 PCR product (with primers 5-dnmtaA and 3-dnmtaA) cloned into KpnI/BstBI-digested pSL1180 | This study |
| pMP54 | Replacement of 166-bp HindIII/KpnI fragment of pMP48 with 1,027-bp HindIII/KpnI fragment of pMP47 | This study |
| pMP64 | AflII/NotI-digested up-mtbA PCR product (with primers AflII-upmtbA and NotI-upmtbA) cloned into AflII/NotI-digested pMP44 | This study |
| pMP65 | NotI/KpnI-digested dn-mtbA PCR product (with primers NotI-dnmtbA and KpnI-dnmtbA) cloned into NotI/KpnI-digested pMP64 | This study |
| pMP68 | Replacement of 52-bp NruI/AvrII fragment of pMP44 with 1,874-bp SmaI/NruI ΔmtaA1 fragment of pMP54 | This study |
| pMP70 | SpeI/SstI-digested up-mtaA2 PCR product (with primers SpeI-upA2 and SacI-upA2) and SstI/NotI-digested dn-mtaA2 PCR product (with primers SacI-dnA2 and NotI-dnA2) cloned into SstI/NotI-digested pMP44 | This study |
| pAMG82 | Vector for construction of promoter fusions to the uidA gene of E. coli | 20 |
| pAB46 | AscI-digested 1,000-bp upstream region of mtaA1 PCR product (using primers mtaA1rev and mtaA1for) was treated with T4 kinase and cloned into Ecl136II/AscI-digested pAMG82 | This study |
| pAB47 | AscI-digested 1,000-bp upstream region of mtaA1 PCR product (using primers mtaA1rev2 and mtaA1for) was treated with T4 kinase and cloned into Ecl136II/AscI-digested pAMG82 | This study |
| pAB48 | AscI-digested 1,000-bp upstream region of mtaA2 PCR product (using primers mtaA2rev and mtaA2for) was treated with T4 kinase and cloned into Ecl136II/AscI-digested pAMG82 | This study |
| pAB49 | AscI-digested 1,000-bp upstream region of mtbA PCR product (using primers mtbArev and mtbAfor) was treated with T4 kinase and cloned into Ecl136II/AscI-digested pAMG82 | This study |
TABLE 2.
Primers used in the study
| Primer | Sequencea | Added site(s) |
|---|---|---|
| 5-upmtaA | GGGGGGAAGCTTCTCGAGAGCCAGGATGTCCTTCACC | HindIII, XhoI |
| 3-upmtaA | GGGGGGGGTACCCATATGGGTCATACC | KpnI, NdeI |
| 5-dnmtaA | GGGGGGGGTACCAGATCTTGAAAACGCATAAAAACCC | KpnI, BglII |
| 3-dnmtaA | GGGGGGTTCGAACCTAGGTAACAGCGCAGGTTCTCTCC | BstBI, AvrII |
| SpeI-upA2 | GCCGCCACTAGTTTCGAGGATAGTTTTACGTTACGA | SpeI |
| SacI-upA2 | GGCGCCGAGCTCCATATGACTCATCTTCAATCTTCCCCAAAT | SstI, NdeI |
| SacI-dnA2 | GCCGCCGAGCTCGGATCCTGAAGAACCTGAAAAATAGAAA | SstI, BamHI |
| NotI-dnA2 | GCCGCCGCGGCCGCTCCGAGACTCTTGCATACGA | NotI |
| AflII-upmtbA | CCGCCGCTTAAGAAAAATCTACTCCCAAACTA | AflII |
| NotI-upmtbA | CCGCCGGCGGCCGCACATGTTATAATCCCTCTCTAAGTTCTA | NotI, BspLU11I |
| NotI-dnmtbA | CCGCGGGCGGCCGCAGATCTGTAAACAAGTACTGAGTAATGC | NotI, BglII |
| KpnI-dnmtbA | CCGCCGGGTACCATTTATCAAAAATCTCTTGG | KpnI |
| A1L | GCACTATCTGCCCCCATTTA | None |
| A1R | AAGCTGCCCTTATGGGTTTT | None |
| A2L | TCGTTGCCCAAACAATGATA | None |
| A2R | GCAGTTCTTTCTCCCTGTGC | None |
| mtbA up | TAAATTGTGCACGGAACTGC | None |
| mtbA down | CTTCCGGAAAATTTGAGAGC | None |
| mtaA1for | GGCGCGCCTCCGTCCCTGAAGACTTTTG | AscI |
| mtaA1rev | TTTTTTACTTTTATCTTAGTACATATATTTTTATTGC | None |
| mtaA1rev2 | GGTCATACCTTTTTTACTTTTATCTTAGTACATATATTTTTATTG | None |
| mtaA2for | GGCGCGCCAATATCTGAAAGGACATTCTAAATCC | AscI |
| mtaA2rev | CAATCTTCCCCAAATATTCAATTCAC | None |
| mtbAfor | GGCGCGCCCACCATACCGCCCAGTATTT | AscI |
| mtbArev | TAATCCCTCTCTAAGTTCTATAGACAGC | None |
Added restriction sites are underlined.
Construction of mtaA and mtbA deletion mutants.
The markerless deletion method (33) was used to create deletion mutants of the mtaA1, mtaA2, and mtbA genes in the Δhpt (WWM1) background of M. acetivorans (Table 3). The plasmids pMP68, pMP70, and pMP65 were used to delete mtaA1, mtaA2, and mtbA, respectively. The ΔmtaA1 ΔmtaA2 mutant was constructed from the ΔmtaA2 (WWM27) mutant by deleting the mtaA1 gene. The ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 ΔmtaA1 (WWM346) and ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 ΔmtaA2 (WWM28) deletion mutants were constructed using the previously constructed mutant ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 (WWM15) that is incapable of growth on methanol (32). The ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 ΔmtaA1 ΔmtaA2 (WWM147) deletion mutant was constructed by using the ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 ΔmtaA2 (WWM28) background and subsequently deleting mtaA1. The mtaA1, mtaA2, and mtaCB deletions were isolated on TMA while the mtbA deletion was isolated on methanol. These substrates were selected as we expected the mtaA genes to be dispensable for growth on TMA and the mtbA gene to be dispensable for growth on methanol.
TABLE 3.
