Abstract
Geranylgeranyl reductase from Sulfolobus acidocaldarius was shown to catalyze the reduction of geranylgeranyl groups in the precursors of archaeal membrane lipids, generally reducing all four double bonds. However, when geranylgeranyl diphosphate was subjected to the reductase reaction, only three of the four double bonds were reduced. Mass spectrometry and acid hydrolysis indicated that the allylic double bond was preserved in the partially reduced product derived from geranylgeranyl diphosphate. Thus, the reaction product was shown to be phytyl diphosphate, which is a substrate for archaeal prenyltransferases, unlike the completely reduced compound phytanyl diphosphate.
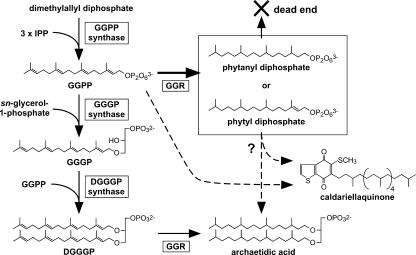
Biosynthesis of the archaeal membrane lipids (13) starts with the formation of precursors for the glycerol moiety and the isoprenoid chains, i.e., sn-glycerol-1-phosphate (18, 19) and, usually, geranylgeranyl diphosphate (GGPP) (4, 21), respectively (Fig. 1). Geranylgeranyl chains are sequentially transferred from GGPPs to the sn-3 and sn-2 positions of sn-glyceryl-1-phosphate to form 3-O-geranylgeranylglyceryl phosphate (GGGP) (5, 17, 23) and then 2,3-di-O-geranylgeranylglyceryl phosphate (DGGGP) (10). Subsequently, various biosynthetic processes, such as modification of polar head groups and reduction or cyclization of prenyl chains, are required for the production of archaeal membrane lipids.
FIG. 1.
Biosynthesis of membrane lipid and caldariellaquinone in Sulfolobus sp. Each dashed arrow indicates a biosynthetic route catalyzed by multiple enzymes. In Sulfolobus sp., GGPP is thought to be the common precursor of hydrophobic isoprenoid compounds such as archaeal membrane lipid and caldariellaquinone (8, 11). In the largest box, two hypothetical products from GGPP obtained from the reaction with GGR from S. acidocaldarius are shown. Phytyl diphosphate, which is the final product of plant GGR, may be utilized by archaeal prenyltransferases for the biosynthesis of the isoprenoid compounds, while phytanyl diphosphate must be a dead-end product because it cannot be a prenyl donor. 3 × IPP, three molecules of IPP.
One of the modifications—the complete reduction of double bonds in the isoprenoid chains—is thought to be important for the survival of archaea under extreme conditions, such as high temperature, because the existence of double bonds affects the properties of the membrane (6). The archaeal enzyme that catalyzes the reduction of geranylgeranyl groups in lipid precursors, geranylgeranyl reductase (GGR), was recently found in two thermophilic euryarchaeotes, Thermoplasma acidophilum (20) and Archaeaoglobus fulgidus (16). The GGR from both organisms can reduce all of the double bonds in the geranylgeranyl chains of DGGGP or of its structural analogues with modified polar head groups. In addition, GGR from A. fulgidus exhibited activity, albeit lower, for the upstream precursors of DGGGP, such as GGGP and GGPP, although the final products of these reactions are yet to be identified. The observation that the GGRs from these euryarchaeotes were capable of reducing all double bonds led to the following question. If all four double bonds in GGPP are reduced by GGR, can the product be a prenyl donor substrate for prenyltransferases? The loss of the double bond at position 2 of GGPP prevents the formation of the allylic carbocation that accompanies the detachment of the diphosphate-leaving group, thereby rendering the reactions catalyzed by prenyltransferases impossible (14, 15). Interestingly, archaeal GGRs are homologues of plant and bacterial GGRs, which specifically reduce three of the four double bonds in the geranylgeranyl side chain of chlorophyll or bacteriochlorophyll, yielding phytyl groups (1-3, 12). GGR from Arabidopsis thaliana can also reduce GGPP to phytyl diphosphate, which retains the allylic double bond and is a substrate for prenyltransferases (12). Thus, the purpose of the present study was to determine the product resulting from GGR reduction of GGPP in order to ascertain whether the archaeal enzyme can reduce all four double bonds.
MATERIALS AND METHODS
Materials.
