Abstract
Mycobacterium tuberculosis is predicted to subsist on alternative carbon sources during persistence within the human host. Catabolism of odd- and branched-chain fatty acids, branched-chain amino acids, and cholesterol generates propionyl-coenzyme A (CoA) as a terminal, three-carbon (C3) product. Propionate constitutes a key precursor in lipid biosynthesis but is toxic if accumulated, potentially implicating its metabolism in M. tuberculosis pathogenesis. In addition to the well-characterized methylcitrate cycle, the M. tuberculosis genome contains a complete methylmalonyl pathway, including a mutAB-encoded methylmalonyl-CoA mutase (MCM) that requires a vitamin B12-derived cofactor for activity. Here, we demonstrate the ability of M. tuberculosis to utilize propionate as the sole carbon source in the absence of a functional methylcitrate cycle, provided that vitamin B12 is supplied exogenously. We show that this ability is dependent on mutAB and, furthermore, that an active methylmalonyl pathway allows the bypass of the glyoxylate cycle during growth on propionate in vitro. Importantly, although the glyoxylate and methylcitrate cycles supported robust growth of M. tuberculosis on the C17 fatty acid heptadecanoate, growth on valerate (C5) was significantly enhanced through vitamin B12 supplementation. Moreover, both wild-type and methylcitrate cycle mutant strains grew on B12-supplemented valerate in the presence of 3-nitropropionate, an inhibitor of the glyoxylate cycle enzyme isocitrate lyase, indicating an anaplerotic role for the methylmalonyl pathway. The demonstrated functionality of MCM reinforces the potential relevance of vitamin B12 to mycobacterial pathogenesis and suggests that vitamin B12 availability in vivo might resolve the paradoxical dispensability of the methylcitrate cycle for the growth and persistence of M. tuberculosis in mice.
Mycobacterium tuberculosis is an obligate human pathogen that is expected to adapt metabolically to conditions that are often hostile and nutrient poor during successive cycles of infection, replication, persistence, and transmission. In particular, glucose deficiency and an abundance of fatty acids are thought to dictate mycobacterial metabolism during infection (3, 35), consistent with the complex repertoire of genes involved in lipid metabolism in the M. tuberculosis genome (10). Subsistence on fatty acids requires the sequential action of the catabolic β-oxidation cycle and, where glycolytic substrates are limiting, the anaplerotic glyoxylate cycle, which enables the assimilation of derivative two-carbon (C2) acetyl-coenzyme A (CoA) subunits (37). In addition to producing acetyl-CoA, β-oxidation of odd- and branched-chain fatty acids yields the C3 subunit propionyl-CoA. This metabolite can also be generated by the catabolism of branched-chain amino acids (24) and cholesterol. Recently, a cassette of genes involved in the catabolism of the A and B rings of cholesterol to propionyl-CoA, pyruvate, and other metabolites was identified in actinomycetes, including members of the M. tuberculosis complex (27, 52). Although the relevance of cholesterol as a carbon source for M. tuberculosis in vivo has yet to be established, the likely action of this catabolic pathway during intracellular growth and survival of M. tuberculosis (52) suggests that it may constitute an additional, and potentially significant, source of propionyl-CoA in this pathogen.
Propionyl-CoA is a key precursor in several lipid biosynthetic pathways in M. tuberculosis (28); however, while providing a high-energy metabolite, the accumulation of propionate is toxic to the cell, and as such, efficient mechanisms are required for its disposal (5). This dual nature implies a central role for propionate metabolism in the growth and persistence of M. tuberculosis in vivo (18, 37). Evidence that a shift to catabolism of host lipids potentiates M. tuberculosis virulence through the increased biosynthesis of the virulence factors phthiocerol dimycocerosate and sulfolipid 1 (25) strengthens this contention. Recently, the possibility that the methylcitrate cycle might constitute the dominant pathway for propionate metabolism in vivo was investigated (37). The two key findings that motivated this investigation were the observed upregulation of methylcitrate cycle genes in the intracellular environment and in the mouse lung (34, 48) and the inability of a Δicl1 Δicl2 double mutant of M. tuberculosis Erdman to grow on propionate in vitro or establish an infection in mice (36). The unusual involvement of icl1 and icl2 in both the methylcitrate cycle (as 2-methylisocitrate lyase [MCL]) and the glyoxylate cycle (as isocitrate lyase [ICL]) (18, 37) in M. tuberculosis, however, complicates any interpretation of the relative importance of these pathways to M. tuberculosis metabolism. Moreover, the demonstration by Muñoz-Elías et al. that a mutant of M. tuberculosis Erdman lacking two earlier genes in the methylcitrate pathway, prpD, encoding methylcitrate dehydratase (MCD), and prpC, encoding methylcitrate synthase (MCS), is unable to grow on propionate in vitro but establishes a wild-type infection in mice suggested the possibility that propionate might be oxidized via an alternative route in vivo (37).