M. acetivorans C2A strains used
| Straina | Genotype or description | Source or reference |
|---|---|---|
| C2A | Wild type (DSM 2834) | 37 |
| WWM1 | Δhpt | 33 |
| WWM13 | ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 Δhpt | 32 |
| WWM21 | ΔmtaA1 Δhpt | This study |
| WWM27 | ΔmtaA2 Δhpt | This study |
| WWM28 | ΔmtaA2 ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 Δhpt | This study |
| WWM33 | ΔmtaA1 ΔmtaA2 Δhpt | This study |
| WWM40 | ΔmtbA Δhpt | This study |
| WWM147 | ΔmtaA1 ΔmtaA2 ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 Δhpt | This study |
| WWM346 | ΔmtaA1 ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 Δhpt | This study |
| WWM82 | Δhpt::φC31 int-attP | 20 |
| WWM347 | Δhpt::φC31 int-attR PmtaA1-uidA (annotated start codon)-attL | This study |
| WWM348 | Δhpt::φC31 int-attR PmtaA1-uidA (alternate start codon)-attL | This study |
| WWM349 | Δhpt::φC31 int-attR PmtaA2-uidA (annotated start codon)-attL | This study |
| WWM350 | Δhpt::φC31 int-attR PmtbA-uidA-attL | This study |
All strains are derivatives of M. acetivorans WWM1 and were constructed by markerless exchange as described in Materials and Methods. Plasmids used for introduction of the ΔmtaA1, ΔmtaA2, and ΔmtbA alleles were pMP68, pMP70, and pMP65, respectively. The construction of WWM15 has been previously published (32) and was used to construct WWM28, WWM147, and WWM346.
Determination of growth characteristics.
To determine the growth parameters of M. acetivorans mtaA deletion mutants on TMA, DMA, acetate, and methanol, mid-exponential phase, TMA-grown cells (optical density at 600 nm [OD600] of 0.4 to 0.5) were collected anaerobically by centrifugation (for 10 min at 5,000 × g), washed once with 5 ml of HS medium, and resuspended in 10 ml of HS medium. For MMA, cells were adapted once from TMA to MMA, and growth parameters were then determined on this substrate as described above. Ten-milliliter cultures of HS medium containing 50 mM trimethylamine, 125 mM methanol, 50 mM dimethylamine, 50 mM monomethylamine, or 120 mM acetate were inoculated with 0.3 ml of washed cells (in triplicate) and incubated at 37°C. Cell growth was monitored at 600 nm in a Bausch and Lomb Spectronic 21. The growth parameters of the mtbA deletion mutant were determined in the same way as above except that mid-exponential methanol-grown cells were used. Lag time was defined as the time required to achieve a half-maximal OD600 value.
Rate of methane production.
A 250-ml methanol- or TMA-grown culture (OD600 of 0.4 to 0.5) was pelleted anaerobically by centrifugation (10 min at 5,000 × g), washed with an equal volume of HS medium, and resuspended in HS medium supplemented with puromycin at a concentration of 1 × 109 cells/ml as determined by visible count using a Petroff-Hauser counting chamber. A total of 2 × 109 cells (2 ml of resuspended cells per tube) were aliquoted into Balch tubes on ice, and the headspace was exchanged for 250 kPa N2-CO2 (80%/20%). The reaction was started by the addition of 500 μmol of methanol for methanol-grown cells or 500 μmol of TMA for TMA-grown cells. Samples (50 μl or 100 μl) of headspace gas were removed every 8 to 10 min and analyzed on a Hewlett Packard 5890 Series II gas chromatograph using an 80- to 120-mesh Carbopack B column (Supelco, Bellefonte, PA). To determine protein concentration, 1 ml of the resuspended cells was centrifuged, and the resulting pellet was lysed by resuspending it in 100 μl of double-distilled H2O with 1 μg/ml of RNase and DNase. Protein concentration was determined by the method of Bradford using the Pierce protein assay kit following the manufacturer's guidelines. Specific activity was calculated from a CH4 standard curve and reported as milliunits (mU is calculated as nmol of CH4 produced min−1 mg−1 of protein).
Integrating promoter fusions on the M. acetivorans chromosome.
All plasmids constructed in either pAMG82 or pJK200 were integrated on the M. acetivorans chromosome using site-specific recombination between the φC31 attB site on the plasmid with the φC31 attP site on the chromosome as described previously (20).
Extract preparation and β-glucuronidase assay.
The preparation of cell extracts and the β-glucuronidase assay method were as previously described (34).
Determination of transcription start site.
Transcription start sites were determined using 5′ RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE) as previously described (3). The primers used for amplification are listed in Table 4. The products were then treated with ExoSAP-IT (USB, Cleveland, OH) to remove primers as per the manufacturer's guidelines. The PCR products were sequenced at the W. M. Keck Center for Comparative and Functional Genomics, University of Illinois.
TABLE 4.
Primers used for 5′ RLM-RACE
| Primer | Purpose | Sequence |
|---|---|---|
| 5′ RACEouter | 5′ RLM- RACE, sequencing | GCTGATGGCGATGAATGAACACTG |
| 5′ RACEinner | Sequencing | CATCAAAGCCAGCAAACGCAGTGTTCGGATCCGCG |
| mtaA1raceinner | Sequencing | TCTGCAGGAGGTCTGCAGGCACGGCTGC |
| mtaA1raceouter | 5′ RLM- RACE, sequencing | TCTGTAGTCTGGGCTGGAGGTACTG |
| mtaA1rev2 | Sequencing | GGTCATACCTTTTTTACTTTTATCTTAGTACATATATTTTTATTG |
| mtaA2raceinner | Sequencing | TCCCCAGCAAATTATCGGGCATTTTCAG |
| mtaA2raceouter | 5′ RLM- RACE, sequencing | TCTGCAGCCGCGGCTCAAGTTCGGT |
| mtaA2rev | Sequencing | CAATCTTCCCCAAATATTCAATTCAC |
| mtbAinner | 5′ RLM- RACE, sequencing | ATCTTGATAGCTTCAAGGATTAAAC |
| mtbAouter | Sequencing | GCCGGAACGATCTCCTCGCCTGTGAAAAG |
RESULTS
In silico analysis of the mtaA and mtbA genes in M. acetivorans, M. mazei, and M. barkeri.
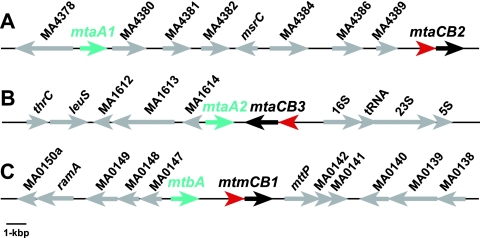
The proximity of the mtaA and mtbA genes to the mtaCB2 and mtmCB1 operons, respectively, supports their proposed in vivo roles in methanol and methylamine metabolism. In all sequenced Methanosarcina genomes, the mtaA1 gene is located about 16 kbp upstream of mtaCB2 whereas mtaA2 is directly downstream of mtaCB3. The mtbA gene is located upstream of the genes encoding an MMA MT1 (mtmB and mtmC) (Fig. 1 shows these loci in M. acetivorans).
FIG. 1.
Physical maps of the mtaA genes in M. acetivorans. A 20-kbp DNA region surrounding mtaA1 (A), mtaA2 (B), and the mtbA gene mtaA2 (C) is shown. mtaA1, mtaA2, and mtbA are shown as aquamarine arrows; mtaC2 and mtaC3 are shown as red arrows; and mtaB2 and mtaB3 are shown as blue arrows. Other open reading frames are shown as gray arrows.
