Abstract
Neisseria gonorrhoeae releases peptidoglycan fragments during growth. The majority of the fragments released are peptidoglycan monomers, molecules known to increase pathogenesis through the induction of proinflammatory cytokines and responsible for the killing of ciliated epithelial cells. In other gram-negative bacteria such as Escherichia coli, these peptidoglycan fragments are efficiently degraded and recycled. Peptidoglycan fragments enter the cytoplasm from the periplasm via the AmpG permease. The amidase AmpD degrades peptidoglycan monomers by removing the disaccharide from the peptide. The disaccharide and the peptide are further degraded and are then used for new peptidoglycan synthesis or general metabolism. We examined the possibility that peptidoglycan fragment release by N. gonorrhoeae results from defects in peptidoglycan recycling. The deletion of ampG caused a large increase in peptidoglycan monomer release. Analysis of cytoplasmic material showed peptidoglycan fragments as recycling intermediates in the wild-type strain but absent from the ampG mutant. An ampD deletion reduced the release of all peptidoglycan fragments and nearly eliminated the release of free disaccharide. The ampD mutant also showed a large buildup of peptidoglycan monomers in the cytoplasm. The introduction of an ampG mutation in the ampD background restored peptidoglycan fragment release, indicating that events in the cytoplasm (metabolic or transcriptional regulation) affect peptidoglycan fragment release. The ampD mutant showed increased metabolism of exogenously added free disaccharide derived from peptidoglycan. These results demonstrate that N. gonorrhoeae has an active peptidoglycan recycling pathway and can regulate peptidoglycan fragment metabolism, dependent on the intracellular concentration of peptidoglycan fragments.
As Neisseria gonorrhoeae grows and divides, the cell wall is remodeled by peptidoglycanases that degrade intact peptidoglycan (PG) into smaller fragments (16, 23, 43). The principal PG fragments released from N. gonorrhoeae during growth are PG monomers, the basic subunits of the bacterial cell wall. These PG monomers are released in the anhydro form (1,6-anhydro disaccharide tetrapeptide and 1,6-anhydro disaccharide tripeptide) and are liberated by the action of lytic transglycosylases (7, 25, 43, 47). Purified anhydro PG monomers from N. gonorrhoeae reproduce the tissue damage of gonococcal infections, causing ciliated cell death when applied to human fallopian tubes in the organ culture model of pelvic inflammatory disease (37). The 1,6-anhydro disaccharide tetrapeptide monomers are identical to the tracheal cytotoxin of Bordetella pertussis and have been shown to induce the production of inflammatory cytokines interleukin-1 (IL-1) and IL-6 in human monocytes (8, 10, 24, 34, 45). The 1,6-anhydro disaccharide tripeptide monomers are identical to the PG fragments shown to stimulate Nod1 and lead to IL-8 production (4, 12, 13).
Gram-negative bacteria have evolved mechanisms for recycling PG fragments generated during normal growth (27, 42). PG monomers are translocated back into the cytoplasm via the AmpG transporter (6, 27, 32). Once inside the cytoplasm, these anhydro PG monomers are degraded, yielding glucosamine, alanine, and stem tripeptides. AmpD is a cytoplasmic N-acetylmuramyl-l-alanine amidase that is specific to anhydro-muropeptides and acts to remove the stem peptide from the sugar residues (26, 28). LdcA is an l,d-carboxypeptidase that removes terminal d-Ala residues from stem tetrapeptides to form tripeptides (49). Murein tripeptides are then reincorporated into the PG biosynthetic pathway for the production of pentapeptide monomers to be incorporated back into the growing cell wall (15, 38). NagZ, a beta-N-acetylglucosaminidase removes N-acetylglucosamine from PG monomers or free disaccharide (5, 54, 55). Anhydromuramic acid kinase (AnmK) and MurQ, an N-acetylmuramic acid-6-phosphate etherase (51, 52), convert liberated N-acetylmuramic acid to N-acetylglucosamine phosphate.
Both Escherichia coli and gonococci break down up to 50% of their PG each generation (15, 23). However, because the PG fragments liberated by E. coli are efficiently recycled, only 3 to 10% of the PG fragments are released into the environment per generation (17). When they compared the PG turnover rate of E. coli with that of gonococci, Greenway and Perkins observed similar rates (9 to 15%) for gonococci and E. coli (18). Unlike E. coli, however, gonococci release immunologically relevant amounts of PG monomers into the extracellular milieu (36, 37). Significant release of PG monomers has been observed for other gram-negative bacterial pathogens including B. pertussis and Haemophilus influenzae (1, 2, 14). The question why these pathogens release greater amounts of PG fragments than E. coli remains unanswered. In B. pertussis, it was shown that the expression of E. coli ampG greatly reduces PG monomer release (39). This result suggested that gram-negative bacteria that release PG monomers may do so because of defects in PG fragment uptake and recycling.
To examine PG recycling in N. gonorrhoeae, we made mutations in ampG and ampD and asked whether these mutants showed increased PG fragment release. Our results show that gonococcal AmpG is functional and proficient for the uptake of anhydro PG fragment monomers and free disaccharides. An ampD mutant showed decreased PG fragment release, a buildup of PG monomers in the cytoplasm, and an increase in the metabolism of imported PG fragments. These results demonstrate a functional PG recycling system in N. gonorrhoeae and suggest that the concentration of PG monomers within the cytoplasm is sensed by the cell for the regulation of PG metabolism.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Table 1 contains a list of all N. gonorrhoeae strains used in this study. Piliated gonococcal variants of strain MS11 or its derivatives were used for transformation, whereas nonpiliated variants were used for all other procedures. N. gonorrhoeae strains were grown with aeration in gonococcal base liquid medium (GCBL) containing Kellogg's supplements (29) and 0.042% NaHCO3 (40) or on GCB agar plates (Difco) with Kellogg's supplements and 5% CO2 (9). E. coli was grown in Luria broth or on Luria agar plates (46). Antibiotics erythromycin and chloramphenicol were both used at a concentration of 10 μg/ml for N. gonorrhoeae, and erythromycin was used at 500 μg/ml and chloramphenicol at 25 μg/ml for E. coli.
TABLE 1.