The methylmalonyl pathway offers a potentially attractive alternative to the methylcitrate cycle (8, 38, 49, 51, 54, 55); however, the function of this pathway and its role in propionate metabolism in M. tuberculosis has remained unexplored. The final step in the methylmalonyl pathway is the reversible intramolecular rearrangement of (R)-methylmalonyl-CoA to succinyl-CoA (Fig. 1). This reaction is catalyzed by the mutAB-encoded methylmalonyl-CoA mutase (MCM), a vitamin B12-dependent enzyme (33). We sought to address whether the mutAB-encoded MCM is functional in M. tuberculosis and to investigate the possibility that the methylmalonyl pathway provides an alternative to the methylcitrate cycle during growth on propionate. During concurrent studies on other vitamin B12-dependent enzymes in M. tuberculosis (56), we demonstrated the functionality of the B12-dependent methionine synthase (MetH) and the operation of a B12-dependent regulatory mechanism (a B12 riboswitch) (56), potentially implicating vitamin B12 metabolism in M. tuberculosis pathogenesis. Importantly, those studies revealed that M. tuberculosis does not produce vitamin B12 in vitro but has the capacity to transport and utilize this cofactor when exogenously supplied in the form of cyanocobalamin. Although the extent to which vitamin B12 availability dictates the activity of the B12-dependent enzymes in vivo remains unclear, the implication of these observations for the function of the vitamin B12-dependent MCM was immediately evident. In this paper, we demonstrate the functionality of the methylmalonyl pathway in M. tuberculosis under conditions in which vitamin B12 is not limiting and discuss the implications of these findings for the growth of M. tuberculosis on fatty acid substrates.
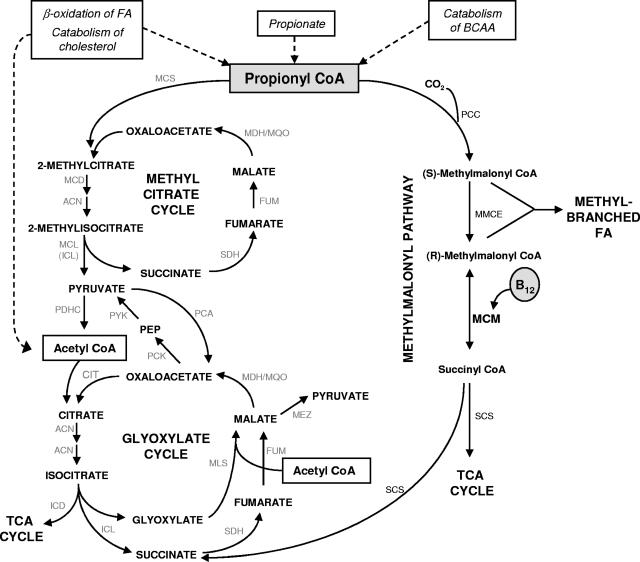
FIG. 1.