Precoated reversed-phase thin-layer chromatography (TLC) plates (LKC18) were purchased from Whatman International Ltd., United Kingdom. (All-E)-farnesyl diphosphate was donated by Kyozo Ogura and Tanetoshi Koyama, Tohoku University. Nonlabeled isopentenyl diphosphate (IPP) was donated by C. Ohto, Toyota Motor Co. [1-14C]IPP was purchased from GE Healthcare, United Kingdom. Nonlabeled GGPP, GGGP, and DGGGP were synthesized enzymatically from farnesyl diphosphate, IPP, and racemic glyceryl-1-phosphate, as previously described (10). All other chemicals were analytical grade.
General procedures.
Restriction enzyme digestion, transformation, and other standard molecular biology techniques were carried out as described by Sambrook et al. (22).
Cloning, expression, and purification of recombinant GGR.
The saci_0986 gene was amplified by PCR using the genome of Sulfolobus acidocaldarius as a template and primers 5′-AATGGCATATGAAGGAACTTTAAATATGACGTTC-3′ and 5′-TAGTAGGATCCTTAACTTAAACTTTTGTTAAACTCTG-3′. The amplified gene was excised using NdeI and BamHI for insertion into a pET16b vector (Novagen, United States). The vector was introduced into Escherichia coli Rosetta 2(DE3), and the transformant was grown at 37°C in 250 ml LB medium supplemented with 50 mg/liter ampicillin and 30 mg/liter chloramphenicol. When the optical density at 600 nm reached 0.45, 1 mM isopropyl-β-d-thiogalactoside was added to induce protein expression. After additional overnight cultivation, the cells were harvested and sonicated in 3 ml binding buffer for a HisTrap column (GE Healthcare, United Kingdom). The cell homogenate was centrifuged at 15,000 × g for 30 min, and the resulting supernatant was recovered and heated at 55°C for 1 h. After centrifugation at 15,000 × g for 30 min, the supernatant was passed through a 0.45-μm filter and then purified using a HisTrap column according to the manufacturer's protocol. The purity of the S. acidocaldarius enzyme was confirmed using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. UV-visible analysis of the purified enzyme solution was performed with a Shimadzu UV-2450 spectrophotometer.
GC-MS analysis of the product of GGR.
A 3-ml reaction mixture, which contained 0.5 mmol of 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pH 6.0), 250 μmol of sodium dithionite, approximately 50 nmol of the enzymatically synthesized DGGGP, and an excess amount (∼100 nmol) of GGR, was incubated at 55°C for 2 h. Reaction products were extracted with 3 ml of 1-butanol saturated with H2O. The compounds in the butanol layer were enzymatically dephosphorylated using the method of Fujii et al. (7). The resulting alcohols were extracted with n-pentane, and the pentane layer was completely evaporated. The residual lipids or the authentic archaeol was dissolved in 30 μl anhydrous pyridine. After the pyridine solution was mixed with 30 μl of 1-trimehylsilylimidazole (Wako, Japan) for at least 30 min at 60°C, an aliquot of the solution was subjected to gas chromatography (GC)-mass spectrometry (MS) analysis performed with a Hewlett-Packard 6890 gas chromatograph interfaced with an MStation JMS-700 MS system (JOEL, Japan) using a J&B DB-1 capillary column (30 m by 0.25 mm; film thickness, 0.25 μm). Samples were injected at 70°C, and the temperature was increased to 220°C at a rate of 50°C/min and then to 320°C at a rate of 8°C/min. The temperature was then kept constant at 320°C for 6 min. Helium was used as the carrier gas. Electron impact MS was performed at 70 eV with a mass range from m/z 50 to 750 and a cycle time of 1 s in the positive ion mode.
The product that resulted from the reduction of GGGP was analyzed using the same procedure that was used for the analysis of the product from DGGGP, except for the following differences in the GC conditions: samples were injected at 50°C, and the temperature was increased to 220°C at a rate of 50°C/min and then to 300°C at a rate of 4°C/min. The GC-MS analysis of the final product derived from GGPP was performed using the pentane-extracted alcoholic derivative resulting from phosphatase treatment, without trimethylsilylation. The pentane layer was evaporated to concentrate the product, and an aliquot was analyzed using the GC-MS system with the equipment described above. The samples were injected into the GC at 50°C, and the temperature was increased to 190°C at a rate of 50°C/min and then to 215°C at a rate of 1°C/min. A mixture of phytol isomers (Sigma-Aldrich, United States) was employed as an authentic sample.
Radio-TLC analyses of the products from GGPP and GGGP.