Strains and plasmids used in this study
| Plasmid or strain | Description | Reference or source |
|---|---|---|
| Plasmids | ||
| pIDN3 | N. gonorrhoeae insertion-duplication plasmid (Ermr) | 22 |
| pKH35 | Gonococcal complementation vector (Camr) derived from pGCC6 | 21 |
| pKH37 | Gonococcal complementation vector (Camr) derived from pGCC6 | 30 |
| pDG017 | 1,341-bp fragment containing amiC in pKH37 (Camr); amiC under lac promoter control | |
| pDG131 | 677-bp 5′ ampG fragment in pIDN3 (Ermr) | This work |
| pDG132 | ampG in-frame deletion construct; 722-bp 3′ ampG fragment in pDG131 (Ermr) | This work |
| pDG133 | ampG complementation; 1,331-bp fragment containing ampG of N. gonorrhoeae under lac promoter control in pKH37 (Camr) | This work |
| pDG140 | 1,892-bp ampD region in pIDN3 (Ermr) | This work |
| pDG141 | ampD in-frame deletion constructed in pDG140; contains 504-bp deletion internal to ampD | This work |
| pDG143 | ampD complementation; 680-bp fragment containing ampD under lac promoter control in pKH37 (Camr) | This work |
| Strains | ||
| MS11 | Wild-type N. gonorrhoeae (Strr) | 48 |
| DG132 | MS11 transformed with pDG132; ampG deletion mutant | This work |
| DG133 | MS11 transformed with pDG133; ampG complemented (Camr) | This work |
| DG142 | MS11 transformed with pDG141; ampD deletion mutant | This work |
| DG143 | DG142 transformed with pDG143; ampD complemented (Camr) | This work |
| DG147 | DG142 transformed with pDG017; amiC overexpression | This work |
| DG152 | DG132 transformed with pDG141; ampD ampG deletion mutant | This work |
Plasmid construction.
Plasmids used in this study are listed in Table 1. The primers, which include added restriction enzyme recognition sites (underlined below), were designed based on the N. gonorrhoeae strain FA1090 genome sequence (GenBank accession no. AE004969), unless indicated otherwise.
For amplification of ampD and the surrounding region, the primers 5′ ACATCAAGCTTTGTAGGCGGTTTGGTAAATCTGCA 3′ and 5′ TTCTACTAGTGCTCGACCTTCGACAGCTCTTT 3′ were used with MS11 chromosomal DNA as the template. The amplicon contained the region from 614 bp upstream to 683 bp downstream of the ampD coding sequence. The PCR product was digested with HindIII and SpeI, ligated into the plasmid pIDN3 (22) to produce pDG140, and transformed into competent E. coli TAM1 cells. Plasmids from the resulting Ermr transformants were screened for the predicted size and restriction digest patterns. To make a deletion in ampD, pDG140 was amplified with divergent primers containing BsaI restriction sites, 5′ GTCATAGGTCTCCAGACGTGATTGTCCATGATGTTCTTCCTGTC 3′ and 5′GTCATAGGTCTCCGTCTTCGACTGGCGGCGGATA3′. The resulting PCR product was digested with BsaI, self-ligated, and transformed into E. coli. Plasmids from the resulting Ermr transformants were screened for the predicted size and for the expected restriction digest patterns. One of the plasmids with the correct predicted size was confirmed to contain the expected deletion by DNA sequencing and was named pDG141.
For complementation, the ampD gene lacking its predicted intrinsic promoter was PCR amplified from MS11 by using the primers 5′ TTCTACTAGT CGCCGGACAGGAAGAACATC 3′ (SpeI) and 5′ ACATCAAGCTTGCAGGGTCTGACAGCAGTGT 3′ (HindIII). The resulting 680-bp product was cut and ligated into pKH37 at the SpeI and HindIII restriction sites to generate pDG143. Transformants containing pDG143 were selected for chloramphenicol resistance. Correct plasmid construction was verified by PCR amplification and restriction digest mapping.
To construct the ampG deletion mutant, both the 5′ and the 3′ ends of ampG, including their flanking sequences, were PCR amplified separately using two primer pairs containing restriction sites. The 677-bp fragment containing the 5′ portion (8 bp) of the ampG sequence was amplified with primers 5′ ACATCAAGCTTAACACGCCAAAGCCCTGAAC 3′ (HindIII) and 5′ AGTAGGATCC GCAGTCATCGTTCGTACAGCCAA 3′ (BamHI). The product was cut and ligated into pIDN3 at the HindIII and BamHI restriction sites to generate pDG131. The 722-bp fragment containing the 3′ portion (79 bp) of ampG was amplified with the primers 5′ TTCTACTAGTCGGCGAGGGCTGTACCGTAT 3′ (SpeI) and 5′ AGTAGGATCCACTTGCCCTGCCGGGTATG 3′ (BamHI). The product was cut and ligated into pDG131 at the SpeI and BamHI restriction sites to generate pDG132. Ermr transformants were screened for plasmids of the predicted size and the expected restriction digest patterns.
For complementation using ampG, the gene lacking its predicted promoter was PCR amplified from strain MS11 chromosomal DNA by using the primers 5′ TTCTACTAGT TGGCTGTACGAACGATGACTGCA 3′ (SpeI) and 5′ ACATCAAGCTT CAATATCAGGTAAACGCTCCAGTTTGA 3′ (HindIII). The resulting 1,340-bp product was cut and ligated into pKH37 at the SpeI and HindIII restriction sites to generate pDG133. Transformants containing pDG133 were selected for chloramphenicol resistance. Correct plasmid construction was verified by PCR amplification, restriction digest mapping, and DNA sequencing.
ampG and ampD mutants of N. gonorrhoeae.
To construct a deletion mutation in ampD, the wild-type gonococcal strain MS11 was transformed with NheI-linearized pDG141. Transformants were screened for the absence of ampD by PCR, using both intragenic and ampD-flanking primer pairs to identify an ampD deletion mutant (DG142). Complementation of the ampD mutant was generated by transformation with pDG143 containing a gonococcal ampD allele under the control of the lac promoter-operator. Plasmid pDG143 contains portions of the gonococcal aspC and lctP genes that facilitate insertion of the complementation construct between these genes in the chromosome (Table 1). Complemented ampD strains were selected for their resistance to chloramphenicol and were screened by PCR for incorporation of the additional ampD allele and retention of the ampD deletion mutation.
To create a deletion mutation in ampG, pDG132 was linearized with NheI and introduced into N. gonorrhoeae by spot transformation (20). Potential transformants were screened for the absence of ampG by PCR, using intragenic and ampG-flanking primers. DNA sequencing confirmed the expected deletion. Complementation of the ampG mutant DG132, was performed by spot transformation using plasmid pDG133. After transformants were selected with chloramphenicol, they were screened by PCR, using primers to verify retention of the ampG deletion and insertion of the ampG allele at the region of homology. Sequencing of the introduced ampG allele confirmed the expected sequence.
For the creation of an ampD ampG double mutant, strain DG132 was transformed with pDG141 linearized with NheI. Transformants were screened for a deletion in ampD by PCR using both intragenic and ampD-flanking primer sets. To express amiC in an ampD background, strain DG142 was transformed using pDG017, a plasmid that contains amiC under the control of the lac promoter (11). Transformants resistant to chloramphenicol were screened by PCR using primers specific to amiC and flanking the chromosomal genes aspC and lctP.
Characterization of released PG fragments.
