Abstract
Mycobacterium tuberculosis maintains a large genetic capacity necessary for growth in different environments during infection and survival upon aerosol transmission to new hosts. Screening for bacterial RNAs produced in response to host interactions produced candidate lists where we noted proXVWZ, annotated as encoding a putative glycine betaine or proline transporter. As high surface-to-volume ratios make bacterial cells particularly vulnerable to changes in water availability, we investigated the contributions of this transporter to the ability of M. tuberculosis to colonize macrophages. An H37Rv proXVWZ mutant was impaired for initial survival and intracellular growth and exhibited reduced growth at elevated medium osmolarity. This defect could be complemented by restoring proXVWZ and was attributable to a failure to accumulate the compatible solute glycine betaine. We then demonstrated that ProXVWZ allows M. tuberculosis to obtain betaine from host macrophages and thereby contributes to early steps in colonizing this niche.
A genome predicted to encode more than 4,200 bacterial factors allows Mycobacterium tuberculosis to survive and grow within a variety of different environments encountered during the progressive course of human infection. Recognition of this capacity has led to descriptions of M. tuberculosis as a “master adaptor” (5) among intracellular bacterial pathogens. Among the different environments encountered during infection are those at the epithelial surface of the lung, within phagosomes of alveolar macrophages and other phagocytes, and likely in a necrotic milieu at the centers of solid granulomas and in aerated liquid in pulmonary cavities (16). Tubercle bacilli must then survive at the nuclei of evaporated airborne droplets and can remain viable in sputum outside the body for extended periods (2). This versatility in adapting to multiple niches and surviving transfer to new hosts has clearly allowed M. tuberculosis to be among the most successful of human pathogens. Infection remains both ubiquitous in many populations and a leading cause of preventable death from communicable disease (13, 18).
A fundamental environmental challenge faced by all types of cells, and particularly unicellular organisms, is maintenance of a suitable osmotic balance between the cytoplasm and the external environment. High surface-to-volume ratios make bacterial cells particularly vulnerable to osmotic stress. Bacteria compensate for changes in environmental osmolarity by accumulation or efflux of solutes or by movement of water across the cytoplasmic membrane. In response to decreased water availability, bacteria typically accumulate compatible solutes or osmotically active particles (osmolytes) that are relatively nondisruptive of protein folding and hydration (1). Common compatible bacterial osmolytes among nonhalophilic eubacteria include potassium cations, the amino acid proline, and betaines, particularly glycine betaine or 2-trimethylammonioacetate (8). Transport is the energetically preferred method of osmolyte accumulation, but glutamic acid and trehalose are often also synthesized when necessary.
Osmoregulation has previously been shown to be important for bacterial pathogens in the context of infection. In Staphylococcus aureus PutP proline transport activity contributes to colonization of host tissues (4, 29, 30). Although S. aureus PutP mutants are able to acquire proline, uptake and therefore use as an osmolyte (15) are limited. Other studies have linked osmotic stress and virulence gene expression in Pseudomonas aeruginosa (11, 26) and compatible solute transport and colonization in Listeria monocytogenes (32, 36) and identified a role for Escherichia coli ProP in colonization of the mouse bladder (9). Osmoregulation by compatible solute accumulation has not previously been described for in bacterial pathogens that proliferate by intracellular growth. The different in vivo environments encountered by M. tuberculosis during infection likely vary widely in available water (i.e., water activity), and yet none of the relevant bacterial osmoregulatory mechanisms have been described.
A genomic screening method developed to identify M. tuberculosis mRNAs that are expressed upon interactions with host cells (SCOTS) previously identified genes with important roles in bacterial pathogenesis (14). Subsequent applications of this approach yielded lists of numerous potentially differentially expressed genes (A. Bukka and J. E. Graham, not shown). Among plasmid cDNA clones obtained in several different experiments similar to those previously reported, we noted regions within the proXVWZ operon. proXVWZ is predicted to encode the subunits of a mycobacterial osmolyte ABC transporter. Given the known role of glycine betaine (or betaine) in bacterial osmoregulation, we initiated studies of the role of ProXVWZ in compatible solute accumulation and growth in human macrophages. Here, we show that M. tuberculosis proXVWZ RNA levels are elevated in response to phagocytosis and that the transcript encodes a glycine betaine transporter capable of acquiring betaine from host cells. This activity contributes to the ability of M. tuberculosis to grow at elevated osmolarity and in the human macrophage phagosome.
MATERIALS AND METHODS
Mycobacterial culture.