Predicted pathways of propionate metabolism in M. tuberculosis. Catabolism of alternative carbon sources including odd- and branched-chain fatty acids (FA), branched-chain amino acids (BCAA), and cholesterol generates propionyl-CoA as a three-carbon (C3) terminal product. Previous studies have established the importance of the glyoxylate and methylcitrate cycles for anaplerosis and propionyl-CoA metabolism, respectively, during fatty acid catabolism by M. tuberculosis (36, 37). Glyoxylate cycle enzymes are the isocitrate lyases (ICL1/ICL2) and malate synthase (MLS); ICL1 and ICL2 also provide MCL activity in M. tuberculosis (18, 37). Other enzymes of the methylcitrate cycle include MCS and MCD. Methylmalonyl pathway enzymes are PCC, MMCE, and MCM. Pyruvate is produced from malate by malic enzyme (MEZ) or from oxaloacetate by the sequential action of pyruvate carboxykinase (PCK) and pyruvate kinase (PYK); the coupled decarboxylation of pyruvate by the pyruvate dehydrogenase complex (PDHC) yields acetyl-CoA. Anaplerosis during carbohydrate catabolism is by carboxylation of pyruvate to oxaloacetate by pyruvate carboxylase (PCA). ACN, aconitase; CIT, citrate synthase; FUM, fumarase; ICD, isocitrate dehydrogenase; MDH, malate dehydrogenase; MQO, malate:quinine oxidoreductase; PEP, phosphoenolpyruvate; SCS, succinate synthase; SDH, succinate dehydrogenase.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains and plasmids used in this study are detailed in Table S1 in the supplemental material. Wild-type M. tuberculosis strain H37Rv (ATCC 25618) and mutant derivatives thereof were grown standing at 37°C in Middlebrook 7H9 medium (Merck) supplemented with 0.2% glycerol, oleic acid-albumin-dextrose-catalase enrichment (Merck), and 0.05% Tween 80. Sodium propionate, valeric acid, and heptadecanoic acid were purchased from Sigma. For carbon utilization experiments, bacteria were grown in 7H9 medium containing 0.5% albumin, 0.085% NaCl, 0.05% Tween 80, and sodium propionate or valeric acid at a concentration of 0.1% (10 mM in both cases). The pH of the valeric acid-containing medium was adjusted to 6.8 with 10 M NaOH prior to use. In the case of heptadecanoic acid, a prewarmed 0.2% stock solution of heptadecanoic acid was added to the medium at a final concentration of 0.007% (0.25 mM). The lower final concentration of the carbon source in this case was attributable to the poor solubility of heptadecanoic acid in water, which limited the concentration that could be achieved. Unless otherwise indicated, vitamin B12 supplement (cyanocobalamin; Sigma) was included at a concentration of 10 μg/ml. Hygromycin and kanamycin were used in M. tuberculosis cultures at final concentrations of 50 and 25 μg/ml, respectively, and where indicated, 3-nitropropionate (3NP) (Sigma) was used at a concentration of 0.1 mM (36).
Construction of mutant strains.
A 7,660-bp EcoRI fragment of M. tuberculosis genomic DNA carrying the mutAB genes was obtained from the H37Rv bacterial artificial chromosome library clone Rv58 (7) and cloned into p2NIL (43) to form p2mutAB. An internal, 2,342-bp region of mutAB was deleted from p2mutAB by digestion with AscI and BglII. The fragment was blunt ended with Klenow fragment (Roche) and religated to create p2ΔmutAB. The ΔmutAB mutation created an out-of-frame fusion at the AscI/BglII junction and eliminated 213 amino acids from the C terminus of the 615-amino-acid MutA and 566 amino acids from the N terminus of the 750-amino-acid MutB (see Fig. S1 in the supplemental material). The lacZ-sacB marker gene cassette from pGOAL17 (43) was then inserted into the PacI site of p2ΔmutAB to create the suicide plasmid p2ΔmutAB17, which was used to construct the mutAB mutant of M. tuberculosis H37Rv by standard two-step allelic exchange mutagenesis using previously described methods (17, 43). Genetic reversion of the mutAB mutation in the ΔmutAB mutant strain was carried out by knock-in allelic exchange mutagenesis using the suicide plasmid p2mutAB17, which contains the full-length mutAB genes plus 1,431 bp of 3′- and 2,228 bp of 5′-flanking chromosomal sequences (see Table S1 in the supplemental material) and was produced by cloning the lacZ-sacB cassette from pGOAL17 into the PacI site of p2mutAB. The ΔprpDC, ΔmutAB ΔprpDC, and ΔmutAB::mutAB ΔprpDC mutants were constructed by the deletion of prpDC in the H37Rv, ΔmutAB, and ΔmutAB::mutAB backgrounds, respectively, using the previously described suicide plasmid pAU100 (37). The ΔprpDC mutant was complemented genetically by the integration of pPRPDC at the attB locus (37). All mutant strains were genotypically confirmed by Southern blot analysis, as previously described (17; data not shown) (see Fig. S1 in the supplemental material).
Gene expression analysis by real-time qRT-PCR.