Labeling, purification, and characterization of gonococcal PG were performed after the method described by Rosenthal and Dziarski (44), with modifications described by Cloud and Dillard (7). Gonococcal strains were pulse-labeled by growth in GCB medium containing [6-3H]glucosamine and lacking glucose. To allow for quantitative comparisons, the amount of radioactivity in the cells was determined for each culture, after the labeling period, and the cultures were diluted to achieve equivalent amounts of radioactivity in the cells for the chase period. PG fragments released into the medium during the chase period were separated by size-exclusion chromatography and detected by liquid scintillation counting. The relative amounts of PG monomers released were quantified by determining the area under the curve for the PG monomer peaks, using the method described by Vossoughi (53). High-performance liquid chromatography (HPLC) analysis was carried out as described by Kohler et al. (30). Fractions from the size-exclusion column containing PG monomers were pooled, desalted, and lyophilized. PG monomers were separated by C18 reversed-phase HPLC using a 4 to 13% acetonitrile gradient for 30 min.
Preparation of hot-water extracts.
Cell-free hot-water extracts were prepared as described by Jacobs et al. (27). Each sample represented the hot-water extract from approximately 3 × 109 logarithmic phase cells. Gonococci were grown with aeration for 35 min in GCBL medium lacking glucose and containing 0.4% pyruvate plus [6-3H]glucosamine at a concentration of 10 μCi/ml to label the PG. After samples were centrifuged for 5 min at 1,800 × g, supernatants were discarded, and cells were washed with GCBL and resuspended in 6 ml of GCBL with glucose and without label. Triplicate 100-μl samples were taken from each culture to measure cpm, to normalize the amount of label incorporated into the cells. Cultures were grown with aeration for 45 min, quickly chilled to 4°C, centrifuged at 1,800 × g, and washed once with 1.5 ml of cold water. Cells were then suspended in 2 ml of boiling water and maintained at 100°C for 5 min. To remove particulate matter, samples were centrifuged at 12,000 × g for 10 min at 4°C, and supernatants (hot-water extracts) were collected. For direct comparison, the amount of sample loaded onto the column was adjusted to account for the total amount of label incorporated by each individual culture.
Uptake of purified PG fragments.
Metabolically labeled PG monomers and free disaccharide were purified from MS11 culture supernatant by size-exclusion chromatography, as described above. Purified PG monomers (4.8 × 106 cpm) or free disaccharide (1.6 × 106 cpm) suspended in 400 μl water were added to 3 ml wild-type or mutant gonococcal strains growing in log-phase culture. The amount of labeled PG monomers or free disaccharide was equivalent to 60% of that found in log-phase culture. After 1 h of growth, cells were harvested by centrifugation for 10 min at 1,800 × g. Radioactivity levels in the cell pellets were determined by liquid scintillation counting and were normalized to total protein content. Protein concentrations in cell pellets were determined by the Lowry method using the Bio-Rad DC protein assay as outlined in the manufacturer's instructions (33).
RESULTS
Peptidoglycan recycling genes in N. gonorrhoeae.
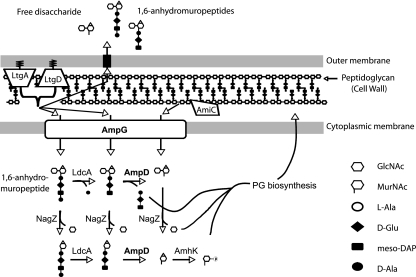
Although N. gonorrhoeae releases PG fragments during growth, the genes for PG recycling proteins are found in the N. gonorrhoeae genome (GenBank accession no. AE004969) (Fig. 1). Homologues were found for AmpG (NGO1599), a permease that acts to transport PG fragments across the inner membrane (6, 32); for LdcA (NGO1274), a carboxypeptidase that removes the terminal alanine from PG tetrapeptide monomers (49); for AmpD (NGO0237), an amidase that splits PG monomers into disaccharide and peptide portions (26); and for NagZ (NGO0135), a beta-glucosaminidase that removes the N-acetylglucosamine from PG monomers or free disaccharide (5, 54, 55). A homologue for AnmK was also found (NGO1583). AnmK acts to phosphorylate anhydro-N-acetylmuramic acid and eliminates the 1,6-anhydro bond (52). However, no homologue was found for MurQ, which in E. coli, acts to convert N-acetylmuramic acid-phosphate to N-acetylglucosamine-phosphate (51).
FIG. 1.
Model of peptidoglycan recycling in Neisseria gonorrhoeae. As the bacterial cell grows and divides, the sacculus is remodeled by peptidoglycanases including lytic transglycosylases LtgA and LtgD and amidase AmiC. The liberated PG fragments include 1,6-anhydro PG monomers and free disaccharide. The gonococcal genome was found to encode homologues of multiple PG recycling proteins, and their predicted functions are shown here. PG fragments are taken into the cytoplasm through the AmpG permease to be further degraded by a carboxypeptidase (LdcA), an N-acetylmuramyl-l-alanine amidase (AmpD), and an N-acetylglucosaminidase (NagZ). The order in which these reactions occur in gonococci is not known. The resulting tripeptide is incorporated within the PG biosynthetic pathway leading to UDP-pentapeptide monomers for incorporation into the growing sacculus. The symbols used to represent amino acids and sugar residues were chosen to mirror those used by Jacobs et al. (27).
The gonococcal AmpG coding sequence is significantly shorter than that of E. coli. An amino acid alignment of the gonococcal and E. coli AmpG proteins shows gonococcal AmpG is shorter at the C-terminal end by 77 amino acids, approximately 15% of the coding sequence. Chahboune et al. proposed a topological model of E. coli AmpG inner membrane protein showing 10 membrane-spanning regions (3). A topological profile of the predicted membrane-spanning regions for gonococcal and E. coli AmpG protein homologues shows the former lacking two membranes, one periplasmic and two cytoplasmic regions at the C-terminal end. It is also notable that the helical regions 5 and 6 of gonococcal AmpG have lower prediction probabilities for membrane spanning and are assigned a probable and transient residence in the periplasm or cytosol (data not shown). These results suggested that the gonococcal AmpG might not function or might not function as well as E. coli AmpG.
Mutation of ampG increases the release of PG monomers.
To test the functionality of AmpG and its role in PG recycling, we constructed an in-frame deletion of ampG in the gonococcal chromosome and characterized PG fragments released by the ampG mutant. During growth, N. gonorrhoeae sheds soluble PG fragments including PG multimers, PG monomers, and free disaccharide (43, 47). PG monomers are the predominant soluble fragments found in gonococcal culture supernatants and are the molecules that cause inflammatory cytokine induction and the death of human ciliated cells (10, 37, 47).