Radiolabeled GGPP was enzymatically synthesized in a 200-μl reaction mixture containing 0.2 nmol of [14C]IPP (1.85 GBq/mmol), 1 nmol of farnesyl diphosphate, 20 μmol of MES buffer (pH 6.0), 2 μmol of MgCl2, and an excess amount of GGPP synthase prepared as described in a previous paper (10). After incubation at 55°C for 30 min, the GGR reaction was initiated by addition of an excess amount (∼1 nmol) of S. acidocaldarius GGR and 25.6 μmol of sodium dithionite dissolved in N2-bubbled water to a 250-μl (final volume) mixture. The mixture was then incubated at 55°C for 30 min, and the reaction was stopped by adding 200 μl of a cold, saturated NaCl solution. The mixture was extracted with 600 μl of 1-butanol saturated with H2O, and the butanol-extracted compounds were hydrolyzed with potato acid phosphatase (Sigma, United States) using the method of Fujii et al. (7). The resulting alcohols were extracted with n-pentane and analyzed by reversed-phase TLC using precoated plates (Partisil LKC18; Whatman, United Kingdom), which were developed with a mixture of acetone and H2O (9:1). The distribution of radioactivity was detected using a BAS2000 bioimaging analyzer (Fujifilm, Japan). When GGGP was used as the substrate for the GGR reaction, an excess amount of GGGP synthase, which was prepared as previously described (10), and 100 nmol of racemic glycerol-1-phosphate were added to the first reaction mixture. When A. fulgidus GGR was assayed, the second reaction mixture was prepared as follows: 200 μmol of 3-(N-morpholino)propanesulfonic acid buffer (pH 7.5), 460 μmol of sodium dithionite, and 0.2 nmol of the recombinant enzyme, prepared as previously described (16), were added to a 920-μl (final volume) mixture.
Acid hydrolysis of the product derived from GGPP.
Radiolabeled GGPP and the final product derived from GGPP were synthesized enzymatically as described above. The compounds extracted with 1-butanol were evaporated under a flow of N2 gas and then were subjected to acid hydrolysis in 100 μl of concentrated HCl-methanol (1:3, vol/vol) at 37°C for 10 min. After hydrolysis, 100 μl of H2O and 100 μl of chloroform were added. The chloroform layer was recovered and then evaporated for analysis by normal-phase TLC using Partisil K6F silica gel plates (Whatman, United Kingdom), which were developed with 2-propanol-aqueous NH4OH-H2O (6/3/1). Concentrated diphosphate compounds and compounds treated with acid phosphatase using the method of Fujii et al. (7) were analyzed at the same time.
Determination of the substrate preferences of archaeal prenyltransferases.
Recombinant GGGP synthase and hexaprenyl diphosphate (HexPP) synthase from S. solfataricus were expressed and purified as described in previous reports (9, 10). For the reaction of GGGP synthase, the same amount (∼40 pmol) of radiolabeled GGPP or GGPP which was completely reduced by an excess amount of GGR, both of which were synthesized as described above, was added as the prenyl donor substrate to a 200-μl mixture containing 20 μmol of MES buffer (pH 6.0), 2 μmol of MgCl2, an appropriate amount of purified GGGP synthase, and 100 nmol of α-glycerophosphate (Sigma Aldrich, United States) as the acceptor. The mixture was incubated at 55°C for 15 min, after which the reaction was stopped by addition of 200 μl of a cold, saturated NaCl solution. The mixture was extracted with 600 μl of 1-butanol saturated with H2O, and the butanol-extracted compounds were subjected to acid phosphatase treatment. The resulting alcohols were extracted with n-pentane and analyzed by reversed-phase TLC using Partisil LKC18 plates (Whatman, United Kingdom), which were developed with a mixture of acetone and H2O (9:1). The distribution of radioactivity was detected using a BAS2000 bioimaging analyzer (Fujifilm, Japan). For the HexPP synthase reaction, nonlabeled GGPP and GGPP reduced with GGR were synthesized enzymatically by using the same procedure that was used for the synthesis of the radiolabeled compounds, except that 1 nmol of nonlabeled IPP and 0.2 nmol of farnesyl diphosphate were used as substrates. The same amount (<0.2 nmol) of the nonlabeled GGPP or GGPP reduced with GGR was added to a 200-μl mixture containing 20 μmol of MES buffer (pH 6.0), 2 μmol of MgCl2, 0.1% (final concentration) Triton X-100, an appropriate amount of purified HexPP synthase, and 0.4 nmol of [14C]IPP (1.85 GBq/mmol) as the acceptor substrate. The mixture was incubated at 55°C for 15 min, and the reaction products were analyzed using the same methods that were used for the analysis of the products of GGGP synthase, except that the solution used to develop the reversed-phase TLC plates was a mixture of acetone and H2O (19:1).