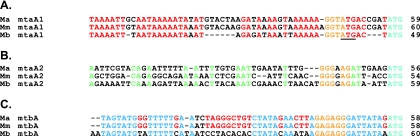
The annotated mtaA1, mtaA2, and mtbA genes of M. acetivorans are 1,029 bp, 1,020 bp, and 1,020 bp in length, respectively, and are predicted to encode MT2 methyltransferase proteins of 342 amino acids (aa), 339 aa, and 339 aa, respectively. All three annotated genes have ATG start codons; however, alignment of these genes with those from the other sequenced Methanosarcina genomes shows that the start codons of the mtaA1 genes from M. acetivorans and M. mazei are not equivalent to the experimentally determined start codon for the M. barkeri mtaA1 gene. Furthermore, the annotated M. acetivorans and M. mazei genes lack potential ribosome-binding sites (RBS), which can be found upstream of the annotated M. barkeri translation start (Fig. 2). Because a homologous ATG codon and RBS are present within the coding sequences of the M. acetivorans and M. mazei mtaA1 genes, we favor this internal start codon and have used the shorter coding sequence for all of the following analyses. This assignment is supported by the gene fusion data reported below.
FIG. 2.
Sequence alignment of the upstream regions of Methanosarcina mtaA1, mtaA1, and mtbA genes. A sequence of approximately 60 bp of DNA upstream of the predicted start site of the M. acetivorans (Ma), M. barkeri (Mb), and M. mazei (Mm) mtaA1 gene (A), mtaA2 gene (B), and mtbA gene (C) was compared using CLUSTALW (42). The predicted start codon for each gene is shown in cyan. The putative RBS is shown in yellow. (A) Conserved bases are shown in red. The annotated start site is underlined. (B) Conserved bases are shown in green. (C) Bases conserved in all three Methanosarcina spp. are shown in blue while those conserved in M. acetivorans and M. mazei are shown in red.
All Methanosarcina MtaA1 and MtaA2 proteins share 88% to 76% identity (Table 5), with most differences being conservative amino acid changes. For comparison, the MtbA and MtaA share only 35 to 37% identity. Biochemical analyses of the MtaA and MtbA proteins have shown that both coordinate zinc, potentially by virtue of a HXCXnC motif (where n is 74 or 76) (24, 36) that is conserved in the MtaA1, MtaA2, and MtbA proteins from all three Methanosarcina spp. (data not shown).
TABLE 5.
Percent amino acid identity of M. acetivorans, M. mazei, and M. barkeri MtaA1, MtaA2, and MtbA proteinsa
| Protein | % Amino acid identity
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| MtaA1Ma | MtaA1Mm | MtaA1Mb | MtaA2Ma | MtaA2Mm | MtaA2Mb | MtbAMa | MtbAMm | MtbAMb | |
| MtaA1Ma | 88 | 82 | 82 | 80 | 78 | 38 | 38 | 37 | |
| MtaA1Mm | 87 | 79 | 77 | 76 | 36 | 36 | 35 | ||
| MtaA1Mb | 76 | 77 | 76 | 36 | 35 | 36 | |||
| MtaA2Ma | 83 | 81 | 38 | 39 | 38 | ||||
| MtaA2Mm | 86 | 35 | 35 | 35 | |||||
| MtaA2Mb | 37 | 38 | 37 | ||||||
| MtbAMa | 90 | 87 | |||||||
| MtbAMm | 87 | ||||||||
| MtbAMb | |||||||||
CLUSTALW was used for calculations (42). The following subscripts were used to identify the proteins: Ma, M. acetivorans; Mm, M. mazei; and Mb, M. barkeri.
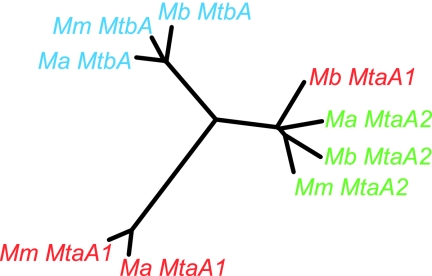
Phylogenetic analyses of the MtaA1, MtaA2, and MtbA proteins from M. acetivorans, M. barkeri, and M. mazei (Fig. 3) show that, with one exception, each of the isozymes forms a monophyletic group, suggesting that they evolved prior to separation of the three Methanosarcina spp. However, the MtaA1 protein from M. barkeri clusters with the MtaA2 proteins, consistent with a gene conversion event in which the M. barkeri Fusaro mtaA1 gene was replaced by a copy of the mtaA2 gene. If true, this suggests that MtaA1 and MtaA2 subfamilies are functionally equivalent.
FIG. 3.
Phylogeny of Methanosarcina MtaA and MtbA proteins. Unrooted neighbor-joining tree generated by the DrawTree program (http://workbench.sdsc.edu) (13, 22, 42) for the MtaA1 (red), MtaA2 (green), and MtbA (blue) proteins from M. acetivorans (Ma), M. mazei (Mm), and M. barkeri (Mb) are shown. Note that in all cases the individual isozymes in the three Methanosarcina spp. are more similar to each other than they are to other isozymes found in the same organism. The M. barkeri MtaA1 protein is an exception as it is more similar to the MtaA2 isozymes from the three Methanosarcina spp.
Construction and phenotypic characterization of mtaA and mtbA deletion mutants.
Mutants carrying deletions of the mtaA1, mtaA2, and mtbA genes were constructed as previously described using the markerless method of gene deletion (33). Various deletion combinations were also constructed, including ones in which the mtaA mutations were combined with previously constructed strains lacking the three methanol specific MT1 operons: mtaCB1, mtaCB2, and mtaCB3 (32) (Table 3).
The resulting deletion mutants were tested for their ability to grow on five methanogenic substrates: methanol, TMA, DMA, MMA, and acetate (Table 6). The mtaA1 single deletion mutant was unable to grow on methanol, indicating that mtaA1 is the sole functional methanol-specific MT2 enzyme and that the presence of mtaA2 does not compensate for lack of mtaA1. Consistent with this result, the mtaA2 single deletion mutant showed similar generation times, lag times, and growth yields to the parental strain on methanol, and, thus, mtaA2 is not required for growth on this substrate. The ΔmtaA1 ΔmtaA2 mutant did not grow on methanol, which is also consistent with the growth phenotypes of the single deletion mutants. Interestingly, some cultures of the ΔmtaA1 and ΔmtaA1 ΔmtaA2 mutants acquired the ability to utilize methanol after extended incubation. When these cultures were readapted to growth on TMA and subsequently inoculated into methanol medium, they showed a substantially shorter lag time than they exhibited during the first transfer from TMA to methanol (data not shown). Thus, it is likely that these cultures had acquired suppressor mutations allowing them to use methanol.