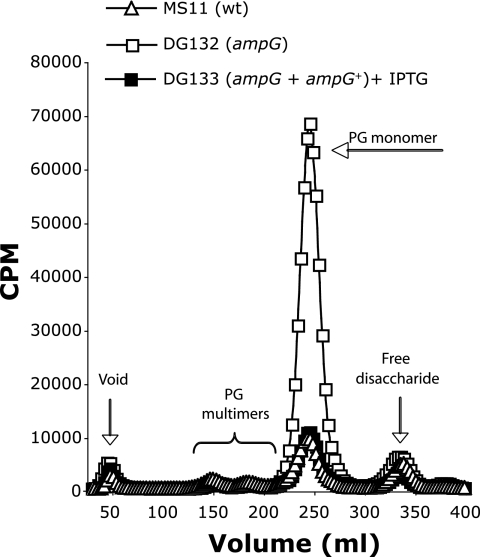
To measure the amount of PG fragments released into the supernatant during growth, each gonococcal strain was pulse-labeled metabolically by growing cells in medium containing [6-3H]glucosamine. Cultures containing equivalent amounts of radiolabel were grown for 2.5 h in medium without label to allow release of PG fragments, and the released fragments were characterized by size-exclusion chromatography. The resulting profile of the ampG deletion mutant (DG132) showed an increase in PG monomer release of approximately sevenfold compared to that of the wild type (Fig. 2). Complementation of the ampG deletion mutant with wild-type ampG at a distant location on the gonococcal chromosome (DG133) restored wild-type levels of PG monomer release.
FIG. 2.
Mutation of ampG causes an increase in PG monomer release. N. gonorrhoeae strains MS11 (wild type), DG132 (ampG), and DG133 (ampG + ampG+, complemented strain) were labeled metabolically by growth in glucose-deficient medium containing [6-3H]glucosamine. Supernatants were collected after 2.5 h of growth, and released PG fragments were separated by size-exclusion chromatography and detected by scintillation counting.
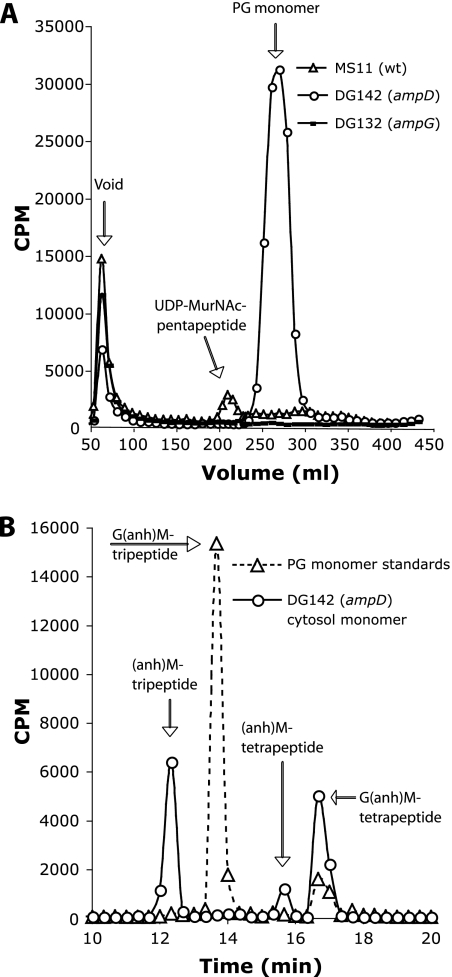
To determine if AmpG acts in the transport of PG fragments across the inner membrane, PG fragments in the cytoplasm of the wild-type or the ampG mutant strain were prepared by hot water extraction, as described by Jacobs et al. (27). Gonococci were pulse-labeled in medium containing [6-3H]glucosamine and lacking glucose. Cells were washed and resuspended in medium containing glucose and allowed to grow for an additional 45 min. Cells were subjected to osmotic shock by washing them with water to remove periplasmic contents. The cells were then boiled in water to obtain the hot-water extracts. The soluble cytosolic material was then separated by size-exclusion chromatography, and PG fragments were detected by scintillation counting. The wild-type strain MS11 showed a peak slightly larger than that of the PG monomer standard (Fig. 3A). This material likely represents the UDP-N-acetylmuramic acid (MurNAc) pentapeptide, the PG biosynthesis precursor, which is the only detectable peak in similar hot-water extracts of E. coli (27). By contrast, the ampG deletion mutant showed no cytoplasmic PG fragments (Fig. 3A). This result demonstrates that AmpG is required for the uptake of PG fragments into the cytoplasm. Overall, these results suggest that gonococcal AmpG is a functional PG fragment permease and indicate that N. gonorrhoeae recycles approximately 85% of liberated PG monomers generated during growth in vitro.
FIG. 3.
Hot-water extracts to detect PG fragments in the cytoplasm. (A) The PG of strains MS11 (wild type), DG132 (ampG), and DG142 (ampD) were labeled by growth in medium containing [6-3H]glucosamine. After a 45-min chase period, cytoplasmic extracts were prepared, and the material was separated by size-exclusion chromatography. The PG monomer peak shown in panel A was analyzed by C18 reversed-phase HPLC (B). The dotted line shows the retention times of PG monomer standards. Identities of the ampD cytosolic monomers are predicted based on their elution from the sizing column and retention times on the C18 column. anh, anhydro; G, N-acetylglucosamine; M, N-acetylmuramic acid.
Mutation of ampD diminishes PG fragment release.
AmpD in E. coli is a cytoplasmic N-acetylmuramyl-l-alanine amidase that specifically recognizes and cleaves anhydro PG monomers (26, 28). We made an ampD deletion mutant so that we could assess effects that the lack of PG recycling might have on growth characteristics. It has been speculated that the purpose of PG recycling is to recover nutrients. However, measurements of growth in culture showed that neither the ampG mutant nor the ampD mutant was deficient in growth under these conditions (data not shown).
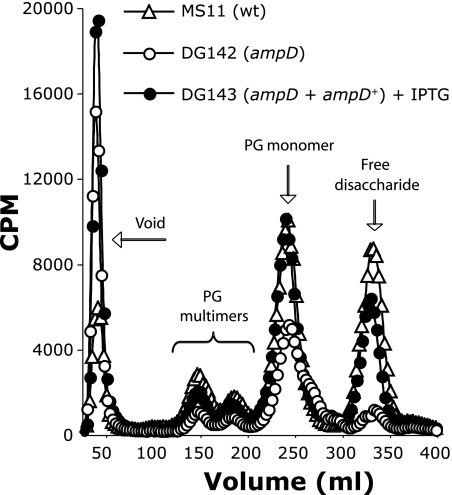
We measured PG fragment release from the ampD mutant and from the wild-type strain. The ampD deletion mutant (DG142) exhibited a decrease in the release of all small PG fragments including a 40% reduction in PG monomers and a dramatic reduction in free disaccharide levels compared to the wild type (Fig. 4). Also, an additional species appeared in the ampD profile as an unresolved peak with a slightly smaller molecular weight compared to that of PG monomers. We speculate that this peak may represent PG monomers from which the N-acetylglucosamine has been cleaved. Complementation with a wild-type copy of ampD at another location on the chromosome restored the wild-type phenotype.
FIG. 4.