RESULTS AND DISCUSSION
In the present study, we first examined the catalytic specificity of archaeal GGR, especially the preference of double-bond reduction toward the precursors of membrane lipids, such as GGPP, using the enzyme from A. fulgidus, which we had cloned previously (16). However, A. fulgidus GGR produced numerous intermediates with unreduced double bonds even when an excess amount of enzyme was used. This enzyme characteristic made product analysis complicated, so we isolated the saci_0986 gene from a thermophilic crenarchaeote, S. acidocaldarius. Among the proteins encoded by the S. acidocaldarius genome, the protein encoded by this gene shows the highest homology with the known archaeal GGRs. However, the sequence identity between this protein and A. fulgidus GGR was only 27%, suggesting that the molecules are phylogenetically distant. The protein was recombinantly expressed in E. coli and purified by heat treatment and by polyhistidine affinity column chromatography. The purity of the protein was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown). The UV-visible spectrum of the protein showed a specific peak for flavin coenzymes at approximately 440 nm (data not shown), as observed in the spectrum of recombinant A. fulgidus GGR, suggesting that the GGR homologue from S. acidocaldarius is a flavoprotein, as are the known GGRs (16, 20).
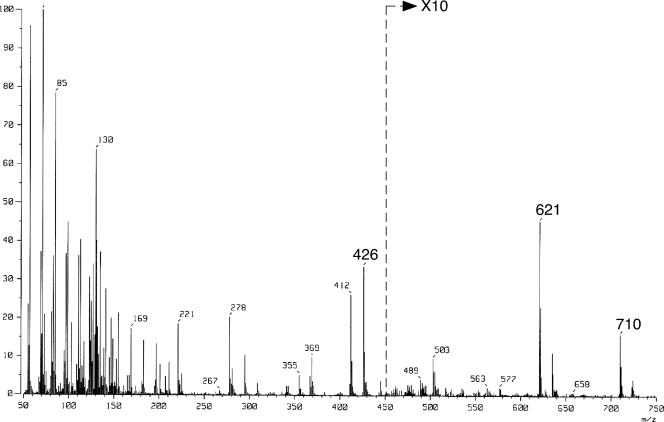
To confirm that the protein actually had GGR activity, the GGR assay was performed using DGGGP synthesized enzymatically as the substrate and sodium dithionite as the reducing agent. The reaction products were extracted with 1-butanol and then treated with acid phosphatase. The resulting alcohols were extracted again with n-pentane and then trimethylsilylated for GC-MS analysis. A compound with the same GC retention time as and an ion spectrum (Fig. 2) similar to that of trimethylsilylated authentic archaeol extracted from Halobacterium salinarum was detected, strongly suggesting that archaetidic acid (2,3-di-O-phytanylglyceryl phosphate) was formed. Thus, the results of the present study demonstrate that the protein encoded by S. acidocaldarius is a GGR. This is the first report describing GGR in the Crenarchaeota. Similar to its homologue from A. fulgidus (16), S. acidocaldarius GGR does not accept NADPH, which can be used as a reducing agent by GGRs from plants and T. acidophilum (12, 20). Because the physiological reducing agents for these enzymes remain unknown, sodium dithionite was used in this study.
FIG. 2.
Mass spectrum of the product from the GGR reaction with DGGGP. DGGGP was reduced with an excess amount of S. acidocaldarius GGR, and the product was treated with phosphatase. The resulting alcohol, trimethylsylilated before the GC-MS analysis, gave a peak with the same GC retention time as the peak of authentic trimethylsylilated archaeol. The ion spectrum of the peak was similar to that of the authentic molecule. The ions at m/z 710, 621, and 426 correspond to [M-CH3]+, [M-CH3OSi(CH3)3]+, and [M-C20H41OH]+, respectively.