M. tuberculosis strain H37Rv, obtained from the ATCC, was grown in Middlebrook 7H9 medium containing 0.05% Tween 80 supplemented with 10% oleic acid, albumin, dextrose, and catalase (OADC) (Becton Dickinson) at 37°C with shaking. When appropriate, hygromycin B (100 mg/liter) and apramycin (50 mg/liter) were added to mycobacterial cultures. For osmotic shock experiments, 20 ml of M. tuberculosis H37Rv or a deletion mutant was used to inoculate 50-ml aliquots of M7H9 or M7H9 supplemented with 0.25 M NaCl in 125-ml Erlenmeyer flasks to an initial optical density at nm (OD600) of 0.015. Cultures were incubated at 37°C with shaking at 150 rpm, with A600 determined for 7 days. For standardized inocula in cell culture models, frozen aliquots of mid-logarithmic-phase M. tuberculosis in Cryovials containing 0.1 mm zirconia silica beads (Biospec) were thawed and incubated at 37°C for 2 days to resuscitate frozen M. tuberculosis. Vials were then vortexed and then allowed to settle for 1 h prior to withdrawing the upper aqueous phase containing uniform single-cell suspensions.
Construction of a proXVWZ operon knockout mutant.
The entire M. tuberculosis H37Rv proXVWZ operon was deleted by homologous recombination using phAE87 mycobacteriophage supplied by Stoyan Bardarov, with minor modifications to the previously described procedure (3). Briefly, 700-bp regions precisely flanking proXVWZ open reading frames (ORFs) were ligated into polylinker regions on each side of the hygromycin resistance marker in plasmid pYUB854. The resulting plasmid was then linearized with PacI and ligated to a PacI-linearized phAE87 vector prepared as closed circular plasmid DNA from E. coli HB101. Lambda phage packaging (Gigapack XL; Stratagene) was then used to select the desired large product, which was transduced into E. coli HB101. Mycobacterium smegmatis mc2155 was then electroporated with plasmid DNA from pooled colonies and plated at 30°C to generate plaques. Phage temperature sensitivity at 37°C was verified prior to generating high-titer plate lysates at 30°C. M. tuberculosis H37Rv was then transduced at a multiplicity of infection (MOI) of 10:1, and putative mutants were selected on agar containing hygromycin. Deletions were confirmed by PCR of novel chromosomal junctions. A complemented strain containing the operon with and without the native promoter was ligated either downstream of or in place of the constitutive hsp60 promoter in plasmid pLou3apr, a vector containing the L5 mycobacteriophage attP, derived from pLUC10 (7), and the apramycin resistance marker from pMP399 (6).
Accumulation of compatible solutes in response to osmotic stress.
M. tuberculosis H37Rv and H37RvΔproXVWZ were grown with shaking at 37°C to an OD of 0.3 A600 units in 50 ml M7H9 medium supplemented with 1 mM betaine, l-proline, or choline. Cultures were split into two 20-ml volumes, and the medium osmolarity of one was raised by the addition of solid NaCl to 0.25 M. Triplicate 0.5-ml aliquots were taken at various time points following simultaneous addition of radio label (1 μCi of either [methyl-14C]betaine from American Radiochemicals or [U14C]l-proline or [methyl-14C]choline chloride from MP Biomedicals) and NaCl and rapidly filtered through a 0.45 μM hemagglutination filter (Millipore) before three washes with 2 ml of 0.5 M NaCl (22). Counts per minute on dried filters were determined with a liquid scintillation counter (Wallac 1410 scintillation counter; Pharmacia).
Infection of primary human macrophages and THP-1 macrophages.
Human monocyte-derived macrophages (MDMs) were obtained from two healthy donors and cultured specifically as previously described by Schlesinger and Horwitz (14, 27). Briefly, mononuclear cells were obtained by a Ficoll gradient and incubated in Teflon wells for 5 days prior to allowing monocytes to adhere to plastic for 3 days in RPMI medium. Adherent cells were harvested and plated at a density of 2.5 × 105 cells in standard 48-well plates and incubated for a further 3 days prior to infection. RPMI 1640 (Gibco) medium was supplemented with l-glutamine, nonessential amino acids, 10 mM HEPES, and unless otherwise indicated 10% (vol/vol) heat-inactivated fetal bovine serum (FBS) at 37°C and 5% CO2. The human leukemic macrophage cell line THP-1 was used in an M. tuberculosis infection model described by Theus et al. (33). THP-1 cells were diluted to 2.5 × 105 cells/0.5 ml and incubated for 3 days in the presence of 100 nM phorbol myristate acetate and human recombinant gamma interferon (150 U) to generate mature macrophage monolayers in standard 48-well plates (33). Bacteria were then mixed with native FBS (final concentration, 10% [vol/vol]) and incubated for a further 30 min. Opsonized M. tuberculosis was then diluted in RPMI supplemented with 2% (vol/vol) FBS as appropriate for an MOI of either 1:1 or 1:50 (bacteria to cells) and transferred to washed macrophage monolayers. Monolayers were washed after overnight incubation with Hanks buffer, and the medium was replaced with fresh RPMI supplemented with 2% (vol/vol) FBS. At various time points, monolayers in three wells for each strain studied were lysed with 0.5 ml 1% (vol/vol) Triton X-100 and diluted in Hanks buffer. Serial dilutions were plated on M7H9 agar plates supplemented with 10% (vol/vol) OADC to determine numbers of M. tuberculosis CFU.
