Abstract
We report on the identification of Mycobacterium tuberculosis HtdZ (Rv0130), representing a novel 3-hydroxyacyl-thioester dehydratase. HtdZ was picked up by the functional complementation of Saccharomyces cerevisiae htd2Δ cells lacking the dehydratase of mitochondrial type II fatty acid synthase. Mutant cells expressing HtdZ contained dehydratase activity, recovered their respiratory ability, and partially restored de novo lipoic acid synthesis.
The availability of the complete genome sequence of Mycobacterium tuberculosis (6) has substantiated the presumption that this organism contains all of the components of the type II fatty acid synthase (FASII) system (16). The latest FASII enzyme to be identified is 3-hydroxyacyl-acyl carrier protein dehydratase, represented by heterodimers of Rv0635-Rv0636 HadAB and Rv0636-Rv0637 HadBC (5, 15) but potentially also by Rv0130 (11) and Rv0098 (16). Here, we have exploited Saccharomyces cerevisiae as a surrogate host for expressing the latter two mycobacterial proteins (8). The yeast htd2Δ mutant used is devoid of the mitochondrial FASII enzyme 3-hydroxyacyl-thioester dehydratase Htd2p and therefore lacks the corresponding activity, contains abnormally small mitochondria, fails to assemble respiratory complexes or produce sufficient levels of lipoic acid, and is exclusively fermentative (12). Mutant cells expressing ectopic Rv0130 or Rv0098 were examined for hydratase activity, growth on glycerol medium, the assembly of cytochrome complexes, respiration, and lipoic acid production. We discuss our findings with reference to the identification of a novel M. tuberculosis FASII-like 3-hydroxyacyl-thioester dehydratase.
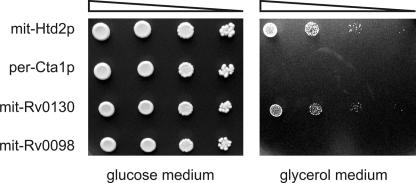
To assess whether these two mycobacterial proteins can compensate in vivo for the missing dehydratase activity in mitochondria of the yeast BY4741htd2Δ mutant strain (EUROSCARF), they were expressed as fusion constructs that were preceded by the Coq3p mitochondrial leader sequence (mit-Rv0130 and mit-Rv0098), which was demonstrated previously to be sufficient for targeting proteins to yeast mitochondria (10). As controls, isogenic htd2Δ cells were transformed with plasmids expressing either native mitochondrial Hdt2p (12) (mit-Hdt2p) or peroxisomal catalase A (per-Cta1p) (7). All four constructs were tethered behind the yeast oleic acid-inducible CTA1 promoter on multicopy plasmids. The four transformants were propagated overnight in synthetic glucose medium lacking uracil for plasmid maintenance, and following adjustment of the cell concentration to an A600 of 0.5 and serial 10-fold dilution, cells were spotted onto solid 2% glucose or 3% glycerol medium, and the plates were incubated at 30°C until single colonies were detectable.
The results shown in Fig. 1 demonstrated that htd2Δ mutant cells expressing mit-Rv0130 resembled the self-complemented strain producing mit-Htd2p, since they could grow on glycerol as the sole carbon source. On the other hand, cells expressing mit-Rv0098 resembled the strain enriched for per-Cta1p in that they were completely incapable of growth on this medium. Therefore, we were able to show here for the first time that when expressed within the context of a heterologous FASII framework, Rv0130 can function as a physiological 3-hydroxyacyl-thioester dehydratase.
FIG. 1.
Growth of S. cerevisiae htd2Δ mutants expressing M. tuberculosis Rv0130. Yeast htd2Δ cells expressing native mitochondrial 3-hydroxyacyl-thioester dehydratase (mit-Htd2p) (positive control), native peroxisomal catalase A (per-Cta1p) (negative control), mitochondrially targeted Rv0130 (mit-Rv0130), or mitochondrially targeted Rv0098 (mit-Rv0098) were applied to solid glucose or glycerol medium following a serial 10-fold dilution ( ).