An ampD deletion mutant exhibits a decrease in the release of PG multimers, PG monomers, and free disaccharide. N. gonorrhoeae strains MS11 (wild type), DG142 (ampD), and DG143 (ampD + ampD+, complemented strain) were labeled by growth in glucose-deficient medium containing [6-3H]glucosamine. Supernatants containing radiolabeled PG fragments were collected after 2.5 h of growth and passed over tandem size-exclusion columns. Fractions containing radiolabeled PG fragments were detected by scintillation counting.
Examination of the cytoplasmic PG fragments by using hot-water extracts showed that the ampD mutant accumulated PG monomers (Fig. 3A). The ampD mutant was predicted to build up 1,6-anhydro-N-acetylmuramic acid (anhMurNAc) tripeptide monomers, since the mutant should not be able to degrade imported PG monomers further than the removal of GlcNAc and the terminal alanine (Fig. 1). Reversed-phase HPLC separation of the monomer peak from the ampD hot-water extracts showed that this material was composed of three separate monomers (Fig. 3B). The major peak is the expected anhMurNAc tripeptide. A minor peak is likely the related anhMurNAc tetrapeptide. A third peak was identified, with the same retention time as GlcNAc-anhMurNAc tetrapeptide. The absence of the PG biosynthesis precursor seen in the wild-type profile and the accumulation of PG monomers in the cytoplasm of the ampD mutant demonstrate that AmpD is required for the breakdown and reuse of imported PG monomers.
Mutation of ampD results in increased metabolism of PG fragments.
The large reduction in free disaccharide release by the ampD mutant (Fig. 4) was surprising since we had previously found that a different amidase, AmiC, is responsible for the generation of free disaccharide in N. gonorrhoeae. In the amiC mutant, free disaccharide release was completely abolished (11). The generation of free disaccharide should occur in the periplasm by the combined actions of a lytic transglycosylase and an amidase. Free disaccharide would then diffuse across the outer membrane. Why then should a mutation affecting a cytoplasmic amidase affect the generation or release of a PG fragment in the periplasm? We considered three possibilities to explain these results. (i) AmiC might be inhibited or produced in lower amounts in the absence of ampD. (ii) AmpD might be responsible for the production of free disaccharide. (iii) A buildup of PG monomers in the cytoplasm might affect the transcription of genes for PG fragment degradation, or it might affect the activity of enzymes involved in PG fragment breakdown.
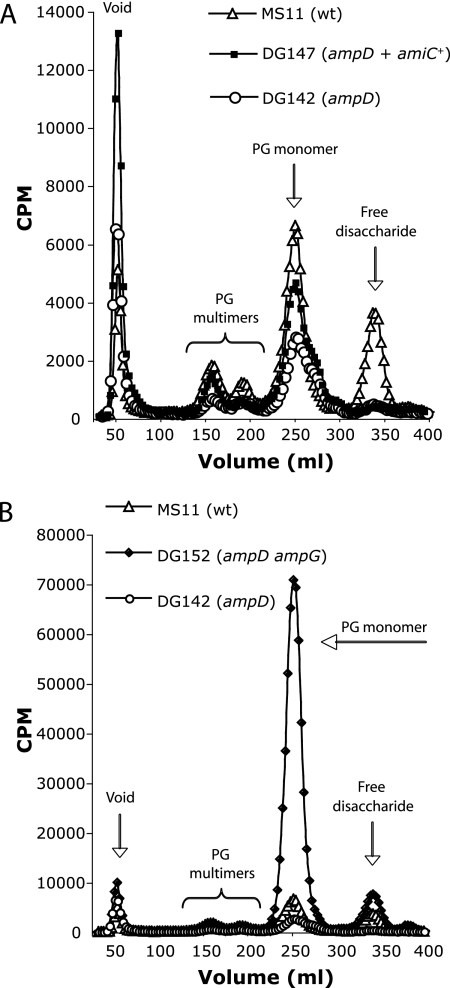
If the amounts of AmiC were reduced or if the activity of AmiC were inhibited in the ampD mutant, then the overexpression of amiC might restore free disaccharide production. Therefore, we transformed the ampD mutant strain DG142 with a construct that carries amiC under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible lac promoter-operator pDG017 (11). PG fragment release was not restored by the overexpression of amiC (Fig. 5A), suggesting that the loss of free disaccharide release was not due to effects on AmiC.
FIG. 5.
Free disaccharide release by the ampD mutant is not restored by the expression of amiC but is restored by the mutation of ampG. PG fragments released from strains MS11 (wild type), DG142 (ampD), and DG147 (ampD + amiC+) induced with IPTG and from the strain DG152 (ampD ampG) were separated by size and detected by scintillation counting. The expression of amiC does not significantly alter the release of PG fragments from the ampD mutant (A). Mutation of ampG restores the amount of free disaccharide released by the ampD mutant to levels that are slightly higher than those observed with the wild-type strain (B).
By introducing an ampG mutation into the ampD background, we were able to separate events occurring in the periplasm that affect PG breakdown from those that occur in the cytoplasm. An ampG mutant should be unable to transport PG monomers or free disaccharide into the cytoplasm, and thus no buildup of PG monomers should occur in the double mutant. The PG release profile for the ampG ampD double mutant showed that free disaccharide release was restored to wild-type levels (Fig. 5B). This result shows that ampD is not required for free disaccharide production. This result also demonstrates that transport of PG fragments into the cytoplasm is necessary for the reduced release of PG fragments seen with the ampD mutant.
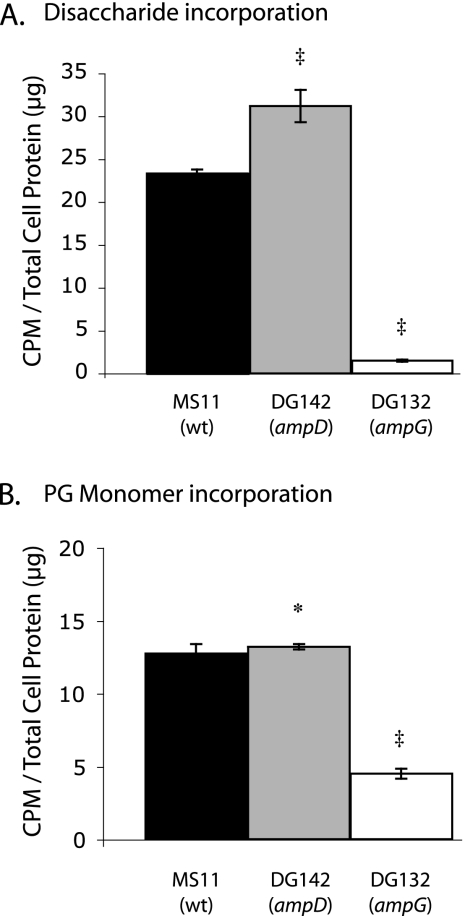
The decreased release of free disaccharide by the ampD mutant suggested that this mutant was metabolizing more of the PG fragments that were generated by PG breakdown; i.e., free disaccharide from PG degradation was not being released at wild-type amounts because these PG fragments were being transported into the cytoplasm, broken down into N-acetylglucosamine and N-acetylmuramic acid, and incorporated into non-cell wall material to a greater extent than in the wild-type strain. To test this hypothesis, we added purified radiolabeled PG fragments to gonococci and measured the incorporation of the labeled PG material into the cells. Labeled free disaccharide or PG monomers were purified and added to the wild-type, ampD, or ampG gonococci growing in log-phase culture. Free disaccharide was incorporated to a greater extent by the ampD strain than by the wild-type strain, confirming increased PG fragment metabolism in the ampD mutant (Fig. 6A). PG monomers were incorporated in equal amounts by the wild-type and ampD strains (Fig. 6B). The ampG mutant showed minimal incorporation of free disaccharide or PG monomers, consistent with the requirement for AmpG for transport of anhydro-PG fragments into the cytoplasm (6). The results shown in Fig. 6A should not be compared to those shown in Fig. 6B to evaluate the relative ability of AmpG to transport PG monomers versus free disaccharide, because different amounts of radioactivity were used in the two experiments and because the abilities of PG monomers and free disaccharide to cross the outer membrane are likely different.