Isolation of RNA from bacteria, infected cells, and tissues.
Total RNA from bacterial mid-log-phase broth cultures, infected primary human MDMs, or frozen mouse lung tissue specimens was isolated using an RNeasy mini kit, following the manufacturer's instructions with minor modifications (Qiagen). Quick-frozen mouse lung tissues were obtained as approved by the Upstate Medical University IACUC from experimentally infected C57BL6 mice 7 days following intranasal infection using a previously described active infection model (31). Samples in Qiagen lysis buffer were first heated at 70°C for 1 min to assist in lysing mycobacteria and then transferred to tubes containing 0.1-mm zirconia silica beads (Biospec) and processed in a Fast-A homogenizer (Bio101) prior to being applied to RNeasy columns. Following column washes and elution, RNA was treated with DNase I (Ambion) to remove contaminating chromosomal DNA, verified by gel electrophoresis, and stored at −80°C in aliquots as ethanol precipitates.
cDNA synthesis and qPCR.
First-strand cDNA was prepared using 5 μg of total RNA, random nonomers (NEB), and Superscript III, following the manufacturer's instructions (Invitrogen). Primers specific for the sigA (5′-GACGAAGACCACGAAGACC-3′ and 5′-CATCCCAGACGAAATCACC-3′) and proV (5′-ACGATGTCAGCAAGGTGT-3′ and 5′-AGTGATGGTGCCCGAGGT-3′) genes of M. tuberculosis were selected using Primer3 software and rigorously tested for optimal PCR efficiency with dilutions of genomic DNA template. M. tuberculosis H37Rv is a natural mutant lacking normal regulation of sigA gene expression, allowing sigA RNA to be used as an internal control for quantitative PCR (qPCR) experiments, as previously described (21, 37). Fluorescence-monitored PCR was carried out with an Opticon detection system (MJ Research), using Sybr green as the indicator. Primers (250 nM final concentration) were added to 2-μl aliquots of diluted cDNA, and water was added to make a total volume of 10 μl. Ten microliters of 2× master mix (Dynamo Sybr green qPCR kit; Finnzymes) was then added, and the plates were transferred to the Opticon system. Each gene was measured two times in triplicate for all cDNAs. Following PCR, the cycle threshold was calculated for each sample by subtracting background fluorescence (10 standard deviations over the mean), using Opticon Monitor software (MJ Research). The changes in steady-state proV mRNA levels were calculated and statistically analyzed for significance with REST-XL software, using a P threshold of <0.05 (24).
Betaine accumulation during intracellular growth.
THP-1 monolayers in standard six-well plates (1.2 × 106 cells/well) were prepared as described above and then preloaded with 0.5 μCi of [methyl-14C]betaine for 2 hours. Loaded monolayers were extensively washed with Hanks buffer before addition of fresh unlabeled RPMI medium supplemented with 2% (vol/vol) FBS and incubated overnight. Washed, betaine-loaded monolayers were infected with H37Rv, H37RvΔproXVWZ, and complemented H37RvΔproXVWZ as described above, except the MOI used was 5:1 and bacterial adherence was allowed to proceed for 1 h at 4°C. Nonadherent bacteria were removed by washing monolayers three times with Hanks buffer. Fresh RPMI medium was added, and then infected monolayers were transferred to 37°C and 5% CO2. At 24 h postinfection, monolayers were again washed extensively with Hanks buffer and lysed with 1% (vol/vol) Triton X-100 supplemented with 0.5 M NaCl. Following lysis, cell debris (THP-1 cell fragments and intact intracellular bacteria) was pelleted by centrifugation. The pellets were washed three times with 0.5 ml of 0.5 M NaCl, which resulted in the loss of radioactivity associated with THP-1 cell debris but not bacteria. Radioactivity remaining in the pellets was determined using liquid scintillation counting.
RESULTS AND DISCUSSION
M. tuberculosis proXVWZ mRNA levels increase upon phagocytosis.
proXVWZ is annotated in the M. tuberculosis H37Rv genome as a putative osmolyte transporter. cDNAs for these ORFs were among many identified by SCOTS as expressed during growth in different intracellular environments. We initiated studies of the contributions of this transporter to growth in human macrophages by using fluorescence-monitored qPCR to compare mRNA expression levels in intracellular bacteria to those in standardized mid-logarithmic-phase cultures.