TABLE 6.
Growth of ΔmtaA and mtbA mutants in various methanogenic substratesa
| Strain genotype | Substrate generation time (h)
|
Substrate lag time (h)
|
Substrate growth yield (maximum OD600)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methanol | TMA | DMA | MMA | Acetate | Methanol | TMA | DMA | MMA | Acetate | Methanol | TMA | DMA | MMA | Acetate | |
| Δhpt | 7.2 ± 0.2 | 6.5 ± 0.3 | 15.3 ± 0.6 | 11.7 ± 1.1 | 50.8 ± 2.9 | 29 ± 2 | 20 ± 1 | 44 ± 1 | 28 ± 1 | 124 ± 6 | 0.72 ± 0.01 | 0.82 ± 0.01 | 0.51 ± 0.03 | 0.39 ± 0.02 | 0.42 ± 0.1 |
| ΔmtaA1 Δhpt | NG | 8.5 ± 0.4 | 24.9 ± 3.0 | 12.8 ± 0.3 | 55.6 ± 0.8 | NG | 27 ± 2 | 53 ± 5 | 30 ± 2 | 159 ± 10 | NG | 0.85 ± 0.02 | 0.51 ± 0.02 | 0.38 ± 0.02 | 0.44 ± 0.1 |
| ΔmtaA2 Δhpt | 6.5 ± 0.6 | 8.8 ± 0.6 | 32.8 ± 0.9 | 21.6 ± 0.9 | 55.7 ± 3.1 | 18 ± 2 | 29 ± 1 | 68 ± 14 | 52 ± 3 | 138 ± 6 | 0.80 ± 0.04 | 0.83 ± 0.02 | 0.50 ± 0.02 | 0.39 ± 0.0 | 0.42 ± 0.0 |
| ΔmtaA1 ΔmtaA2 Δhpt | NG | 9.7 ± 0.9 | 28.5 ± 0.7 | 25.7 ± 0.6 | 57.1 ± 1.7 | NG | 34 ± 2 | 67 ± 2 | 56 ± 1 | 124 ± 2 | NG | 0.84 ± 0.04 | 0.51 ± 0.02 | 0.39 ± 0.01 | 0.44 ± 0.0 |
| ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 Δhpt | NG | 8.7 ± 0.7 | 27.9 ± 0.3 | 30.2 ± 0.4b | 72.7 ± 3.3 | NG | 25 ± 3 | 71 ± 3 | 134 ± 4b | 241 ± 7 | NG | 0.85 ± 0.00 | 0.52 ± 0.01 | 0.36 ± 0.01b | 0.4 ± 0.1 |
| ΔmtaA1 ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 Δhpt | NG | 8.3 ± 0.1 | 25.1 ± 0.5 | 13.9 ± 0.4 | NG | NG | 33 ± 1 | 64 ± 2 | 31 ± 2 | NG | NG | 0.89 ± 0.01 | 0.46 ± 0.04 | 0.38 ± 0.02 | NG |
| ΔmtaA2 ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 Δhpt | NG | 10.5 ± 0.9 | 26.6 ± 3.8 | 29.6 ± 0.6 | 85.2 ± 2.4 | NG | 40 ± 2 | 76 ± 2 | 21 ± 1 | 233 ± 18 | NG | 0.84 ± 0.01 | 0.51 ± 0.01 | 0.38 ± 0.02 | 0.4 ± 0.1 |
| ΔmtaA1 ΔmtaA2 ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 Δhpt | NG | 8.4 ± 0.9 | 25.1 ± 0.5 | 17.3 ± 1.1 | NG | NG | 28 ± 3 | 64 ± 5 | 38 ± 2 | NG | NG | 0.84 ± 0.02 | 0.44 ± 0.02 | 0.36 ± 0.01 | NG |
| ΔmtbA Δhpt | 9.5 ± 1.4 | 21.2 ± 0.5 | NG | NG | 46.5 ± 1.1 | 29 ± 2 | 107 ± 5 | NG | NG | 109 ± 5 | 0.72 ± 0.01 | 0.42 ± 0.01 | NG | NG | 0.40 ± 0.02 |
Growth was measured as indicated in Materials and Methods. Lag time represents the time required to reach one-half of the maximum OD value. Values represent the average and standard deviations of triplicate measurements. NG, no growth was seen for at least 3 months.
Growth parameters for this strain on MMA were determined during a TMA-to-MMA substrate switch growth experiment.
We previously showed that a mutant lacking the operons encoding all three methanol-specific MT1 enzymes (mtaCB1, mtaCB2, and mtaCB3) does not grow on methanol (32). Therefore, the inability of the ΔmtaA1 ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 mutant to grow on methanol was not surprising; however, we were surprised to observe that this mutant failed to grow on acetate, whereas the ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 mutant does not have any observable growth defect on acetate (32). Relative to this mutant, the ΔmtaA2 ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 mutant did grow on acetate, though with a slightly longer generation time. Thus, analogous to the methanol growth phenotype, the mtaA2 gene does not appear to be required for growth on acetate. Results with the deletion mutant lacking all five methanol-specific MT1 and MT2 enzymes are consistent with this interpretation. All the mtaA mutants tested in this study showed a modest but reproducible increase in generation time and lag time on TMA, DMA, and MMA compared to the parental strain, the significance of which is not clear (Table 6).
The ΔmtbA mutant is incapable of growth on DMA and MMA, and, thus, MtbA is probably the sole MT2 enzyme for use of these substrates. The ΔmtbA strain is able to grow on TMA; however, the generation time of the mutant was threefold longer, the lag phase when switching from methanol to TMA was fivefold longer, and the growth yield was only half relative to the parent strain. Interestingly, this growth yield is similar to that achieved by the wild-type strain on MMA, suggesting that only a single methyl group resulting from the demethylation of TMA to DMA is being channeled into the methylotrophic pathway in this mutant (i.e., the data suggest that the product DMA is not further catabolized in this mutant). Growth of the ΔmtbA mutant is unlikely to be due to suppressor mutations because this mutant retained the characteristic lag time, slow growth rate, and low yield after being switched from TMA to methanol and back to TMA (data not shown). Thus, MtbA is very important, but not essential, for growth on TMA. The ΔmtbA mutant had only slight growth defects on methanol or acetate.
Methanogenesis from various substrates in mtaA and mtbA deletion mutants.