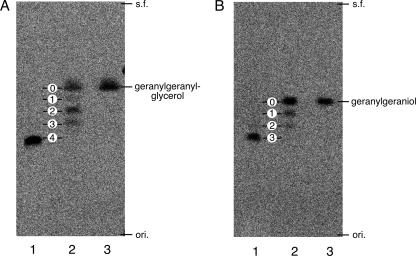
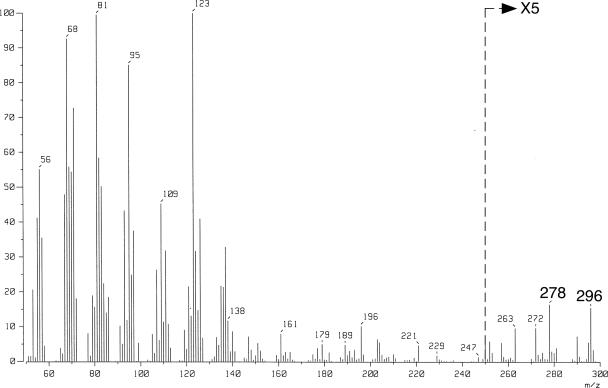
Next, the activity of S. acidocaldarius GGR for the more upstream precursors of membrane lipid biosynthesis (GGGP and GGPP) was examined. The resulting reduced products were analyzed using reversed-phase radio-TLC after hydrolysis of the phosphate or diphosphate group with acid phosphatase. As shown in Fig. 3A and B, the formation of a single product was observed in each reaction that used an excess amount (∼1 nmol) of S. acidocaldarius GGR. It should be noted that even a much smaller amount of S. acidocaldarius GGR (∼0.05 nmol) was enough to obtain the single, final products without producing partially reacted intermediates (data not shown). To determine the number of reduced double bonds in the final products, similar reducing reactions using ∼0.2 nmol of A. fulgidus enzyme were run simultaneously. The reduction catalyzed by the enzyme was partial. In addition to the alcohols from unreacted substrate, many other intermediates were observed, primarily because A. fulgidus GGR readily produces intermediates under the conditions used (Fig. 3A and B). The spots on the TLC plates that were located at nearly regular intervals below the substrate-derived spot were considered to have arisen from the partially reduced intermediates possessing different numbers of double bonds. Thus, the number of double bonds in the final products of the S. acidocaldarius GGR reactions were estimated using the results of the TLC analysis. When GGGP was used as the substrate, no double bonds appeared to be intact, indicating that there was formation of 3-O-phytanylglyceryl phosphate (Fig. 3A). To confirm that there was complete reduction of GGGP, the product produced with the same reaction steps from nonlabeled substrates was analyzed by GC-MS by using the same pretreatments used for the analysis of the DGGGP-derived substrate (i.e., phosphatase treatment and trimethylsilylation). The mass spectrum of the product gave an ion peak at m/z 501, which was attributed to [M-CH3]+ of ditrimethylsilylated 3-O-phytanylglycerol (data not shown). In contrast, the result of the radio-TLC analysis clearly showed that one double bond remained intact in the final product when GGPP was used as the substrate (Fig. 3B). The final product derived from nonlabeled GGPP was hydrolyzed with acid phosphatase and then directly analyzed by GC-MS. The GC retention time was identical to that of a component present in the mixture of phytol isomers (Sigma, United States), which gave a few GC peaks. In addition, the ion spectrum of the product was similar to those observed for the phytol isomers, clearly indicating that the product had one double bond (Fig. 4).
FIG. 3.
Radio-TLC analyses of the products from the GGR reactions with GGGP (A) and GGPP (B). After hydrolysis with phosphatase, the compounds were analyzed by reversed-phase TLC developed with acetone/H2O (9:1). Lane 1, phosphatase-treated product from the reaction with S. acidocaldarius GGR; lane 2, phosphatase-treated product from the reaction with A. fulgidus GGR; lane 3, authentic geranylgeranylglycerol (A) and geranylgeraniol (B). s.f., solvent front; ori., origin.
FIG. 4.
Mass spectrum of the product from the GGR reaction with GGPP. GGPP was reduced with an excess amount of S. acidocaldarius GGR, and the product was treated with phosphatase. The retention time of the GC peak of the resulting alcohol corresponded with that of one of the peaks of authentic phytol isomers. The ion spectrum of the peak was similar to those of the authentic compounds. The ions at m/z 296 and 278 correspond to M+ and [M-H2O]+, respectively.