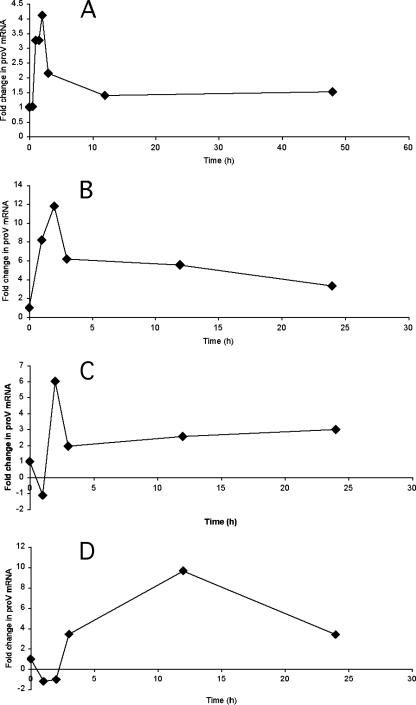
Using a previously described THP-1 macrophage infection model (33), we observed a transient increase in bacterial proV mRNA levels (fourfold) at 2 h postphagocytosis (Fig. 1A) relative to those in bacteria at mid-logarithmic phase in laboratory cultures. proV mRNA levels showed greater increases on phagocytosis by cultured human primary macrophages (Fig. 1B to D). Independent experiments with different donor macrophages showed variable kinetics, with increases up to 12-fold at 2 h postinfection and levels remaining elevated until termination of the experiments. Transcript levels were also elevated in bacteria growing in the lungs of mice in a previously described active-infection model (31), as shown in Table 1. Increased expression of proXVWZ upon interactions with host cells suggested that the encoded transporter might contribute to bacterial adaptation to the environment within phagocytic cells. However, proXVWZ mRNA levels did not rise when bacteria in the laboratory medium were subjected to increased osmolarity (data not shown). These data suggest that proXVWZ is transcribed in response to additional signals encountered in phagosomes that are not present in typical laboratory cultures subjected to mild osmotic shock. Potential signals include, for example, reduced oxygen availability, increased reactive oxygen species, and alternate or limiting nutrients (28). Recent data (25) indicate that M. tuberculosis proXVWZ transcription is regulated by alternate sigma factor SigF. proXVWZ expression is also likely mediated by additional uncharacterized transcriptional and posttranscriptional mechanisms (see below).
FIG. 1.
proV mRNA levels in intracellular M. tuberculosis. THP-1 cells (A) or human primary macrophages (B to D) containing M. tuberculosis H37Rv were lysed at various time points postinfection to determine steady-state transcript levels relative to those in bacteria at mid-logarithmic phase in broth culture. Levels were normalized relative to a sigA mRNA internal standard as previously described (21, 37) and reported as determined by the method of reference 24, as described in the text (P < 0.05).
TABLE 1.
proV mRNA levels in intracellular bacteriaa
| Infection model | Fold increase in proV mRNA level |
|---|---|
| Human MDM no. 1 | 4.46 |
| Human MDM no. 2 | 6.08 |
| Mouse lung (7 day) no. 1 | 7.54 |
| Mouse lung (7 day) no. 2 | 2.15 |
qPCR was used to compare transcript levels in M. tuberculosis bacteria growing in primary human macrophages (48 h postinfection) or mouse lung tissues relative to levels in mid-logarithmic-phase cultures. Increased steady-state transcript levels are given relative to H37Rv sigA mRNA levels calculated by the method of reference 24, as described in Materials and Methods (P < 0.05).
M. tuberculosis transports glycine-betaine in response to osmotic stress.
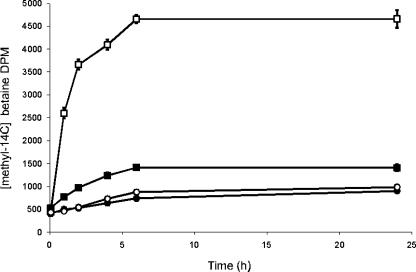
We next examined the cytoplasmic accumulation of the putative ProXVWZ substrates glycine betaine, proline, and choline following a modest osmotic shock in broth cultures. M. tuberculosis accumulated radiolabeled betaine rapidly in response to 0.25 M added NaCl (Fig. 2). In contrast to other gram-positive and -negative species, M. tuberculosis did not accumulate proline- or choline-derived betaine under these experimental conditions (not shown). Proline and choline are therefore unlikely to function as compatible solutes, potentially reflecting a lack of availability in the macrophage phagosome. As proXVWZ mRNA levels did not increase under these conditions, it appears that basal expression levels were sufficient to allow osmoregulation by activation or increasing the activity of the ProXVWZ transporter. Posttranscriptional regulation facilitates rapid accumulation of betaine in other bacteria (12, 20, 35).
FIG. 2.