Because growth can be affected by a variety of factors, we also measured the rate of methane production by mutant and wild-type cell suspensions (Table 7). We were unable to measure the rate of methane production by the ΔmtaA1, the ΔmtaA1 ΔmtaCB1 ΔmtaCB2 ΔmtaCB3, the ΔmtaA2 ΔmtaCB1 ΔmtaCB2 ΔmtaCB3, and the ΔmtaA1 ΔmtaA2 ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 mutants on methanol as these strains do not grow on this substrate. We attempted to circumvent this problem by measuring methane production from methanol in TMA-grown cells but were unsuccessful. This is likely due to a lack of MT1 expression under these growth conditions (4). Interestingly, the rate of methane production from methanol by resting cell suspensions in methanol-grown ΔmtaA2 cells was nearly twofold slower than wild-type. Thus, despite the lack of observable growth phenotypes in this mutant, MtaA2 clearly plays a role in methanol metabolism. The ΔmtbA mutant had no significant effect on methane production from methanol, and neither the ΔmtaA1 nor the ΔmtaA2 mutation affected the ability of TMA-grown cells to produce methane from TMA. In contrast, the ΔmtbA mutant displayed a 50-fold reduction in growth rate, relative to wild-type, on TMA. Therefore, the slow growth of the ΔmtbA mutant is probably due to the slow rate of substrate catabolism.
TABLE 7.
Rate of methane production of the mtaA and mtbA single deletion mutants
| Strain genotype | Specific activity (mU/mg of protein)a
|
|
|---|---|---|
| Methanol | TMA | |
| Δhpt | 161 ± 2 | 24 ± 3 |
| ΔmtaA1 Δhpt | NGb | 21 ± 2 |
| ΔmtaA2 Δhpt | 78 ± 4 | 21 ± 2 |
| ΔmtbA Δhpt | 197 ± 7 | 0.4 ± 0.04 |
Activity was determined as nmol of CH4 produced min−1 mg−1 protein. Values represent the average and standard deviations of four independent measurements.
NG, no growth occurs on this substrate.
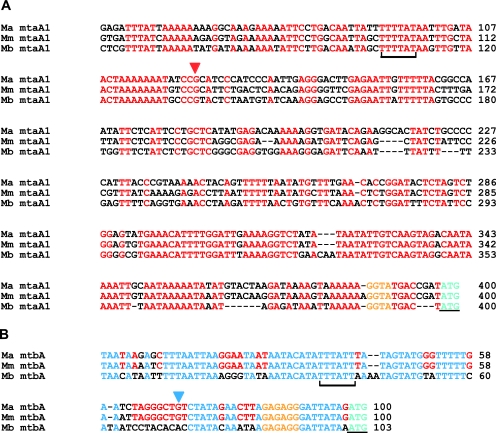
Identification of the TSS of mtaA1 and mtbA.
We determined the transcription start site (TSS) of mtaA1 and mtbA (Fig. 4) using 5′ RACE as described previously. The mtaA1 TSS is a G residue 275 bp upstream of the putative start codon. Twenty-four base pairs upstream of the TSS lies a putative TATA-box adjacent to a purine rich element representing a potential transcription factor B recognition element (BRE). These elements are conserved in all three Methanosarcina spp. In sharp contrast, the mtbA TSS is a G residue only 28 bp upstream of the putative start codon. Twenty-eight base pairs upstream of that lies a putative TATA-box next to a poorly recognizable potential BRE. Interestingly, the mtbA TSS was not conserved in M. barkeri Fusaro. We were unable to determine the TSS of mtaA2, possibly due to the extremely low levels of expression of this gene (see below).
FIG. 4.
The TSS of mtaA1 and mtbA in M. acetivorans determined by 5′ RLM-RACE. The 5′ mRNA leader region upstream of the putative start codon (cyan and underlined bases) for the three genes along with the experimentally determined TSS(arrow) and the putative TATA box (black bracket) and RBS (orange text) are shown. Panel A shows this region for the mtaA1 promoter; the red letters represent bases conserved in all three Methanosarcina spp., namely M. acetivorans (Ma), M. mazei (Mm), and M. barkeri (Mb). Panel B shows the 5′ mRNA leader region for the mtbA promoter; the blue letters represent bases conserved in all three Methanosarcina spp. while the red letters represent bases conserved between M. acetivorans and M. mazei.
Expression analysis of mtaA1, mtaA2, and mtbA genes.
To gain further insight into their in vivo functions we examined the substrate-dependent regulation of the mtaA1, mtaA2, and mtbA genes using reporter gene fusions. Accordingly, translational gene fusions were constructed such that a 1-kbp region immediately upstream of each gene including the start codon was fused to the uidA gene, which encodes the easily assayable enzyme β-glucuronidase (33). Based on the mapped TSSs for each gene (Fig. 4), we believe that this region should include all necessary regulatory elements required for controlled gene expression, although it remains formally possible that distally located elements or elements within the coding sequence could also be required for expression. These translational fusions were integrated into the chromosome in single copies as described previously (20) and assayed for β-glucuronidase activity after growth on methanol, TMA, DMA, MMA, and acetate (Table 8).
TABLE 8.
β-Glucuronidase activities of uidA translational fusions to mtaA1, mtaA2 and mtbA in cells grown on various methanogenic substrates
| Fusion | Activity on the indicated substrate (mU/mg of protein)a
|
||||
|---|---|---|---|---|---|
| Methanol | TMA | DMA | MMA | Acetate | |
| mtaA1 (annotated) | 8 ± 0.3 | 6 ± 0.3 | 10 ± 1.8 | 7 ± 0.7 | 1.8 ± 0.5 |
| mtaA1 (alternate) | 157 ± 10 | 42 ± 3 | 65 ± 9 | 13 ± 3 | 68 ± 11 |
| mtaA2 | 8 ± 0.2 | 1 ± 0.1 | 2 ± 0.1 | 1 ± 0.5 | 2 ± 0.9 |
| mtbA | 418 ± 37 | 381 ± 36 | 460 ± 39 | 164 ± 38 | 22 ± 2 |
Activity was determined as nmol of β-glucuronidase produced min−1 mg−1 of protein Values represent the average and standard error of nine independent measurements. The limit of detection for this assay is 0.1 mU/mg of protein.
Each of the three gene fusions was expressed on all substrates tested. Expression of the mtaA1 fusion was strongly induced by methanol (10-fold relative to MMA) but remained at relatively high levels on TMA, DMA, and acetate (only two- to threefold lower than methanol). The mtbA fusion was expressed at equally high levels on methanol, TMA, and DMA, with two- to threefold lower expression on MMA and 20-fold lower expression on acetate. These observations are in contrast to those made by previous workers in M. barkeri using Northern blot analysis and immunochemical approaches (19, 21). These differences might be species specific and/or might be a reflection of the different techniques used by us and these groups to assess expression. The mtaA2 gene was expressed at very low levels on all methanogenic substrates tested but did show an eightfold increase during growth on methanol. We also observed that the translational fusion constructed using the annotated start codon for the mtaA1 gene was expressed at very low levels on all substrates, consistent with the annotation's being incorrect, as suggested above.