).
The recovery of htd2Δ cells from their respiratory deficiency phenotype by complementation is accompanied by the restoration of assembled cytochrome complexes and the regeneration of the electron transport chain (12). Hence, the above-mentioned yeast strains were monitored spectrophotometrically for cytochrome assembly using the cold-temperature cuvette method (13). The results presented in Fig. 2 showed that htd2Δ cells expressing mit-Rv0130 or mit-Htd2p were much more efficient than those expressing mit-Rv0098 or per-Cta1p at assembling cytochrome complexes c, c1, b, a, and a3. In addition, the application of 0.1% 2,4,5-triphenyltetrazolium chloride as a low-melting-temperature agarose overlay (3) to the four strains following growth on solid rich-glucose medium demonstrated that the former two strains were able to metabolize this compound to generate the red chromophore, whereas the latter two were not (not shown). The combined results showed that a mitochondrially localized Rv0130 can reverse the effects of the lesion caused by the htd2Δ mutation.
FIG. 2.
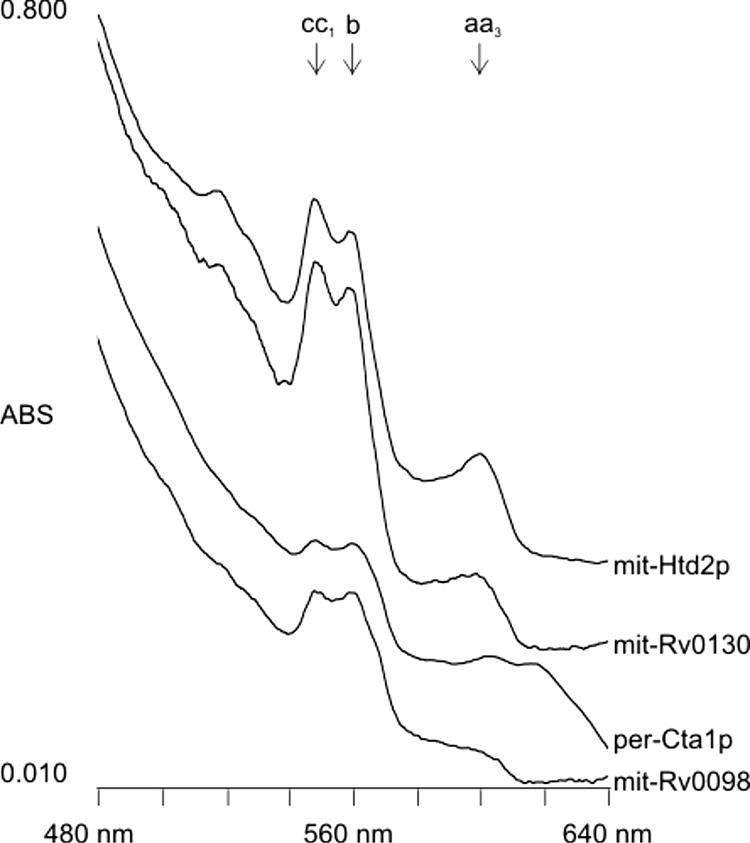
Spectrophotometric assay for mitochondrial cytochrome complexes. Cell pastes from the indicated strains were collected from colonies grown on solid rich-glucose medium that were left for 4 days at room temperature and applied to the glass face of otherwise aluminum cold-temperature cuvettes that were immersed in liquid nitrogen prior to being placed into a spectrophotometer for analysis. The pastes were scanned at wavelengths of between 480 nm and 640 nm. Numbers to the left indicate units of absorbance (ABS); those at the bottom refer to wavelengths in nm. Peaks corresponding to known cytochromes are indicated (letters with arrows).