Accumulation of betaine in response to osmotic stress. Closed squares indicate [methyl-14C]betaine accumulation in H37Rv, open squares indicate accumulation by H37Rv in medium with 0.25 M NaCl, closed circles indicate accumulation by H37RvΔproXVWZ, and open circles indicate accumulation by H37RvΔproXVWZ in medium with 0.25 M NaCl. Data points are the means of three individual samples, and error bars indicate standard deviations.
A proXVWZ mutant fails to accumulate betaine and is sensitive to elevated osmolarity.
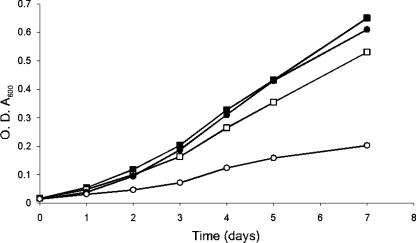
To examine the role of ProXVWZ in adaptation to reduced water availability, the proXVWZ ORFs in strain H37Rv were precisely deleted by allelic exchange using the temperature-sensitive pHAE87 mycobacteriophage as previously described by Bardarov et al. (3). Growth of the mutant was slowed by osmotic stress to a greater extent than that of the isogenic parent (Fig. 3). This difference increased at higher medium osmolarities (data not shown), and growth of the mutant was not impaired at conventional medium osmolarity.
FIG. 3.
Reduced osmotolerance in an M. tuberculosis H37Rv ΔproXVWZ mutant. Bacteria were inoculated into M7H9 medium either with or without supplemental 0.25 M NaCl. Closed squares indicate OD600 values for H37Rv in M7H9, open squares for H37Rv in M7H9 plus 0.25 M NaCl, closed circles for H37RvΔproXVWZ in M7H9, and open circles for H37RvΔproXVWZ in M7H9 plus 0.25 M NaCl. Results for a representative experiment are shown.
We next characterized the ability of the mutant to accumulate betaine in response to osmotic challenge. Following a 0.25 M NaCl shock, cytoplasmic betaine pools in the parent strain rose to an estimated compensatory intracellular concentration of 519 mM, based on a cell water content estimate of 2 × 10−15 liters per bacterium, as described by Tian et al. (34). The mutant strain lacked this capacity (Fig. 2), and the inability to raise intracellular betaine levels reduced growth rate at elevated osmolarity (Fig. 3). ProXVWZ therefore transports glycine betaine, and this confers an osmoprotective effect on the growth of M. tuberculosis.
Loss of proXVWZ delays growth in human macrophages.
Glycine betaine is a ubiquitous cellular compatible solute and functions as a principal osmolyte in many plant, animal, and bacterial cells (19, 38). The M. tuberculosis ProXVWZ activity characterized was next evaluated in terms of its contribution in adaptation to the central environment encountered by mycobacteria during infection, that within the human macrophage phagosome. Initial experiments with the proXVWZ mutant indicated reduced intracellular growth in primary human macrophages from two donors, resulting in only two divisions in a previously described 4-day infection model (14). In this model, a 1:1 MOI allows for three doublings of the H37Rv parent prior to loss of monolayers by day 5 (14, 17). Complementation of proXVWZ reversed this defect.
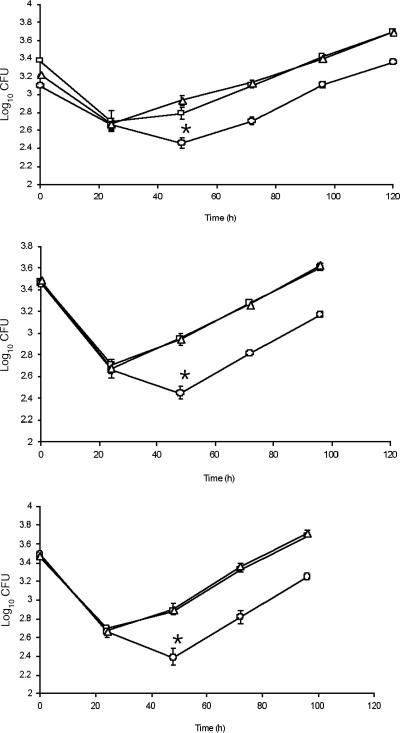
As this was a rather modest phenotype, we further confirmed it in the THP-1 infection model described by Theus et al. (33) and then examined intracellular growth more closely, using a lower (1:50) MOI and this model. The proXVWZ mutant exhibited significantly reduced intracellular survival in the first 48 h following phagocytosis (0.5-log reductions in CFU at 48 h [P < 0.005] for all experiments), as shown in Fig. 4. During this initial lag, a single round of cell division occurred with the wild type but not the mutant. At 48 h postinfection, the mutant strain was able to begin dividing at a normal rate and then continued as seen for the wild-type and complemented strains (Fig. 4). This partial impairment in initial survival and division suggests that ProXVWZ contributes to the early adaptation to the phagosomal microenvironment. Other, as-yet-uncharacterized osmoregulatory mechanisms then appear to provide necessary osmotic balancing as bacteria in the phagosome begin to grow.