The level of expression of the prpD gene (Rv1130) in H37Rv cells cultured under various conditions was determined by real-time quantitative reverse transcription-PCR (qRT-PCR). Cultures were grown to mid-log phase (optical density at 600 nm [OD600] of 0.4), bacteria were harvested, and RNA was extracted using TRIzol (Sigma). RNA (0.5 to 2.5 μg) was used to synthesize cDNA using previously described methods (13, 26). Real-time qRT-PCR was carried out using 2 μl of cDNA for amplification with the LightCycler FastStart DNA Master Sybr green I kit with Roche LightCycler software (version 1.5), and absolute quantifications of transcript levels using standard curves were performed with LightCycler software (version 4.0) (26). The primers used to determine prpD transcript levels were PRPDF (5′-GGTCTGGTAACCGCCTATGA) and PRPDR (5′-ATCGCGTGGTAGATGGTCTC), and those used to determine sigA transcript levels for normalization were described previously by Dawes et al. (12).
Statistics.
The paired t test was used to assess the statistical significance of pairwise comparisons using GraphPad Prism software (http://www.graphpad.com/quickcalcs/ttest1.cfm).
RESULTS
Identification of genes encoding the methylmalonyl pathway.
The key elements of propionate metabolism deduced from the genome sequence of M. tuberculosis H37Rv and determined experimentally (18, 37) are illustrated in Fig. 1. The methylcitrate cycle converts propionyl-CoA and oxaloacetate to pyruvate and succinate and was described previously (37). It comprises MCS and MCD enzymes encoded by prpC and prpD, respectively. M. tuberculosis is unusual in not encoding a dedicated MCL and, instead, relies on the glyoxylate cycle enzyme Icl1 (and Icl2, in strains of M. tuberculosis possessing a functional version of this enzyme) for both ICL and MCL activity (18, 37).
The methylmalonyl pathway, on the other hand, converts propionyl-CoA to succinyl-CoA via a methylmalonyl-CoA intermediate. In the first step, propionyl-CoA carboxylase (PCC) synthesizes (S)-methylmalonyl-CoA from propionyl-CoA. The PCC complex in M. tuberculosis, which has been characterized biochemically, comprises α, β, and ɛ subunits encoded by accA3 (Rv3285), accD5 (Rv3280), and accE5 (Rv3281), respectively (14, 32); notably, both accA3 and accE5 are predicted to be essential for the optimal growth of M. tuberculosis in vitro (47). Methylmalonyl-CoA epimerase (MMCE) then catalyzes the conversion of (S)-methylmalonyl-CoA to (R)-methylmalonyl-CoA, the epimer necessary for subsequent B12-dependent MCM activity. Based on a BLAST analysis (1), we have assigned Rv1322A as the M. tuberculosis MMCE with approximately 40% identity and 60% similarity to characterized MMCEs from other bacteria (2, 30). The final reaction of the methylmalonyl pathway, the isomerization of (R)-methylmalonyl-CoA to succinyl-CoA, is catalyzed by MCM, a heterodimer comprising subunits encoded by mutA (Rv1492) and mutB (Rv1493) (33). The α-subunit, MutB, contains the binding domain for the vitamin B12-derived cofactor adenosylcobalamin. A GTPase, MeaB, functions in the assembly and protection of MCM in other bacteria (22, 29, 40); a BLAST homology search (1) identified Rv1496 as being the putative M. tuberculosis meaB ortholog (57% identity and 70% similarity to MeaB from other organisms). Consistent with this designation, Rv1496 is located only 626 bp downstream of mutB, with the two genes separated by a predicted MazEF-type toxin-antitoxin module (Rv1494 and Rv1495) (42, 57).
Vitamin B12 supplementation enables growth of a prpDC mutant of H37Rv on propionate.
A prpDC deletion mutant of M. tuberculosis Erdman was described previously (37). M. tuberculosis Erdman carries a single icl2 (or aceA) gene encoding a functional Icl2 protein (18, 36). In H37Rv, this gene is split into two open reading frames, aceAa (Rv1915) and aceAb (Rv1916) (10, 37), which precludes the formation of Icl2 in H37Rv either as a single protein or through the association of the separate aceAa and aceAb modules (20). Consequently, ICL (and MCL) activity in H37Rv is provided exclusively by Icl1, unlike M. tuberculosis Erdman, where both Icl1 and Icl2 function as such (18, 36). Therefore, to enable a direct comparison of mutant strains of H37Rv disrupted in the methylmalonyl pathway (ΔmutAB) and/or the methylcitrate cycle (ΔprpDC), we constructed a prpDC deletion mutant of H37Rv using the previously described suicide plasmid pAU100 (37). As shown in Fig. 2A, the prpDC mutant of H37Rv was unable to grow in liquid medium containing propionate as the sole carbon source, recapitulating precisely the phenotype of the corresponding prpDC mutant of Erdman (37). Furthermore, complementation of the prpDC mutant of H37Rv with prpDC integrated at the attB site (ΔprpDC::prpDC) restored growth (Fig. 2A).