To characterize Rv0130 and Rv0098 biochemically, their enzyme activities were measured in yeast extracts. Following overnight propagation in liquid oleic acid medium, under which conditions the CTA1 promoter driving the expression of the four open reading frames is highly induced, yeast cells from three independent inductions were broken with glass beads, and their contents were examined for hydratase activity (14) using 2-trans-butyryl (crotonyl-)-, -hexenoyl-, and -decenoyl-coenzyme A (CoA) as substrates. The results of the enzyme assays showed that any potential hydratase activity in soluble protein extracts made from cells overexpressing either mit-Htd2p, per-Cta1p, or mit-Rv0098 was below the detection limit of the assay used, i.e., <0.05 nmol substrate metabolized per mg protein per min. On the other hand, soluble protein extracts produced from mutant cells overexpressing mit-Rv0130 contained a 3-hydroxyacyl-thioester hydratase activity equivalent to 1.81 ± 0.39 nmol/mg·min−1 (mean ± standard deviation) (n = 3) using 2-trans-butyryl-CoA as a substrate, 5.80 ± 0.49 nmol/mg·min−1 using 2-trans-hexenoyl-CoA, and 0.36 ± 0.07 nmol/mg·min−1 using 2-trans-decenoyl-CoA. Hence, in agreement with previously published data on the hydratase activity of recombinant Rv0130 (11), the overexpression of Rv0130 in mutant htd2Δ cells was coincidental with soluble protein extracts containing detectable levels of 3-hydroxyacyl-thioester hydratase activity.
To link Rv0130 expression with fatty acid biosynthesis in yeast mitochondria, the production of lipoic acid (4, 9) was examined in the four strains, and the values reported represent an average of data for three independent bacterial growth responses. The results showed that extracts derived from htd2Δ mutant cells expressing mit-Htd2p supported a level of bacterial growth that was equivalent to 233 ± 5 ng lipoic acid per gram (wet weight) yeast cells (mean ± standard deviation) (n = 3), whereas those enriched for per-Cta1p supported only background-level growth corresponding to 10 ± 1 ng lipoic acid per gram. The production of mit-Rv0130 or mit-Rv0098 in these cells resulted in bacterial growth that correlated with 43 ± 4 and 11 ± 2 ng lipoic acid per gram, respectively. In a separate representative experiment for lipoic acid production, BY4741 wild-type cells contained 254 ng lipoic acid per gram (duplicates). Hence, the expression of mit-Rv0130 gave rise to a fourfold-greater amount of lipoic acid than did the expression of mit-Rv0098 or per-Cta1p. Since previous expressions of heterologous dehydratases in the htd2Δ mutant successfully corrected the mutant's defective respiration, including bacterial FabZ and FabA (12) as well as human (2) and Trypanosoma brucei (1) HTD2, we suggest that due to its similar success at restoring respiration to this mutant, Rv0130 represents a bona fide M. tuberculosis FASII-like 3-hydroxyacyl-thioester dehydratase, and we propose naming it HtdZ.
Acknowledgments
We thank Johanna Makinen from the Mycobacterial Reference Laboratory at the National Public Health Institute in Turku, Finland, for DNA.
This work was supported by grants from the Academy of Finland and the Sigrid Jusélius Foundation to J.K.H. and grants P19378-B03 and P19399-B03 from the Austrian Science Fund (FWF) to A.G.
Footnotes
Published ahead of print on 28 March 2008.