FIG. 6.
The ampD mutant incorporates more radiolabeled free disaccharide (A) than the wild type. The ampG mutant is deficient in the uptake of both PG monomers (B) and free anhydro disaccharides (A). The N. gonorrhoeae MS11 (wild-type), DG142 (ampD), and DG132 (ampG) strains were labeled by growth in medium containing glucose and purified PG fragments for 1 h. Cells were centrifuged and resuspended in water, and radioactivity was measured by scintillation counting to determine the level of incorporation of radiolabeled PG monomers, free disaccharide, or glucosamine (data not shown). Counts per minute were normalized for total cell protein. The values shown are the averages of three cultures per strain with at least three measurements from each culture. Error bars represent the standard deviations. ‡, Student's t test P value of <0.01 compared to that of the wild type; asterisk, not statistically different from that of the wild type.
Together these results suggest a model in which AmpG acts to transport PG fragments across the cytoplasmic membrane, and AmpD breaks down the monomers to peptides and disaccharide that are preferentially incorporated into new cell wall synthesis. In the ampD mutant, a buildup of PG monomers causes increased metabolism of PG fragments, and N-acetylglucosamine released from PG monomers or N-acetylglucosamine and N-acetylmuramic acid derived from free disaccharide, is incorporated into PG and non-PG cell material.
DISCUSSION
It has been speculated that the reason that N. gonorrhoeae sheds PG monomers into the extracellular milieu during growth is because it is deficient in PG fragment uptake and recycling. However, we have shown that gonococci have a functional PG recycling system. Homologues of five PG fragment-recycling proteins were found encoded in the gonococcal genome. No homologue was found for MurQ, which in E. coli, acts to convert MurNAc-phosphate to GlcNAc-phosphate (51). N. gonorrhoeae may not perform the MurQ function and may be able to use MurNAc-phosphate directly, or gonococci may use an unrelated enzyme for this purpose. The mutation of ampG resulted in a sevenfold increase in PG monomer release. This result suggests that gonococci normally recycle 85% of the PG monomers liberated by peptidoglycanases during growth and division.
The role of gonococcal AmpG in the uptake of PG fragments was confirmed by our results showing that exogenously added PG monomers or free disaccharide were taken up efficiently by the wild-type strain but were taken up poorly by the ampG mutant (Fig. 6). Given this result, it is somewhat surprising that the ampG mutant did not show a significant increase in the release of free disaccharide (Fig. 2). Instead, a very minor increase in free disaccharide loss is seen with the ampG mutant compared to that of the wild type, and a similar slight increase in free disaccharide loss is seen with the ampG ampD double mutant compared to that of the wild type (Fig. 5B). Two possible explanations may account for these results. One possibility is that gonococci may normally release most of the free disaccharide produced. The mutation of ampG would then affect only the small amount normally recycled. Perhaps gonococcal AmpG has increased specificity for PG monomers compared to that of free disaccharide. Such a specificity would make sense from an immunological viewpoint, since it could be advantageous for the bacteria to control the release of the proinflammatory PG monomers under some conditions, whereas controlling the release of the apparently immunologically inert free disaccharide would be of less importance. A second possibility is that gonococci have a second transporter for free disaccharide uptake, and it continues to operate in the ampG mutant. This possibility seems less likely, since the ampG mutant is dramatically reduced in free disaccharide uptake (Fig. 6A) and shows no PG fragments in the cytoplasm (Fig. 3A).
A deletion of ampD affected PG fragment recycling but not in the way we expected. The ampD mutants should be unable to cleave the bond between the N-acetylmuramic acid and the stem peptide in imported PG monomers and thus should be unable to use the N-acetylmuramic acid for cell wall biosynthesis or general metabolism. An ampD mutant might show increased release of PG monomers, since the mutant is unable to fully degrade these molecules. The appearance of an additional monomer peak of a smaller molecular size on the ampD fragment release profile is consistent with the buildup of anhydromuramic acid tripeptide monomers in the ampD mutant (Fig. 4). These fragments may be generated by NagZ activity in the cytoplasm and may be released by autolysis in a portion of the population. The surprising result was that the ampD mutant's release of free disaccharide was reduced (Fig. 4). In pulse-chase experiments, the liberated PG fragments were found in the cell as PG monomers in the cytoplasm and as other, non-PG cell constituents (Fig. 3). Since the labeled disaccharide was not released into the medium, we hypothesized that these PG fragments were being metabolized and used for the synthesis of other cell constituents at a higher rate than in the wild-type strain. This hypothesis was confirmed by the greater degree of incorporation of exogenously added free disaccharide by the ampD mutant (Fig. 6A). Thus, it appears that gonococci regulate PG fragment recycling in response to changes in cytoplasmic levels of PG monomer.
The mechanism for the increased utilization of PG free disaccharide fragments by the ampD mutant is unclear. It could be due to increased uptake of PG fragments or increased breakdown of the fragments in the cytoplasm. Increased uptake of disaccharide might occur by an increase in AmpG production or by an increase in a second, hypothetical, free disaccharide transporter. An increase in NagZ levels or function could result in increased levels of N-acetylglucosamine in the cytoplasm that could then be used for general metabolism or cell wall synthesis. It is known that Citrobacter freundii can respond to increased levels of PG monomers by transcriptional regulation. AmpR binds to PG monomers and derepresses the transcription of ampC, encoding a beta-lactamase (31). Gonococci, like many gram-negative bacterial species, do not encode an obvious AmpR orthologue. However, changes in PG fragment metabolism in the ampD mutant suggest that gonococci may have some other transcriptional regulator to control PG fragment metabolism.