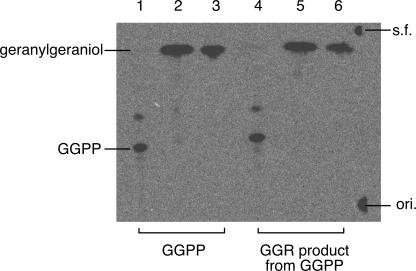
A problem to be solved was the position of the double bond that remained in the final product derived from GGPP. Identification of the alcohol derived from the product as phytol only from the agreement of the GC retention times was not justifiable, because the mixture of phytol isomers was used as the authentic sample. Unfortunately, it was difficult to assign the position from the decomposition fragments in the mass spectrum because of the high background level. If only the double bond at position 2 of GGPP remains intact, the compound is considered to be (7R,11R)-phytyl diphosphate because the reduced prenyl chains of archaeal membrane lipids, like those of chlorophyll/bacteriochlorophyll, have all R stereochemistry. Thus, we tried to determine whether the allylic double bond was present in the final product by investigating the stability of the product in acid. When the double bond is at position 2, the diphosphate group of the final product is a good leaving group because its detachment results in the formation of the relatively stable allylic carbocation. Indeed, prenyl diphosphates with the double bond at position 2 (e.g., dimethylallyl diphosphate and GGPP) are easily hydrolyzed by acid treatment. In contrast, compounds that do not possess the allylic double bond are resistant to acid hydrolysis. In fact, IPP, which possesses a double bond at position 3 but not at position 2, was not hydrolyzed at all by the acid treatment used in the present study (data not shown). Thus, we performed either acid treatment or phosphatase treatment on 14C-labeled GGPP as a positive control and on the final product derived from labeled GGPP. The resulting products were extracted with n-pentane and analyzed, along with the untreated diphosphates, by normal-phase radio-TLC (Fig. 5). The results of this analysis clearly showed that there was alcohol formation as a result of acid treatment of the final product, as observed in the reaction with GGPP. This result indicates that the double bond is at position 2 and that the final product of archaeal GGR derived from GGPP is phytyl diphosphate.
FIG. 5.
Hydrolysis of the GGR reaction product from GGPP. Radiolabeled GGPP and GGPP reduced with S. acidocaldarius GGR were hydrolyzed by acid or phosphatase treatment and then analyzed by normal-phase TLC developed with 2-propanol-aqueous NH4OH-H2O (6/3/1). Lane 1, untreated GGPP; lane 2, acid-treated GGPP; lane 3, phosphatase-treated GGPP; lane 4, untreated GGR product from GGPP; lane 5, acid-treated GGR product from GGPP; lane 6, phosphatase-treated GGR product from GGPP. s.f., solvent front; ori., origin.
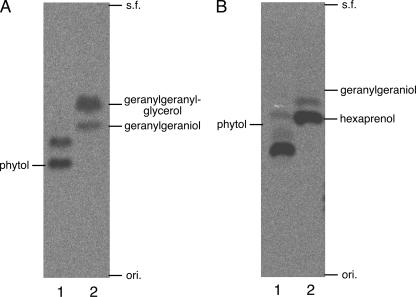
Although phytyl diphosphate is also the final product of A. thaliana GGR when GGPP is the substrate, a phytyl group is also the final enzyme reaction product when a geranylgeranyl group is attached to chlorophyllide (12). A unique and intriguing property of archaeal GGR is that the number of reduced double bonds is different in different substrates (i.e., GGPP and GGGP/DGGGP). For S. acidocaldarius GGR, DGGGP is the most preferred substrate among the three compounds. The relative activity of the enzyme with GGPP was limited to approximately 10% of that with GGGP, whereas the activity with GGGP was comparable to that with DGGGP (data not shown). However, even though it might be slight, the reduction of GGPP catalyzed by GGR can occur in S. acidocaldarius cells. Because GGPP has been proposed to be a branch point of biosynthetic pathways toward isoprenoid compounds, such as membrane lipids, respiratory quinones, and sugar carrier lipids, in Sulfolobus sp. (8, 11), it is conceivable that the archaea have a pool of GGPP in their cells. Reduction of all double bonds in GGPP results in phytanyl diphosphate, which should be a dead-end product that cannot be used by prenyltransferases and might inhibit cell growth because of intracellular accumulation. Zhang and Poulter reported that cell extracts from Halobacterium halobium and Methanobacterium thermoautotrophicum have GGGP synthase activity, which can accept phytyl diphosphate but not phytanyl diphosphate as the prenyl donor substrate (24, 25). The reactivity with phytyl diphosphate was ∼20% of that with GGPP. In the present study, we examined the abilities of two prenyltransferases from Sulfolobus sp. to accept phytyl diphosphate as a substrate. GGGP synthase from S. solfataricus accepted phytyl diphosphate like its homologues from H. halobium and M. thermoautotrophicum, and its preference for the substrate was also weaker than that for GGPP (Fig. 6A). On the other hand, the preference of S. solfataricus HexPP synthase for phytyl diphosphate was comparable to that for GGPP, which is the most preferred donor substrate for the prenyltransferase (Fig. 6B). HexPP synthase is involved in the biosynthesis of Sulfolobus-specific caldariellaquinone, which has a completely reduced C30 prenyl chain derived from HexPP (8). This means that the use of phytyl diphosphate for the biosynthesis of caldariellaquinone as an archaeal membrane lipid can finally yield the natural biological compound. Thus, isoprenoid biosynthesis via, at least in part, phytyl diphosphate is possible, and the fact that archaeal GGR reduces all double bonds when geranylgeranyl groups are attached to the glycerol moiety but leaves the allylic double bond of geranylgeranyl diphosphate that is required for prenyl transfer reactions seems biologically advantageous.