DISCUSSION
The data presented here are consistent with the idea that mtaA1 and mtbA play primary roles in methanol and methylamine metabolism, respectively. The observation that mtaA1 is necessary for growth on methanol, in combination with its proven in vitro activity in M. barkeri (43), demonstrates that MtaA1 is a methanol-specific MT2. Furthermore, it shows that no other MT2 enzyme, including the 82% identical homolog MtaA2, can substitute for MtaA1 during growth of M. acetivorans on methanol. Similarly, our data indicate that MtbA is the sole MT2 enzyme used for DMA and MMA metabolism. The proximity of these genes to other genes known to be involved in the metabolism of methanol and methylamines supports these primary functional assignments. Nevertheless, our data also clearly indicate that the various MT2 enzymes play additional roles in the metabolism of nonmethylotrophic substrates and that cross-reactivity between substrates occurs.
An interesting observation that came out of this study is the requirement of MtaCB and MtaA1 for growth on acetate, a substrate for which there is no known or apparent role for the MT1/MT2 methyltransferase pathway. The nature of the MT1/MT2 requirement for growth on acetate remains mysterious at this time but is supported by the relatively high-level expression of mtaA1 on acetate. MtaA1 expression is not simply constitutive but 10-fold lower on MMA than on methanol and, therefore, clearly regulated. Thus, the expression of mtaA1 on acetate is likely a reflection of an important role during growth on this nonmethylotrophic substrate. These data indicate that there is a required flow of methyl groups from acetate through the methanol-specific MT1/MT2 pathway during growth on acetate. Interestingly, the data presented here suggest that this phenomenon may be a general function of MT1/MT2 systems. Other MT1 and MT2 enzymes can take the place of MtaCB and MtaA1 in performing this function as both the ΔmtaCB1 ΔmtaCB2 ΔmtaCB3 mutant and the ΔmtaA1 mutant can grow on acetate. Accordingly, it appears that in the ΔmtaA1 mutant another MT2 enzyme acts to transfer methyl groups either to or from MtaCB, and in the ΔmtaCB mutant another MT1 enzyme acts to transfer methyl groups to or from MtaA. It should be noted that in this study the ΔmtaCB mutants lacked all three isozymes (mtaCB1, mtaCB2, and mtaCB3). Therefore, it is possible that only one of the mtaCB operons in conjunction with mtaA1 is responsible for this phenotype. In this regard it is interesting that both mtaCB2 and mtaCB3 are specifically induced on acetate compared to TMA (4). The possibility that other MT1/MT2 enzymes may be involved is supported by numerous studies showing their expression during growth on acetate. These include genes encoding MtaCB2, MtaCB3 as mentioned above, MtaA, MtbA, MtmC, and MtsB (4, 6, 9, 16, 19, 25, 31, 40, 46).
Two results demonstrate cross-reactivity between MT2 enzymes. First, the ΔmtbA mutant retains the ability to grow on TMA, albeit poorly. Thus, MtbA is not the sole MT2 that can be used for growth on TMA, although it is clearly the predominant one. These genetic data support previous immunochemical experiments showing that MtbA-depleted extracts retain some ability to produce methane from TMA (16). The data also suggest that another MT2 enzyme is capable of transferring the methyl group from methylated MttC to CoM. Although we did not directly test this idea, it is highly likely, based on in vitro biochemistry (15), that the MT2 enzyme responsible for this activity is MtaA1 though it is quite possible that another MT2 enzyme might be responsible for this activity. Second, we observed that strains carrying the ΔmtaA1 mutation, alone or in combination with the ΔmtaA2 mutation, are capable of acquiring suppressor mutations that allow them to utilize methanol. Deletion mutants lacking the genes encoding the three methanol-specific MT1 isozymes (mtaCB1, mtaCB2, and mtaCB3), either alone or in conjunction with ΔmtaA1 or ΔmtaA1 ΔmtaA2, did not acquire suppressor mutations allowing growth on methanol. Therefore, the genes encoding the MT1 isozymes are needed for this suppression, which most likely occurs by activating or modifying another MT2 gene. The existence of 11 additional MT2 genes (17) suggests likely candidates for the locus modified by the suppressor mutation. Surprisingly, mtaA2 is not a candidate, given that suppressors arise with similar frequency in both the ΔmtaA1 and ΔmtaA1 ΔmtaA2 strains.
Our data do not provide any clear indications of the function of MtaA2. Unlike mtaA1, mtaA2 is neither necessary nor sufficient for growth on methanol. This was surprising, given the proximity of mtaA2 to the mtaCB3 operon, which we have previously shown encodes a methanol-specific MT1 isozyme (32). However, MtaA2 does appear to contribute to methanogenesis from methanol because this deletion mutant had a slower rate of methane production from methanol. Methane production from TMA was not affected, indicating that this is a substrate-specific effect. The mtaA2 gene was expressed at very low levels on all methanogenic substrates tested, although it is up-regulated eightfold on methanol. These observations are in accordance with proteomic and microarray studies done on methanol-grown M. thermophila and M. acetivorans (12, 25). Interestingly, phylogenetic analysis shows that the biochemically characterized methanol-specific MT2 enzyme in M. barkeri Fusaro is an MtaA2 protein but in an identical genomic context as the mtaA1 genes of M. acetivorans and M. mazei. Thus, it seems probable that the MtaA2 proteins from all the other Methanosarcina species are capable of activity with the methanol-specific MT1 enzymes.
Finally, we along with other workers have observed large 5′ untranslated regions (UTRs) for a number of methanogenic genes (3, 8, 35, 39). This was the case for the mtaA1 gene as well, which has a large 5′ UTR (275 nucleotides). The significance of these long leader sequences is not completely understood, but deletion analysis shows that these regions can play an important role in regulating expression (3). The 5′ UTR for the M. acetivorans mtbA transcript is very short (28 nucleotides) and is completely conserved in M. mazei, along with appropriately spaced putative TATA-box and BREs immediately upstream. While the putative TATA-box and BRE are conserved in M. barkeri Fusaro, the TSS is not. Although M. barkeri Fusaro is reported to grow on methylamines, the strain maintained in our laboratory, which was also the source of DNA used for genome sequencing, does not grow on TMA and DMA (data not shown) (26). The lack of conservation in the mtbA promoter therefore reflects a potential inactivating mutation that might explain this phenotype.
The genetic experiments presented in this study confirmed the implications of biochemical studies and underscored the importance of the MT2 methyltransferases in methanogenesis. This study also raised new questions, the answers to some of which we are seeking presently in our laboratory. These include the role of MT1/MT2 systems during growth on nonmethylotrophic substrates, the nature of the suppressor mutations that arise in the ΔmtaA1 backgrounds, and the potential regulatory proteins that might affect expression of these genes. We would also like to determine the MT2 enzyme(s) that substitute for mtbA in TMA utilization and also determine the potential differences in the interactions of MtaA1 and MtbA with the various MT1 isozymes specific for methanol and TMA.
Acknowledgments
We thank Gargi Kulkarni, Rina Opulencia, and Nicole Buan for critical review of the manuscript.