FIG. 4.
Intracellular growth of M. tuberculosis ΔproXVWZ and complemented strains. THP-1 monolayers were infected with H37Rv, H37RvΔproXVWZ, or the complemented mutant at a final MOI of 1:50 as described in the text. Macrophage monolayers in wells were lysed every 24 h for 5 days, and lysates were plated for CFU determination. Squares are for H37Rv, circles for H37RvΔproXVWZ, and triangles for the complemented strain. Time points indicate the mean numbers of CFU for three individual wells, and error bars indicate standard deviations. Results for three independent experiments are shown. Reduced survival of the mutant relative to that of the isogenic parent in all experiments was significant (P < 0.005) at 48 h as determined by an unpaired t test.
M. tuberculosis acquires host cell betaine via ProXVWZ.
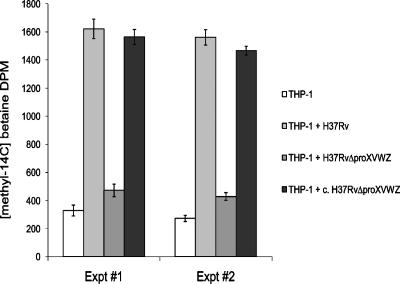
Glycine betaine is an important free cytoplasmic constituent of eukaryotic cells, including human peripheral-blood-derived macrophages (10), and is present at 20 to 60 μM in human serum (23). To demonstrate the relevance of ProXVWZ-mediated betaine transport in interactions between M. tuberculosis and macrophages, we examined betaine uptake by intracellular bacteria. Host cells were first incubated with radiolabeled betaine prior to infection. THP-1 macrophages took up betaine rapidly, reaching a steady state after 2 h, and cytoplasmic pools remained constant over the course of the experiments (data not shown). Washed, pelleted cellular debris from lysed, uninfected macrophages had little labeled betaine, confirming its presence as a free cytoplasmic osmolyte. Macrophages were then infected with wild-type, mutant, and complemented M. tuberculosis strains. A large increase in betaine was measured in lysed cell debris at 24 h when pelleted material contained the intact H37Rv parent. Intracellular acquisition of betaine in the proXVWZ mutant was greatly diminished as previously described, and the complemented strain regained this ability (Fig. 5). These experiments demonstrate that M. tuberculosis uses ProXVWZ to obtain beneficial betaine from host cells. Intracellular bacteria also appeared to experience osmotic stress, as they accumulated betaine. As the proXVWZ mutant was impaired in this ability and showed modest defects in early interactions with macrophages, ProXVWZ-dependent acquisition of betaine is shown contribute to the ability of M. tuberculosis to colonize a niche central in all types of infection.
FIG. 5.
Accumulation of host betaine by intracellular M. tuberculosis. Acquisition of betaine by bacteria at 24 h postinfection was determined as described in Materials and Methods. Results for two representative experiments are shown, and each data point is the mean of three individual samples, with error bars indicating standard deviations.
Concluding remarks.
An M. tuberculosis proXVWZ mutant was impaired only during the initial stage of macrophage colonization, when a transient increase in corresponding transcript level was normally seen. Lack of the encoded betaine transporter delayed initiating growth, and some bacteria perished before other uncharacterized effective osmoadaptive mechanisms were employed. Bacteria inhibited by osmotic stress can also remain viable but must restore suitable osmotic balance and turgor pressure before they can resume macromolecule synthesis and resume growth. Although the magnitude of the defect shown was not great in this infection model, natural respiratory infections are initiated by only a few individual bacteria. Droplet nuclei small enough to reach the alveoli of the human lung are capable of carrying only an estimated 1 to 3 bacilli. Therefore, we believe that this is an adaptive mechanism in the initial stages of colonization of the phagosome and likely contributes to the ability of M. tuberculosis to initiate respiratory infection.
Infectious disease and pathogenesis can be viewed as possible consequences of certain microbes having evolved to survive and multiply within specific environmental niches found only in certain host cells and tissues. These niches include various epithelial and endothelial surfaces and cellular vacuoles that can change dramatically in permeability and content as infection progresses. These in vivo niches are typically both dynamic and transient habitats, and their inhabitants therefore require efficient strategies to tolerate and compensate for corresponding environmental changes. While the roles of environmental variables as signals (for example, temperature, pH, oxygen availability, etc.) in regulating the expression of bacterial factors have been widely appreciated, microbial adaptive responses to these challenges are less frequently described as contributing to disease. This is particularly true of the osmoregulatory mechanisms that allow saprophytic species to survive catastrophic changes in water availability and bacterial pathogens to transition from reservoirs or transient states in the environment to multiple host environments during progressive infection. Our studies indicate that the ability of M. tuberculosis to acquire host cellular betaine immediately following phagocytosis is important to counter changes in water availability that follow phagocytosis, and this activity contributes to colonization of host cells. This aspect of the complex interaction between bacterial pathogens and phagocytic cells has not previously been described but is likely to be important in other microbial pathogens that must adapt to multiple environments encountered during the progressive course of infectious disease.