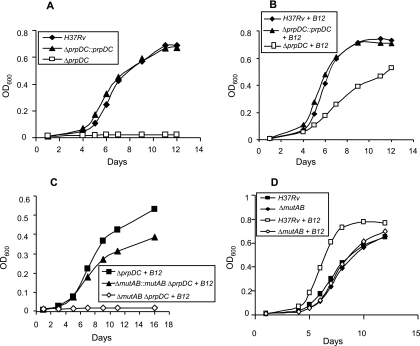
FIG. 2.
Vitamin B12 supplementation enables a prpDC deletion mutant of M. tuberculosis H37Rv to grow on propionate through the action of the mutAB-encoded MCM. (A) Growth on propionate. (B) Growth on propionate supplemented with 10 μg/ml vitamin B12. ⧫, H37Rv; □, ΔprpDC; ▴, complemented ΔprpDC mutant (ΔprpDC::prpDC). (C) Vitamin B12 supplementation enables mutAB-dependent growth of a prpDC-deficient mutant of H37Rv on propionate. ▪, ΔprpDC; ⋄, ΔmutAB ΔprpDC; ▴, ΔmutAB ΔprpDC double mutant containing the reverted mutAB allele (ΔmutAB::mutAB ΔprpDC). (D) Effect of loss of mutAB function on growth of H37Rv on propionate supplemented with vitamin B12. Shown are data for H37Rv with (□) and without (▪) vitamin B12 and the ΔmutAB mutant with (⋄) and without (⧫) vitamin B12. The growth of the complemented ΔmutAB mutant was equivalent to that of the wild type in the presence of vitamin B12 (data not shown). Data are OD600 values for a single representative experiment from three independent biological replicates.
The phenotype of the ΔprpDC mutant strongly implied the essentiality of the methylcitrate cycle for propionate metabolism in M. tuberculosis (37). Recently, we showed that supplementation of the growth medium with vitamin B12 allowed M. tuberculosis to overcome the loss of the apparently essential (B12-independent) methionine synthase MetE by enabling the activity of the alternative, B12-dependent, methionine synthase MetH (56). To establish that vitamin B12 limitation in vitro was similarly crippling the mutAB-encoded MCM (and, hence, the last step in the methylmalonyl pathway), we supplemented the propionate-containing growth medium with vitamin B12 (Fig. 2B). The addition of vitamin B12 (as cyanocobalamin) at a concentration of 10 μg/ml (56) restored the growth of the ΔprpDC mutant. The effect of the vitamin B12 supplement on the growth of the ΔprpDC mutant on propionate was analyzed over a concentration range of 1 to 20 μg/ml. The supplement was found to be saturating for growth at a concentration of 7.5 μg/ml (see Fig. S2 in the supplemental material) and, therefore, was used at a standard concentration of 10 μg/ml in all subsequent experiments. The vitamin B12-enabled growth of the prpDC mutant was significant, as it suggested the capacity of the MCM-dependent methylmalonyl pathway to support the metabolism of propionate independently of the methylcitrate cycle. It also reiterated previously reported evidence that M. tuberculosis does not produce sufficient adenosylcobalamin cofactor in vitro to enable the operation of either B12-dependent MCM or MetH enzymes (56). We also noted that strains in which both pathways for propionate metabolism were active displayed enhanced growth relative to those restricted to only one, suggesting that both the methylcitrate cycle and methylmalonyl pathways are required for optimal growth on propionate (Fig. 2A, B, and D).
Vitamin B12-dependent growth of the prpDC mutant on propionate requires mutAB.