Since it is now clear that gonococci have an efficient PG uptake and recycling system, then why do gonococci produce a PG-related inflammatory response and ciliated cell death, while bacteria such as E. coli do not? One possibility is that PG fragment release is regulated during infection. It is clear from the results shown in Fig. 2 and Fig. 4 that AmpG and AmpD have significant effects on PG fragment release. By regulating the amounts of these proteins, gonococci could promote the inflammatory response or decrease it. Gonococci release multiple proinflammatory molecules including LOS (19), porin (35), DNA (21), and PG (47), suggesting that under some circumstances an inflammatory response favors the bacteria. Gonococci often cause asymptomatic infections in women (41), suggesting that the release of proinflammatory molecules such as PG may be reduced during those infections. A second possibility is that gonococci do not differ from E. coli in the overall amounts of PG fragments released but only in the types of fragments released. It was recently shown that E. coli produces a periplasmic amidase that specifically degrades PG monomers (50). This process would detoxify the liberated fragments (12, 34), and the released fragments would not provoke an immune response.
In summary, we have demonstrated a functional PG recycling pathway in N. gonorrhoeae, where gonococcal AmpG and AmpD proteins are responsible for the translocation and metabolism of PG fragments. Furthermore, we have demonstrated that an ampD mutant deficient in PG monomer degradation has increased metabolism of PG fragments and decreased release of free disaccharide from PG into the medium, suggesting that gonococci regulate PG fragment degradation based on PG fragment levels in the cytoplasm.
Acknowledgments
This work was supported by NRSA AI054325 awarded to D.L.G. and an award from the University of Wisconsin School of Medicine Research Committee to J.P.D.
Footnotes
Published ahead of print on 4 April 2008.
REFERENCES
- 1.Burroughs, M., C. Cabellos, S. Prasad, and E. Tuomanen. 1992. Bacterial components and the pathophysiology of injury to the blood-brain barrier: does cell wall add to the effects of endotoxin in gram-negative meningitis? J. Infect. Dis. 165(Suppl. 1)S82-S85. [DOI] [PubMed] [Google Scholar]
- 2.Burroughs, M., S. Prasad, C. Cabellos, P. M. Mendelman, and E. Tuomanen. 1993. The biological activities of peptidoglycan in experimental Haemophilus influenzae meningitis. J. Infect. Dis. 167464-468. [DOI] [PubMed] [Google Scholar]
- 3.Chahboune, A., M. Decaffmeyer, R. Brasseur, and B. Joris. 2005. Membrane topology of the Escherichia coli AmpG permease required for recycling of cell wall anhydromuropeptides and AmpC β-lactamase induction. Antimicrob. Agents Chemother. 491145-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4702-707. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, Q., H. Li, K. Merdek, and J. T. Park. 2000. Molecular characterization of the β-N-acetylglucosaminidase of Escherichia coli and its role in cell wall recycling. J. Bacteriol. 1824836-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, Q., and J. T. Park. 2002. Substrate specificiy of the AmpG permease required for recycling of cell wall anhydro-muropeptides. J. Bacteriol. 1846434-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cloud, K. A., and J. P. Dillard. 2002. A lytic transglycosylase of Neisseria gonorrhoeae is involved in peptidoglycan-derived cytotoxin production. Infect. Immun. 702752-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cookson, B. T., H. L. Cho, L. A. Herwaldt, and W. E. Goldman. 1989. Biological activities and chemical composition of purified tracheal cytotoxin of Bordetella pertussis. Infect. Immun. 572223-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dillard, J. P. 2006. Genetic manipulation of Neisseria gonorrhoeae, p. 4A.2.1-4A.2.19. In R. Coico, T. Kowalik, J. M. Quarles, B. Stevenson, R. K. Tayor, and A. E. Simon (ed.), Current protocols in microbiology. John Wiley & Sons, Inc., New York, NY. [DOI] [PubMed]
- 10.Dokter, W. H. A., A. J. Dijkstra, S. B. Koopmans, B. K. Stulp, W. Keck, M. R. Halie, and E. Vellenga. 1994. G(Anh)MTetra, a natural bacterial cell wall breakdown product induces interleukin-1beta and interleukin-6 expression in human monocytes. J. Biol. Chem. 2694201-4206. [PubMed] [Google Scholar]
- 11.Garcia, D. L., and J. P. Dillard. 2006. AmiC functions as an N-acetylmuramyl-l-alanine amidase necessary for cell separation and can promote autolysis in Neisseria gonorrhoeae. J. Bacteriol. 1887211-7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girardin, S. E., I. G. Boneca, L. A. Carneiro, A. Antignac, M. Jehanno, J. Viala, K. Tedin, M. K. Taha, A. Labigne, U. Zahringer, A. J. Coyle, P. S. DiStefano, J. Bertin, P. J. Sansonetti, and D. J. Philpott. 2003. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 3001584-1587. [DOI] [PubMed] [Google Scholar]
- 13.Girardin, S. E., R. Tournebize, M. Mavris, A. L. Page, X. Li, G. R. Stark, J. Bertin, P. S. DiStefano, M. Yaniv, P. J. Sansonetti, and D. J. Philpott. 2001. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2736-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman, W. E., D. G. Klapper, and J. B. Baseman. 1982. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect. Immun. 36782-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodell, E. W. 1985. Recycling of murein by Escherichia coli. J. Bacteriol. 163305-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodell, E. W., M. Fazio, and A. Tomasz. 1978. Effect of benzylpenicillin on the synthesis and structure of the cell envelope of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 13514-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodell, E. W., and U. Schwarz. 1985. Release of cell wall peptides into culture medium by exponentially growing Escherichia coli. J. Bacteriol. 162391-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenway, D. L., and H. R. Perkins. 1985. Turnover of the cell wall peptidoglycan during growth of Neisseria gonorrhoeae and Escherichia coli. Relative stability of newly synthesized material. J. Gen. Microbiol. 131253-263. [DOI] [PubMed] [Google Scholar]
- 19.Gregg, C. R., M. A. Melly, C. G. Hellerqvist, J. G. Coniglio, and Z. A. McGee. 1981. Toxic activity of purified lipopolysaccharide of Neisseria gonorrhoeae for human fallopian tube mucosa. J. Infect. Dis. 143432-439. [DOI] [PubMed] [Google Scholar]