Acknowledgments
This work was supported by Public Health Service grant RO1 A148635-01 from the National Institute of Allergy and Infectious Disease.
J.E.G. thanks Brian J. Wilkinson for the initial invitation into this field almost 2 decades ago.
Footnotes
Published ahead of print on 4 April 2008.
REFERENCES
- 1.Arakawa, T., and S. N. Timasheff. 1985. The stabilization of proteins by osmolytes. Biophys. J. 47411-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banda, H. T., A. D. Harries, M. J. Boeree, T. E. Nyirenda, A. Banerjee, and F. M. Salaniponi. 2000. Viability of stored sputum specimens for smear microscopy and culture. Int. J. Tuberc. Lung Dis. 4272-274. [PubMed] [Google Scholar]
- 3.Bardarov, S., S. Bardarov, Jr., M. S. Pavelka, Jr., V. Sambandamurthy, M. Larsen, J. Tufariello, J. Chan, G. Hatfull, and W. R. Jacobs, Jr. 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 1483007-3017. [DOI] [PubMed] [Google Scholar]
- 4.Bayer, A. S., S. N. Coulter, C. K. Stover, and W. R. Schwan. 1999. Impact of the high-affinity proline permease gene (putP) on the virulence of Staphylococcus aureus in experimental endocarditis. Infect. Immun. 67740-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishai, W. 1998. The Mycobacterium tuberculosis genomic sequence: anatomy of a master adaptor. Trends Microbiol. 6464-465. [DOI] [PubMed] [Google Scholar]
- 6.Consaul, S. A., and M. S. Pavelka, Jr. 2004. Use of a novel allele of the Escherichia coli aacC4 aminoglycoside resistance gene as a genetic marker in mycobacteria. FEMS Microbiol. Lett. 234297-301. [DOI] [PubMed] [Google Scholar]
- 7.Cooksey, R. C., J. T. Crawford, W. R. Jacobs, Jr., and T. M. Shinnick. 1993. A rapid method for screening antimicrobial agents for activities against a strain of Mycobacterium tuberculosis expressing firefly luciferase. Antimicrob. Agents Chemother. 371348-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csonka, L. N. 1989. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 53121-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culham, D. E., C. Dalgado, C. L. Gyles, D. Mamelak, S. MacLellan, and J. M. Wood. 1998. Osmoregulatory transporter ProP influences colonization of the urinary tract by Escherichia coli. Microbiology 14491-102. [DOI] [PubMed] [Google Scholar]
- 10.Denkert, C., U. Warskulat, F. Hensel, and D. Haussinger. 1998. Osmolyte strategy in human monocytes and macrophages: involvement of p38MAPK in hyperosmotic induction of betaine and myoinositol transporters. Arch. Biochem. Biophys. 354172-180. [DOI] [PubMed] [Google Scholar]
- 11.D'Souza-Ault, M. R., L. T. Smith, and G. M. Smith. 1993. Roles of N-acetylglutaminylglutamine amide and glycine betaine in adaptation of Pseudomonas aeruginosa to osmotic stress. Appl. Environ. Microbiol. 59473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunlap, V. J., and L. N. Csonka. 1985. Osmotic regulation of l-proline transport in Salmonella typhimurium. J. Bacteriol. 163296-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282677-686. [DOI] [PubMed] [Google Scholar]
- 14.Graham, J. E., and J. E. Clark-Curtiss. 1999. Identification of Mycobacterium tuberculosis RNAs synthesized in response to phagocytosis by human macrophages by selective capture of transcribed sequences (SCOTS). Proc. Natl. Acad. Sci. USA 9611554-11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham, J. E., and B. J. Wilkinson. 1992. Staphylococcus aureus osmoregulation: roles for choline, glycine betaine, proline, and taurine. J. Bacteriol. 1742711-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosset, J. 2003. Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary. Antimicrob. Agents Chemother. 47833-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guinn, K. M., M. J. Hickey, S. K. Mathur, K. L. Zakel, J. E. Grotzke, D. M. Lewinsohn, S. Smith, and D. R. Sherman. 2004. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 51359-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta, R., M. A. Espinal, and M. C. Raviglione. 2004. Tuberculosis as a major global health problem in the 21st century: a WHO perspective. Semin. Respir. Crit. Care Med. 25245-253. [DOI] [PubMed] [Google Scholar]