REFERENCES
- 1.Autio, K. J., J. L. Guler, A. J. Kastaniotis, P. T. Englund, and J. K. Hiltunen. 2008. The 3-hydroxyacyl-ACP dehydratase of mitochondrial fatty acid synthesis in Trypanosoma brucei. FEBS Lett. 582729-733. [DOI] [PubMed] [Google Scholar]
- 2.Autio, K. J., A. J. Kastaniotis, H. Pospiech, I. J. Miinalainen, M. S. Schonauer, C. L. Dieckmann, and J. K. Hiltunen. 2007. An ancient genetic link between vertebrate mitochondrial fatty acid synthesis and RNA processing. FASEB J. 22569-578. [DOI] [PubMed] [Google Scholar]
- 3.Böker-Schmitt, E., S. Francisci, and R. J. Schweyen. 1982. Mutations releasing mitochondrial biogenesis from glucose repression in Saccharomyces cerevisiae. J. Bacteriol. 151303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brody, S., C. Oh, U. Hoja, and E. Schweizer. 1997. Mitochondrial acyl carrier protein is involved in lipoic acid synthesis in Saccharomyces cerevisiae. FEBS Lett. 408217-220. [DOI] [PubMed] [Google Scholar]
- 5.Brown, A. K., A. Bhatt, A. Singh, E. Saparia, A. F. Evans, and G. S. Besra. 2007. Identification of the dehydratase component of the mycobacterial mycolic acid-synthesizing fatty acid synthase-II complex. Microbiology 1534166-4173. [DOI] [PubMed] [Google Scholar]
- 6.Cole, S. T., et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393537-544. [DOI] [PubMed] [Google Scholar]
- 7.Filppula, S. A., R. T. Sormunen, A. Hartig, W.-H. Kunau, and J. K. Hiltunen. 1995. Changing stereochemistry for a metabolic pathway in vivo. Experiments with the peroxisomal β-oxidation in yeast. J. Biol. Chem. 27027453-27457. [DOI] [PubMed] [Google Scholar]
- 8.Gerum, A. B., J. E. Ulmer, D. P. Jacobus, N. P. Jensen, D. R. Sherman, and C. H. Sibley. 2002. Novel Saccharomyces cerevisiae screen identifies WR99210 analogues that inhibit Mycobacterium tuberculosis dihydrofolate reductase. Antimicrob. Agents Chemother. 463362-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayden, M. A., I. Y. Huang, G. Iliopoulos, M. Orozco, and G. W. Ashley. 1993. Biosynthesis of lipoic acid: characterization of the lipoic acid auxotrophs Escherichia coli W1485-lip2 and JRG33-lip9. Biochemistry 323778-3782. [DOI] [PubMed] [Google Scholar]
- 10.Hsu, A. Y., W. W. Poon, J. A. Shepherd, D. C. Myles, and C. F. Clarke. 1996. Complementation of coq3 mutant yeast by mitochondrial targeting of the Escherichia coli UbiG polypeptide: evidence that UbiG catalyzes both O-methylation steps in ubiquinone biosynthesis. Biochemistry 359797-9806. [DOI] [PubMed] [Google Scholar]
- 11.Johansson, P., A. Castell, T. A. Jones, and K. Bäckbro. 2006. Structure and function of Rv0130, a conserved hypothetical protein from Mycobacterium tuberculosis. Protein Sci. 152300-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastaniotis, A. J., K. J. Autio, R. T. Sormunen, and J. K. Hiltunen. 2004. Htd2p/Yhr067p is a yeast 3-hydroxyacyl-ACP dehydratase essential for mitochondrial function and morphology. Mol. Microbiol. 531407-1421. [DOI] [PubMed] [Google Scholar]
- 13.Lindenmayer, A., and R. W. Estabrook. 1958. Low-temperature spectral studies on the biosynthesis of cytochromes in baker's yeast. Arch. Biochem. Biophys. 7866-82. [DOI] [PubMed] [Google Scholar]
- 14.Malila, L. H., K. M. Siivari, M. J. Mäkelä, J. E. Jalonen, P. M. Latipää, W.-H. Kunau, and J. K. Hiltunen. 1993. Enzymes converting D-3-hydroxyacyl-CoA to trans-2-enoyl-CoA. Microsomal and peroxisomal isoenzymes in rat liver. J. Biol. Chem. 26821578-21585. [PubMed] [Google Scholar]
- 15.Sacco, E., A. S. Covarrubias, H. M. O'Hare, P. Carroll, N. Eynard, T. A. Jones, T. Parish, M. Daffe, K. Bäckbro, and A. Quemard. 2007. The missing piece of the type II fatty acid synthase system from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 10414628-14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takayama, K., C. Wang, and G. S. Besra. 2005. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 1881-101. [DOI] [PMC free article] [PubMed] [Google Scholar]