- 20.Gunn, J. S., and D. C. Stein. 1996. Use of a nonselective transformation technique to construct a multiply restriction/modification-deficient mutant of Neisseria gonorrhoeae. Mol. Gen. Genet. 251509-517. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton, H. L., N. M. Domínguez, K. J. Schwartz, K. T. Hackett, and J. P. Dillard. 2005. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol. Microbiol. 551704-1721. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton, H. L., K. J. Schwartz, and J. P. Dillard. 2001. Insertion-duplication mutagenesis of Neisseria: use in characterization of DNA transfer genes in the gonococcal genetic island. J. Bacteriol. 1834718-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hebeler, B. H., and F. E. Young. 1976. Chemical composition and turnover of peptidoglycan in Neisseria gonorrhoeae. J. Bacteriol. 1261180-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heiss, L. N., S. A. Moser, E. R. Unanue, and W. E. Goldman. 1993. Interleukin-1 is linked to the respiratory epithelial cytopathology of pertussis. Infect. Immun. 613123-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Höltje, J.-V. 1998. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol. Mol. Biol. Rev. 62181-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Höltje, J. V., U. Kopp, A. Ursinus, and B. Wiedemann. 1994. The negative regulator of beta-lactamase induction AmpD is a N-acetyl-anhydromuramyl-L-alanine amidase. FEMS Microbiol. Lett. 122159-164. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs, C., L. J. Huang, E. Bartowsky, S. Normark, and J. T. Park. 1994. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for beta-lactamase induction. EMBO J. 134684-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs, C., B. Joris, M. Jamin, K. Klarsov, J. van Beeumen, D. Mengin-Lecreulx, J. van Heijenoort, J. T. Park, S. Normark, and J. M. Frere. 1995. AmpD, esstenial for both beta-lactamase regulation and cell wall recycling, is a novel cytosolic N-acetylmuramyl-L-alanine amidase. Mol. Microbiol. 15553-559. [DOI] [PubMed] [Google Scholar]
- 29.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and C. L. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 851274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohler, P. L., H. L. Hamilton, K. Cloud-Hansen, and J. P. Dillard. 2007. AtlA functions as a peptidoglycan lytic transglycosylase in the Neisseria gonorrhoeae type IV secretion system. J. Bacteriol. 1895421-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindberg, F., L. Westman, and S. Normark. 1985. Regulatory components in Citrobacter freundii ampC beta-lactamase induction. Proc. Natl. Acad. Sci. USA 824620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindquist, S., K. Weston-Hafer, H. Schmidt, C. Pul, G. Korfmann, J. Erickson, C. Sanders, H. H. Martin, and S. Normark. 1993. AmpG, a signal transducer in chromosomal beta-lactamase induction. Mol. Microbiol. 9703-715. [DOI] [PubMed] [Google Scholar]
- 33.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193265-275. [PubMed] [Google Scholar]
- 34.Luker, K., A. Tyler, G. Marshall, and W. Goldman. 1995. Tracheal cytotoxin structural requirements for respiratory epithelial damage in pertussis. Mol. Microbiol. 16733-743. [DOI] [PubMed] [Google Scholar]
- 35.Massari, P., S. Ram, H. Macleod, and L. M. Wetzler. 2003. The role of porins in neisserial pathogenesis and immunity. Trends Microbiol. 1187-93. [DOI] [PubMed] [Google Scholar]
- 36.Melly, M. A., C. R. Gregg, and Z. A. McGee. 1981. Studies of toxicity of Neisseria gonorrhoeae for human fallopian tube mucosa. J. Infect. Dis. 143423-431. [DOI] [PubMed] [Google Scholar]
- 37.Melly, M. A., Z. A. McGee, and R. S. Rosenthal. 1984. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J. Infect. Dis. 149378-386. [DOI] [PubMed] [Google Scholar]
- 38.Mengin-Lecreulx, D., J. van Heijenoort, and J. T. Park. 1996. Identification of the mpl gene encoding UDP-N-acetylmuramate: l-alanyl-γ-d-glutamyl-meso-diaminopimelate ligase in Escherichia coli and its role in recycling of cell wall peptidoglycan. J. Bacteriol. 1785347-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mielcarek, N., A. S. Debrie, D. Raze, J. Quatannens, J. Engle, W. E. Goldman, and C. Locht. 2006. Attenuated Bordetella pertussis: new live vaccines for intranasal immunisation. Vaccine 24(Suppl. 2)S2-54-5. [DOI] [PubMed] [Google Scholar]
- 40.Morse, S. A., and L. Bartenstein. 1974. Factors affecting autolysis of Neisseria gonorrhoeae. Proc. Soc. Exp. Biol. Med. 1451418-1421. [DOI] [PubMed] [Google Scholar]
- 41.Pariser, H. 1972. Asymptomatic gonorrhea. Med. Clin. N. Am. 561127-1132. [DOI] [PubMed] [Google Scholar]
- 42.Park, J. T. 1995. Why does Escherichia coli recycle its cell wall peptides? Mol. Microbiol. 17421-426. [DOI] [PubMed] [Google Scholar]
- 43.Rosenthal, R. S. 1979. Release of soluble peptidoglycan from growing gonococci: hexaminidase and amidase activities. Infect. Immun. 24869-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenthal, R. S., and R. Dziarski. 1994. Isolation of peptidoglycan and soluble peptidoglycan fragments. Method Enzymol. 235235-285. [DOI] [PubMed] [Google Scholar]
- 45.Rosenthal, R. S., W. Nogami, B. T. Cookson, W. E. Goldman, and W. J. Folkening. 1987. Major fragment of soluble peptidoglycan released from growing Bordetella pertussis is tracheal cytotoxin. Infect. Immun. 552117-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 47.Sinha, R. K., and R. S. Rosenthal. 1980. Release of soluble peptidoglycan from growing gonococci: demonstration of anhydro-muramyl-containing fragments. Infect. Immun. 29914-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swanson, J., S. J. Kraus, and E. C. Gotschlich. 1971. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J. Exp. Med. 134886-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Templin, M. F., A. Ursinus, and J. V. Holtje. 1999. A defect in cell wall recycling triggers autolysis during the stationary growth phase of Escherichia coli. EMBO J. 184108-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uehara, T., and J. T. Park. 2007. An anhydro-N-acetylmuramyl-l-alanine amidase with broad specificity tethered to the outer membrane of Escherichia coli. J. Bacteriol. 1895634-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uehara, T., K. Suefuji, T. Jaeger, C. Mayer, and J. T. Park. 2006. MurQ etherase is required by Escherichia coli in order to metabolize anhydro-N-acetylmuramic acid obtained either from the environment or from its own cell wall. J. Bacteriol. 1881660-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uehara, T., K. Suefuji, N. Valbuena, B. Meehan, M. Donegan, and J. T. Park. 2005. Recycling of the anhydro-N-acetylmuramic acid derived from cell wall murein involves a two-step conversion to N-acetylglucosamine-phosphate. J. Bacteriol. 1873643-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vossoughi, J. 1984. A simple, quick and accurate method to measure the area under curves. Exp. Tech. 826-27. [Google Scholar]
- 54.Vötsch, W., and M. F. Templin. 2000. Characterization of a beta-N-acetylglucosaminidase of Escherichia coli and elucidation of its role in muropeptide recycling and beta-lactamase induction. J. Biol. Chem. 27539032-39038. [DOI] [PubMed] [Google Scholar]
- 55.Yem, D. W., and H. C. Wu. 1976. Purification and properties of β-N-acetylglucosaminidase from Escherichia coli. J. Bacteriol. 125324-331. [DOI] [PMC free article] [PubMed] [Google Scholar]