FIG. 6.
Determination of the substrate preferences of S. solfataricus prenyltransferases. (A) Radiolabeled phytyl diphosphate (lane 1) or GGPP (lane 2) was used as a substrate for a GGGP synthase reaction with racemic glyceryl-1-phosphate. After the reaction, radiolabeled compounds were extracted with butanol and then treated with phosphatase. The resulting alcohols were analyzed by reversed-phase TLC developed with acetone/H2O (9:1). s.f., solvent front; ori., origin. (B) Nonlabeled phytyl diphosphate (lane 1) or GGPP (lane 2) was used as a substrate for a HexPP synthase reaction with [14C]IPP. After the reaction, radiolabeled compounds were extracted with butanol and then treated with phosphatase. The resulting alcohols were analyzed by reversed-phase TLC developed with acetone/H2O (19:1).
Acknowledgments
This work was supported in part by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant 18780053 to H.H.) and from the Asahi Glass Foundation (to H.H.).
We are grateful to K. Ogura and T. Koyama, Tohoku University, for providing farnesyl diphosphate. We thank C. Ohto, Toyota Motor Co., for donating the nonlabeled IPP. We are grateful to S. Kitamura, Nagoya University, for his technical assistance with the GC-MS analyses.
Footnotes
Published ahead of print on 28 March 2008.
REFERENCES
- 1.Addlesee, H. A., L. C. Gibson, P. E. Jensen, and C. N. Hunter. 1996. Cloning, sequencing and functional assignment of the chlorophyll biosynthesis gene, chlP, of Synechocystis sp. PCC 6803. FEBS Lett. 389126-130. [DOI] [PubMed] [Google Scholar]
- 2.Addlesee, H. A., and C. N. Hunter. 1999. Physical mapping and functional assignment of the geranylgeranyl-bacteriochlorophyll reductase gene, bchP, of Rhodobacter sphaeroides. J. Bacteriol. 1817248-7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Addlesee, H. A., and C. N. Hunter. 2002. Rhodospirillum rubrum possesses a variant of the bchP gene, encoding geranylgeranyl-bacteriopheophytin reductase. J. Bacteriol. 1841578-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, A., and C. D. Poulter. 1994. Isolation and characterization of idsA: the gene for the short chain isoprenyl diphosphate synthase from Methanobacterium thermoautotrophicum. Arch. Biochem. Biophys. 314399-404. [DOI] [PubMed] [Google Scholar]
- 5.Chen, A., D. Zhang, and C. D. Poulter. 1993. (S)-Geranylgeranylglyceryl phosphate synthase. Purification and characterization of the first pathway-specific enzyme in archaebacterial membrane lipid biosynthesis. J. Biol. Chem. 26821701-21705. [PubMed] [Google Scholar]
- 6.Dannenmuller, O., K. Arakawa, T. Eguchi, K. Kakinuma, S. Blanc, A. M. Albrecht, M. Schmutz, Y. Nakatani, and G. Ourisson. 2000. Membrane properties of archaeal macrocyclic diether phospholipids. Chemistry 6645-654. [DOI] [PubMed] [Google Scholar]
- 7.Fujii, H., T. Koyama, and K. Ogura. 1982. Efficient enzymatic hydrolysis of polyprenyl pyrophosphates. Biochim. Biophys. Acta 712716-718. [PubMed] [Google Scholar]
- 8.Hemmi, H., S. Ikejiri, S. Yamashita, and T. Nishino. 2002. Novel medium-chain prenyl diphosphate synthase from the thermoacidophilic archaeon Sulfolobus solfataricus. J. Bacteriol. 184615-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemmi, H., M. Noike, T. Nakayama, and T. Nishino. 2003. An alternative mechanism of product chain-length determination in type III geranylgeranyl diphosphate synthase. Eur. J. Biochem. 2702186-2194. [DOI] [PubMed] [Google Scholar]
- 10.Hemmi, H., K. Shibuya, Y. Takahashi, T. Nakayama, and T. Nishino. 2004. (S)-2,3-Di-O-geranylgeranylglyceryl phosphate synthase from the thermoacidophilic archaeon Sulfolobus solfataricus. Molecular cloning and characterization of a membrane-intrinsic prenyltransferase involved in the biosynthesis of archaeal ether-linked membrane lipids. J. Biol. Chem. 27950197-50203. [DOI] [PubMed] [Google Scholar]