To confirm that the methylmalonyl pathway alone was responsible for both the vitamin B12-dependent growth of the prpDC mutant on propionate and the vitamin B12-enhanced growth displayed by the wild-type strain, we disrupted MCM function in H37Rv through deletion mutagenesis of mutAB to create the ΔmutAB mutant. As expected, despite supplementation with vitamin B12, this strain displayed growth kinetics similar to those of H37Rv grown in propionate lacking vitamin B12 (Fig. 2D), suggesting that the enhanced growth seen in B12-supplemented propionate was due solely to the operation of the methylmalonyl pathway. In contrast, the abrogation of both methylcitrate cycle and methylmalonyl pathway function in the ΔmutAB ΔprpDC mutant eliminated the ability of M. tuberculosis to metabolize propionate, even in the presence of exogenous vitamin B12 (Fig. 2C). Reversion of the ΔmutAB mutation to wild-type mutAB prior to the introduction of the ΔprpDC mutation yielded a strain that was able to grow on propionate supplemented with vitamin B12 (Fig. 2C), thus confirming that vitamin B12-dependent growth of ΔprpDC on propionate is mediated by the mutAB-encoded MCM. However, the reversion mutant (ΔmutAB::mutAB ΔprpDC) did not grow as well as the ΔprpDC comparator strain (Fig. 2C). The reasons for this difference are unclear, but one possibility is that during the three rounds of allelic exchange mutagenesis required for its construction, the reversion mutant may have inadvertently acquired a second-site mutation(s) that adversely affected its growth on propionate. This difference notwithstanding, these observations nonetheless provided strong evidence for methylmalonyl pathway function in M. tuberculosis and suggested that this pathway provides an alternative to the methylcitrate cycle for growth on propionate where vitamin B12 is not limiting. This notion is consistent with the stimulatory effect of vitamin B12 on the growth of the wild-type strain (Fig. 2D).
A functional methylmalonyl pathway can bypass the requirement for the glyoxylate cycle during growth on propionate.
As mentioned above, ICL and MCL activities are encoded by the same gene(s) in M. tuberculosis, inextricably linking the functions of the glyoxylate and methylcitrate cycles (37). Furthermore, both glyoxylate and methylcitrate cycles utilize enzymes of the tricarboxylic acid (TCA) cycle, including succinate dehydrogenase, fumarase, and aconitase (Fig. 1). The methylmalonyl pathway, in contrast, is reliant on an autonomous set of enzymes, PCC, MMCE, and MCM, to generate the TCA cycle intermediate succinyl-CoA. This raised the possibility that the methylmalonyl pathway might offer a more efficient route for propionate metabolism, perhaps bypassing the need for anaplerosis via the glyoxylate cycle.
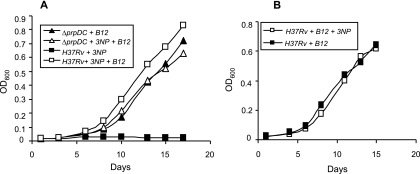
The vitamin B12-dependent growth of the ΔprpDC mutant on propionate established the ability of the methylmalonyl pathway to support growth in the absence of a functional methylcitrate cycle. However, the sufficiency of the methylmalonyl pathway in the absence of both methylcitrate and glyoxylate cycles remained to be determined. To investigate this, we assayed the growth of H37Rv on propionate while inhibiting Icl1 enzymatic function (ICL and MCL activity) through the addition of 3NP (20). As reported previously for strain Erdman (36), H37Rv was unable to metabolize propionate in the presence of 3NP (Fig. 3A), confirming the essentiality of Icl1 (and Icl2) for the growth of M. tuberculosis on propionate as the sole carbon source under the conditions tested. However, the addition of vitamin B12 appeared to alleviate the 3NP-mediated growth inhibition of the wild-type strain, as evidenced by the growth that eventually occurred, albeit after a prolonged (ca. 2-week) delay (Fig. 3A). Abrogation of MCM activity in the ΔmutAB mutant eliminated growth on propionate in the presence of 3NP (Fig. 3B). Together, these observations suggested that the methylmalonyl pathway alone is sufficient for the growth of M. tuberculosis on propionate as the sole carbon source, provided that the vitamin B12 cofactor requirement for MCM activity is met.
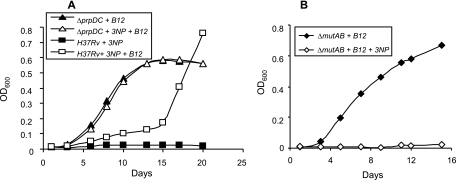
FIG. 3.
The methylmalonyl pathway enables bypass of the glyoxylate shunt during growth of M. tuberculosis on propionate. (A) Growth of H37Rv on propionate in the presence of 3NP with (□) or without (▪) vitamin B12 supplementation versus the growth of the ΔprpDC mutant on vitamin B12-supplemented propionate with (▵) or without (▴) 3NP. (B) Growth of the ΔmutAB mutant on vitamin B12-supplemented propionate with (⋄) or without (⧫) 3NP. Data are OD600 values for a single representative experiment from three independent biological replicates.