- 19.Imhoff, J. F., and F. Rodriguez-Valera. 1984. Betaine is the main compatible solute of halophilic eubacteria. J. Bacteriol. 160478-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kappes, R. M., B. Kempf, and E. Bremer. 1996. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J. Bacteriol. 1785071-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manganelli, R., E. Dubnau, S. Tyagi, F. R. Kramer, and I. Smith. 1999. Differential expression of 10 sigma factor genes in Mycobacterium tuberculosis. Mol. Microbiol. 31715-724. [DOI] [PubMed] [Google Scholar]
- 22.Measures, J. C. 1975. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature 257398-400. [DOI] [PubMed] [Google Scholar]
- 23.Melse-Boonstra, A., P. I. Holm, P. M. Ueland, M. Olthof, R. Clarke, and P. Verhoef. 2005. Betaine concentration as a determinant of fasting total homocysteine concentrations and the effect of folic acid supplementation on betaine concentrations. Am. J. Clin. Nutr. 811378-1382. [DOI] [PubMed] [Google Scholar]
- 24.Pfaffl, M. W., G. W. Horgan, and L. Dempfle. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigue, S., J. Brodeur, P. E. Jacques, A. L. Gervais, R. Brzezinski, and L. Gaudreau. 2007. Identification of mycobacterial sigma factor binding sites by chromatin immunoprecipitation assays. J. Bacteriol. 1891505-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sage, A. E., and M. L. Vasil. 1997. Osmoprotectant-dependent expression of plcH, encoding the hemolytic phospholipase C, is subject to novel catabolite repression control in Pseudomonas aeruginosa PAO1. J. Bacteriol. 1794874-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlesinger, L. S., and M. A. Horwitz. 1991. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-gamma activation inhibits complement receptor function and phagocytosis of this bacterium. J. Immunol. 1471983-1994. [PubMed] [Google Scholar]
- 28.Schnappinger, D., S. Ehrt, M. I. Voskuil, Y. Liu, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwan, W. R., S. N. Coulter, E. Y. Ng, M. H. Langhorne, H. D. Ritchie, L. L. Brody, S. Westbrock-Wadman, A. S. Bayer, K. R. Folger, and C. K. Stover. 1998. Identification and characterization of the PutP proline permease that contributes to in vivo survival of Staphylococcus aureus in animal models. Infect. Immun. 66567-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwan, W. R., K. J. Wetzel, T. S. Gomez, M. A. Stiles, B. D. Beitlich, and S. Grunwald. 2004. Low-proline environments impair growth, proline transport and in vivo survival of Staphylococcus aureus strain-specific putP mutants. Microbiology 1501055-1061. [DOI] [PubMed] [Google Scholar]
- 31.Shoen, C. M., M. S. DeStefano, M. R. Sklaney, B. J. Monica, A. M. Slee, and M. H. Cynamon. 2004. Short-course treatment regimen to identify potential antituberculous agents in a murine model of tuberculosis. J. Antimicrob. Chemother. 53641-645. [DOI] [PubMed] [Google Scholar]
- 32.Sleator, R. D., J. Wouters, C. G. Gahan, T. Abee, and C. Hill. 2001. Analysis of the role of OpuC, an osmolyte transport system, in salt tolerance and virulence potential of Listeria monocytogenes. Appl. Environ. Microbiol. 672692-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theus, S. A., M. D. Cave, and K. D. Eisenach. 2004. Activated THP-1 cells: an attractive model for the assessment of intracellular growth rates of Mycobacterium tuberculosis isolates. Infect. Immun. 721169-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian, J., R. Bryk, M. Itoh, M. Suematsu, and C. Nathan. 2005. Variant tricarboxylic acid cycle in Mycobacterium tuberculosis: identification of alpha-ketoglutarate decarboxylase. Proc. Natl. Acad. Sci. USA 10210670-10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verheul, A., E. Glaasker, B. Poolman, and T. Abee. 1997. Betaine and l-carnitine transport by Listeria monocytogenes Scott A in response to osmotic signals. J. Bacteriol. 1796979-6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wemekamp-Kamphuis, H. H., J. A. Wouters, R. D. Sleator, C. G. Gahan, C. Hill, and T. Abee. 2002. Multiple deletions of the osmolyte transporters BetL, Gbu, and OpuC of Listeria monocytogenes affect virulence and growth at high osmolarity. Appl. Environ. Microbiol. 684710-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu, S., S. T. Howard, D. L. Lakey, A. Kipnis, B. Samten, H. Safi, V. Gruppo, B. Wizel, H. Shams, R. J. Basaraba, I. M. Orme, and P. F. Barnes. 2004. The principal sigma factor sigA mediates enhanced growth of Mycobacterium tuberculosis in vivo. Mol. Microbiol. 511551-1562. [DOI] [PubMed] [Google Scholar]
- 38.Yancey, P. H. 2005. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2082819-2830. [DOI] [PubMed] [Google Scholar]