- 11.Hemmi, H., S. Yamashita, T. Shimoyama, T. Nakayama, and T. Nishino. 2001. Cloning, expression, and characterization of cis-polyprenyl diphosphate synthase from the thermoacidophilic archaeon Sulfolobus acidocaldarius. J. Bacteriol. 183401-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller, Y., F. Bouvier, A. d'Harlingue, and B. Camara. 1998. Metabolic compartmentation of plastid prenyllipid biosynthesis—evidence for the involvement of a multifunctional geranylgeranyl reductase. Eur. J. Biochem. 251413-417. [DOI] [PubMed] [Google Scholar]
- 13.Koga, Y., and H. Morii. 2007. Biosynthesis of ether-type polar lipids in archaea and evolutionary considerations. Microbiol. Mol. Biol. Rev. 7197-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyama, T., and K. Ogura. 1999. Isopentenyl diphosphate isomerase and prenyltransferase, p. 69-96. In D. Cane (ed.), Comprehensive natural product chemistry, vol. 2. Pergamon, Oxford, United Kingdom. [Google Scholar]
- 15.Liang, P. H., T. P. Ko, and A. H. Wang. 2002. Structure, mechanism and function of prenyltransferases. Eur. J. Biochem. 2693339-3354. [DOI] [PubMed] [Google Scholar]
- 16.Murakami, M., K. Shibuya, T. Nakayama, T. Nishino, T. Yoshimura, and H. Hemmi. 2007. Geranylgeranyl reductase involved in the biosynthesis of archaeal membrane lipids in the hyperthermophilic archaeon Archaeoglobus fulgidus. FEBS J. 274805-814. [DOI] [PubMed] [Google Scholar]
- 17.Nemoto, N., T. Oshima, and A. Yamagishi. 2003. Purification and characterization of geranylgeranylglyceryl phosphate synthase from a thermoacidophilic archaeon, Thermoplasma acidophilum. J. Biochem. (Tokyo) 133651-657. [DOI] [PubMed] [Google Scholar]
- 18.Nishihara, M., and Y. Koga. 1997. Purification and properties of sn-glycerol-1-phosphate dehydrogenase from Methanobacterium thermoautotrophicum: characterization of the biosynthetic enzyme for the enantiomeric glycerophosphate backbone of ether polar lipids of Archaea. J. Biochem. (Tokyo) 122572-576. [DOI] [PubMed] [Google Scholar]
- 19.Nishihara, M., and Y. Koga. 1995. sn-Glycerol-1-phosphate dehydrogenase in Methanobacterium thermoautotrophicum: key enzyme in biosynthesis of the enantiomeric glycerophosphate backbone of ether phospholipids of archaebacteria. J. Biochem. (Tokyo) 117933-935. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura, Y., and T. Eguchi. 2006. Biosynthesis of archaeal membrane lipids: digeranylgeranylglycerophospholipid reductase of the thermoacidophilic archaeon Thermoplasma acidophilum. J. Biochem. (Tokyo) 1391073-1081. [DOI] [PubMed] [Google Scholar]
- 21.Ohnuma, S.-I., M. Suzuki, and T. Nishino. 1994. Archaebacterial ether-linked lipid biosynthetic gene. Expression cloning, sequencing, and characterization of geranylgeranyl-diphosphate synthase. J. Biol. Chem. 26914792-14797. [PubMed] [Google Scholar]
- 22.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 23.Soderberg, T., A. Chen, and C. D. Poulter. 2001. Geranylgeranylglyceryl phosphate synthase. Characterization of the recombinant enzyme from Methanobacterium thermoautotrophicum. Biochemistry 4014847-14854. [DOI] [PubMed] [Google Scholar]
- 24.Zhang, D., and C. D. Poulter. 1993. Biosynthesis of archaebacterial lipids in Halobacterium halobium and Methanobacterium thermoautotrophicum. J. Org. Chem. 583919-3922. [Google Scholar]
- 25.Zhang, D., and C. D. Poulter. 1993. Biosynthesis of archaebacterial ether lipids. Formation of ether linkages by prenyltransferases. J. Am. Chem. Soc. 1151270-1277. [Google Scholar]