In contrast to the wild type, no growth delay was observed in the case of the ΔprpDC mutant, which grew equally well in vitamin B12-supplemented propionate in both the presence and absence of 3NP (Fig. 3A). Since the prpDC mutation precluded the flux of propionyl-CoA through the methylcitrate cycle, the differential response of wild-type and prpDC mutant strains to the 3NP-mediated inhibition of both methylcitrate and glyoxylate cycles implicated a buildup of the toxic propionate metabolites 2-methylcitrate and 2-methylisocitrate in the delayed growth of the wild-type strain (21, 44, 50). To investigate whether the growth eventually observed was of wild-type M. tuberculosis or an escape mutant refractory to the inhibitory effects of a methylcitrate cycle intermediate(s), bacteria from the outgrown culture were passaged several times in Middlebrook 7H9 broth to eliminate residual traces of vitamin B12 and then used to inoculate B12-supplemented propionate with or without 3NP. Growth rates in both cases were found to be similar and remained strictly vitamin B12 dependent (data not shown). These observations suggested that a functional methylmalonyl pathway allowed an escape mutant(s) to arise under the pressure imposed by toxic propionate metabolites that accumulated as a result of 3NP-mediated inhibition of the methylcitrate cycle at the step catalyzed by Icl1.
Role of the methylmalonyl pathway in growth of M. tuberculosis on longer odd-chain fatty acids.
The data presented above (Fig. 3A and B) established the ability of the methylmalonyl pathway to metabolize propionate independently of both methylcitrate and glyoxylate cycles. However, growth on longer odd-chain fatty acids might require the dual operation of both the methylmalonyl pathway and the glyoxylate cycle; that is, partitioning the flux of derivative propionyl-CoA and acetyl-CoA subunits through the methylmalonyl pathway and glyoxylate cycle, respectively, could enable the optimal use of such carbon sources.
To test this possibility, we first assessed the growth of H37Rv on valerate, a C5 substrate which yields acetyl-CoA (C2) and propionyl-CoA (C3) subunits in equal proportion. In agreement with recent evidence (9), H37Rv grew poorly on valerate as the sole carbon source (Fig. 4A). However, supplementation of the medium with vitamin B12 improved the growth of the wild-type strain, strongly implying a role for the methylmalonyl pathway in metabolizing the propionyl-CoA derived from this substrate. As observed on propionate-containing medium (Fig. 2A), the prpDC mutant of H37Rv was unable to utilize valerate in the absence of vitamin B12 (Fig. 4A) but could grow in vitamin B12-supplemented medium, again implying propionate toxicity when both methylcitrate cycle and methylmalonyl pathway functions are crippled. Together, these findings strongly suggested the ability of the methylmalonyl pathway to operate as the preferred route for propionate metabolism under the conditions tested.
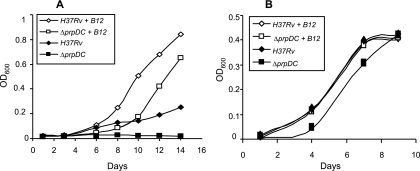
FIG. 4.
Growth of M. tuberculosis on longer odd-chain fatty acids. (A) Stimulatory effect of vitamin B12 on the growth of H37Rv and the ΔprpDC mutant on valerate (C5). Shown are data for H37Rv with (⋄) or without (⧫) vitamin B12 and the ΔprpDC mutant with (□) or without (▪) vitamin B12. (B) Effect of vitamin B12 on growth of H37Rv and the ΔprpDC mutant on heptadecanoate (C17). Shown are data for H37Rv with (⋄) or without (⧫) vitamin B12 and the ΔprpDC mutant with (□) or without (▪) vitamin B12. The growth curves for H37Rv with or without vitamin B12 and ΔprpDC with vitamin B12 are indistinguishable. Data are OD600 values for a single representative experiment from three independent biological replicates.
Growth of H37Rv and the prpDC mutant was then assessed on the longer odd-chain fatty acid heptadecanoate (C17), which produces a much higher ratio of acetyl-CoA to propionyl-CoA subunits (7:1). In contrast to the findings with valerate (Fig. 4A), the wild-type strain displayed robust growth on heptadecanoate (Fig. 4B). Furthermore, the addition of vitamin B12 had no apparent effect, suggesting the dispensability of the methylmalonyl pathway for the metabolism of this carbon source. Significantly, the prpDC mutant also grew on heptadecanoate in the absence of B12 supplement, albeit more slowly than did the wild type (Fig. 4B). However, as observed with valerate, B12 supplementation improved the growth of the prpDC mutant, again implicating the methylmalonyl pathway in the relief of propionate toxicity. Together, these findings illustrate the dominant effect that the molar ratio of derivative propionyl-CoA:acetyl-CoA subunits has in determining the importance of the methylmalonyl pathway for the growth of M. tuberculosis on a given odd-chain fatty acid.