This work was supported by a National Science Foundation Grant (MCB0517419) to W.W.M.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Footnotes
Published ahead of print on 28 March 2008.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1992. Current protocols in molecular biology. John Wiley and Sons, New York, NY.
- 2.Boccazzi, P., J. K. Zhang, and W. W. Metcalf. 2000. Generation of dominant selectable markers for resistance to pseudomonic acid by cloning and mutagenesis of the ileS gene from the archaeon Methanosarcina barkeri Fusaro. J. Bacteriol. 1822611-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bose, A., and W. W. Metcalf. 2008. Distinct regulators control the expression of methanol methyltransferase isozymes in Methanosarcina acetivorans C2A. Mol. Microbiol. 67649-661. [DOI] [PubMed] [Google Scholar]
- 4.Bose, A., M. A. Pritchett, M. Rother, and W. W. Metcalf. 2006. Differential regulation of the three methanol methyltransferase isozymes in Methanosarcina acetivorans C2A. J. Bacteriol. 1887274-7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosius, J. 1989. Superpolylinkers in cloning and expression vectors. DNA 8759-777. [DOI] [PubMed] [Google Scholar]
- 6.Burke, S. A., and J. A. Krzycki. 1995. Involvement of the “A” isozyme of methyltransferase II and the 29-kilodalton corrinoid protein in methanogenesis from monomethylamine. J. Bacteriol. 1774410-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke, S. A., and J. A. Krzycki. 1997. Reconstitution of monomethylamine:coenzyme M methyl transfer with a corrinoid protein and two methyltransferases purified from Methanosarcina barkeri. J. Biol. Chem. 27216570-16577. [DOI] [PubMed] [Google Scholar]
- 8.Burke, S. A., S. L. Lo, and J. A. Krzycki. 1998. Clustered genes encoding the methyltransferases of methanogenesis from monomethylamine. J. Bacteriol. 1803432-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao, X. J., and J. A. Krzycki. 1991. Acetate-dependent methylation of two corrinoid proteins in extracts of Methanosarcina barkeri. J. Bacteriol. 1735439-5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deppenmeier, U., A. Johann, T. Hartsch, R. Merkl, R. A. Schmitz, R. Martinez-Arias, A. Henne, A. Wiezer, S. Baumer, C. Jacobi, H. Bruggemann, T. Lienard, A. Christmann, M. Bomeke, S. Steckel, A. Bhattacharyya, A. Lykidis, R. Overbeek, H. P. Klenk, R. P. Gunsalus, H. J. Fritz, and G. Gottschalk. 2002. The genome of Methanosarcina mazei: evidence for lateral gene transfer between bacteria and archaea. J. Mol. Microbiol. Biotechnol. 4453-461. [PubMed] [Google Scholar]
- 11.Deppenmeier, U., T. Lienard, and G. Gottschalk. 1999. Novel reactions involved in energy conservation by methanogenic archaea. FEBS Lett. 457291-297. [DOI] [PubMed] [Google Scholar]
- 12.Ding, Y. H., S. P. Zhang, J. F. Tomb, and J. G. Ferry. 2002. Genomic and proteomic analyses reveal multiple homologs of genes encoding enzymes of the methanol:coenzyme M methyltransferase system that are differentially expressed in methanol- and acetate-grown Methanosarcina thermophila. FEMS Microbiol. Lett. 215127-132. [DOI] [PubMed] [Google Scholar]
- 13.Felsenstein, J. 1989. Phylogeny inference package (version 3.2). Cladistics 5164-166. [Google Scholar]
- 14.Ferguson, D. J., Jr., N. Gorlatova, D. A. Grahame, and J. A. Krzycki. 2000. Reconstitution of dimethylamine:coenzyme M methyl transfer with a discrete corrinoid protein and two methyltransferases purified from Methanosarcina barkeri. J. Biol. Chem. 27529053-29060. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson, D. J., Jr., and J. A. Krzycki. 1997. Reconstitution of trimethylamine-dependent coenzyme M methylation with the trimethylamine corrinoid protein and the isozymes of methyltransferase II from Methanosarcina barkeri. J. Bacteriol. 179846-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson, D. J., Jr., J. A. Krzycki, and D. A. Grahame. 1996. Specific roles of methylcobamide:coenzyme M methyltransferase isozymes in metabolism of methanol and methylamines in Methanosarcina barkeri. J. Biol. Chem. 2715189-5194. [DOI] [PubMed] [Google Scholar]
- 17.Galagan, J. E., C. Nusbaum, A. Roy, M. G. Endrizzi, P. Macdonald, W. FitzHugh, S. Calvo, R. Engels, S. Smirnov, D. Atnoor, A. Brown, N. Allen, J. Naylor, N. Stange-Thomann, K. DeArellano, R. Johnson, L. Linton, P. McEwan, K. McKernan, J. Talamas, A. Tirrell, W. Ye, A. Zimmer, R. D. Barber, I. Cann, D. E. Graham, D. A. Grahame, A. M. Guss, R. Hedderich, C. Ingram-Smith, H. C. Kuettner, J. A. Krzycki, J. A. Leigh, W. Li, J. Liu, B. Mukhopadhyay, J. N. Reeve, K. Smith, T. A. Springer, L. A. Umayam, O. White, R. H. White, E. Conway de Macario, J. G. Ferry, K. F. Jarrell, H. Jing, A. J. Macario, I. Paulsen, M. Pritchett, K. R. Sowers, R. V. Swanson, S. H. Zinder, E. Lander, W. W. Metcalf, and B. Birren. 2002. The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res. 12532-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gernhardt, P., O. Possot, M. Foglino, L. Sibold, and A. Klein. 1990. Construction of an integration vector for use in the archaebacterium Methanococcus voltae and expression of a eubacterial resistance gene. Mol. Gen. Genet. 221273-279. [DOI] [PubMed] [Google Scholar]
- 19.Grahame, D. A. 1989. Different isozymes of methylcobalamin:2-mercaptoethanesulfonate methyltransferase predominate in methanol- versus acetate-grown Methanosarcina barkeri. J. Biol. Chem. 26412890-12894. [PubMed] [Google Scholar]
- 20.Guss, A. M., M. Rother, J. K. Zhang, G. Kulkarni, and W. W. Metcalf. 2008. New methods for tightly regulated gene expression and highly efficient insertion of foreign genes for Methanosarcina species. [DOI] [PMC free article] [PubMed]
- 21.Harms, U., and R. K. Thauer. 1996. Methylcobalamin: coenzyme M methyltransferase isoenzymes MtaA and MtbA from Methanosarcina barkeri. Cloning, sequencing and differential transcription of the encoding genes, and functional overexpression of the mtaA gene in Escherichia coli. Eur. J. Biochem. 235653-659. [DOI] [PubMed] [Google Scholar]
- 22.Higgins, D. G., A. J. Bleasby, and R. Fuchs. 1992. CLUSTAL V: improved software for multiple sequence alignment. Comput. Appl. Biosci. 8189-191. [DOI] [PubMed] [Google Scholar]
- 23.Keltjens, J. T., and G. D. Vogels. 1993. Conversion of methanol and methylamines to methane and carbon dioxide, p. 253-303. In J. G. Ferry (ed.), Methanogenesis: ecology, physiology, biochemistry and genetics. Chapman and Hall, New York, NY.
- 24.Kruer, M., M. Haumann, W. Meyer-Klaucke, R. K. Thauer, and H. Dau. 2002. The role of zinc in the methylation of the coenzyme M thiol group in methanol:coenzyme M methyltransferase from Methanosarcina barkeri. Eur. J. Biochem. 2692117-2123. [DOI] [PubMed] [Google Scholar]
- 25.Li, L., Q. Li, L. Rohlin, U. Kim, K. Salmon, T. Rejtar, R. P. Gunsalus, B. L. Karger, and J. G. Ferry. 2007. Quantitative proteomic and microarray analysis of the archaeon Methanosarcina acetivorans grown with acetate versus methanol. J. Proteome Res. 6759-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeder, D. L., I. Anderson, T. S. Brettin, D. C. Bruce, P. Gilna, C. S. Han, A. Lapidus, W. W. Metcalf, E. Saunders, R. Tapia, and K. R. Sowers. 2006. The Methanosarcina barkeri genome: comparative analysis with Methanosarcina acetivorans and Methanosarcina mazei reveals extensive rearrangement within methanosarcinal genomes. J. Bacteriol. 1887922-7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metcalf, W. W. 1999. Genetic analysis in members of the domain Archaea, p. 278-326. In M. Smith and L. Sockett (ed.), Methods in microbiology: genetic methods for diverse prokaryotes. Academic Press, London, United Kingdom.
- 28.Metcalf, W. W., J. K. Zhang, E. Apolinario, K. R. Sowers, and R. S. Wolfe. 1997. A genetic system for Archaea of the genus Methanosarcina: liposome-mediated transformation and construction of shuttle vectors. Proc. Natl. Acad. Sci. USA 942626-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metcalf, W. W., J. K. Zhang, and R. S. Wolfe. 1998. An anaerobic, intrachamber incubator for growth of Methanosarcina spp. on methanol-containing solid media. Appl. Environ. Microbiol. 64768-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 1702575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul, L., and J. A. Krzycki. 1996. Sequence and transcript analysis of a novel Methanosarcina barkeri methyltransferase II homolog and its associated corrinoid protein homologous to methionine synthase. J. Bacteriol. 1786599-6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pritchett, M. A., and W. W. Metcalf. 2005. Genetic, physiological and biochemical characterization of multiple methanol methyltransferase isozymes in Methanosarcina acetivorans C2A. Mol. Microbiol. 561183-1194. [DOI] [PubMed] [Google Scholar]
- 33.Pritchett, M. A., J. K. Zhang, and W. W. Metcalf. 2004. Development of a markerless genetic exchange method for Methanosarcina acetivorans C2A and its use in construction of new genetic tools for methanogenic archaea. Appl. Environ. Microbiol. 701425-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rother, M., P. Boccazzi, A. Bose, M. A. Pritchett, and W. W. Metcalf. 2005. Methanol-dependent gene expression demonstrates that methyl-coenzyme M reductase is essential in Methanosarcina acetivorans C2A and allows isolation of mutants with defects in regulation of the methanol utilization pathway. J. Bacteriol. 1875552-5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sauer, K., U. Harms, and R. K. Thauer. 1997. Methanol:coenzyme M methyltransferase from Methanosarcina barkeri. Purification, properties and encoding genes of the corrinoid protein MT1. Eur. J. Biochem. 243670-677. [DOI] [PubMed] [Google Scholar]
- 36.Sauer, K., and R. K. Thauer. 1997. Methanol:coenzyme M methyltransferase from Methanosarcina barkeri. Zinc dependence and thermodynamics of the methanol:cob(I)alamin methyltransferase reaction. Eur. J. Biochem. 249280-285. [DOI] [PubMed] [Google Scholar]
- 37.Sowers, K. R., S. F. Baron, and J. G. Ferry. 1984. Methanosarcina acetivorans sp. nov., an acetotrophic methane-producing bacterium isolated from marine sediments. Appl. Environ. Microbiol. 47971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sowers, K. R., J. E. Boone, and R. P. Gunsalus. 1993. Disaggregation of Methanosarcina spp. and growth as single cells at elevated osmolarity. Appl. Environ. Microbiol. 593832-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sowers, K. R., T. T. Thai, and R. P. Gunsalus. 1993. Transcriptional regulation of the carbon monoxide dehydrogenase gene (cdhA) in Methanosarcina thermophila. J. Biol. Chem. 26823172-23178. [PubMed] [Google Scholar]
- 40.Tallant, T. C., and J. A. Krzycki. 1997. Methylthiol:coenzyme M methyltransferase from Methanosarcina barkeri, an enzyme of methanogenesis from dimethylsulfide and methylmercaptopropionate. J. Bacteriol. 1796902-6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thauer, R. K. 1998. Biochemistry of methanogenesis: a tribute to Marjory Stephenson. 1998 Marjory Stephenson Prize Lecture. Microbiology 1442377-2406. [DOI] [PubMed] [Google Scholar]
- 42.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 224673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Meijden, P., H. J. Heythuysen, A. Pouwels, F. Houwen, C. van der Drift, and G. D. Vogels. 1983. Methyltransferases involved in methanol conversion by Methanosarcina barkeri. Arch. Microbiol. 134238-242. [DOI] [PubMed] [Google Scholar]
- 44.Wanner, B. L. 1986. Novel regulatory mutants of the phosphate regulon in Escherichia coli K-12. J. Mol. Biol. 19139-58. [DOI] [PubMed] [Google Scholar]
- 45.Wassenaar, R. W., P. J. Daas, W. J. Geerts, J. T. Keltjens, and C. van der Drift. 1996. Involvement of methyltransferase-activating protein and methyltransferase 2 isoenzyme II in methylamine:coenzyme M methyltransferase reactions in Methanosarcina barkeri Fusaro. J. Bacteriol. 1786937-6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeliseev, A., P. Gartner, U. Harms, D. Linder, and R. K. Thauer. 1993. Function of methylcobalamin: coenzyme M methyltransferase isoenzyme II in Methanosarcina barkeri. Arch. Microbiol. 159530-536. [DOI] [PubMed] [Google Scholar]
- 47.Zinder, S. H. 1993. Physiological ecology of methanogens, p. 128-206. In J. G. Ferry (ed.), Methanogenesis: ecology, physiology, biochemistry and genetics. Chapman and Hall, New York, NY.