Anaplerotic role for MCM revealed by growth of M. tuberculosis on valerate with vitamin B12 supplementation.
The ability of the methylmalonyl pathway to support growth on valerate (Fig. 4A) raised the possibility that this pathway alone might be sufficient for the growth of M. tuberculosis in the absence of both methylcitrate and glyoxylate cycles. As described above (Fig. 3A), we assayed the growth of M. tuberculosis in the presence of the ICL inhibitor 3NP, but this time, we did so in medium containing valerate as the sole carbon source (Fig. 5A). Interestingly, H37Rv was able to grow in the presence of 3NP, provided that the medium was supplemented with vitamin B12. This observation was significant, as it implied the capacity of the methylmalonyl pathway to perform an anaplerotic function, thereby allowing the organism to overcome the loss of glyoxylate cycle activity (ICL) for the assimilation of derivative acetyl-CoA subunits. The apparent dispensability of Icl1 for the growth of M. tuberculosis on valerate was confirmed by the observation that the addition of 3NP had no effect on growth in the presence of vitamin B12 supplement (Fig. 5B). A similar experiment utilizing the prpDC mutant reinforced that observation (Fig. 5A). However, unlike the situation with propionate (Fig. 3A), no differential phenotype could be detected in the parental H37Rv versus the prpDC mutant strain for growth on valerate in the presence of 3NP (Fig. 5A). These results confirmed an anaplerotic role for the methylmalonyl pathway under conditions in which the prevailing vitamin B12 levels are able to satisfy the cofactor requirements of MCM.
FIG. 5.
Anaplerotic role for MCM revealed by growth of M. tuberculosis on valerate with vitamin B12 supplementation. (A) Growth of H37Rv on valerate in the presence of 3NP with (□) or without (▪) vitamin B12 supplementation versus growth of the ΔprpDC mutant on vitamin B12-supplemented valerate with (▵) or without (▴) 3NP. (B) Growth of H37Rv on vitamin B12-supplemented valerate with (□) or without (▪) 3NP. Data are OD600 for a single representative experiment from three independent biological replicates.
Differential transcriptional response of prpD to propionate versus valerate.
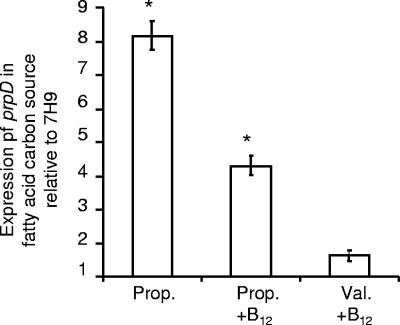
The delayed growth of H37Rv observed on B12-supplemented propionate but not valerate in the presence of 3NP (Fig. 3A versus 5A) suggested that the partitioning of propionyl-CoA between the methylcitrate cycle and the methylmalonyl pathway may differ depending on the carbon source used. To investigate this possibility, we analyzed the expression levels of prpD, normalized to sigA, in various carbon sources (Fig. 6). As observed for other organisms (4, 6, 23, 31, 41), prpD expression in M. tuberculosis was markedly induced in propionate compared to that in a propionate-free control (7H9) medium. In vitamin B12-supplemented propionate, the expression level of prpD remained significantly higher than that of the 7H9 control but was halved in comparison with that of propionate without B12 supplementation (Fig. 6). In contrast to the findings with propionate, the expression level of prpD in valerate supplemented with vitamin B12 was not significantly different from that observed in 7H9 broth (Fig. 6). Importantly, the induction of the prpDC operon in M. tuberculosis grown in propionate relative to that in valerate suggests the preferential routing of propionyl-CoA through the methylcitrate cycle when M. tuberculosis is cultured in propionate and is consistent with the observed delay in the growth of the organism on this carbon source as a result of the accumulation of toxic methylcitrate cycle intermediates (Fig. 3A).
FIG. 6.
Expression of the prpD gene of H37Rv cultured in various carbon sources. H37Rv was grown on propionate (Prop.) or valerate (Val.) in the presence or absence of vitamin B12. Growth on valerate alone was insufficient to support any further downstream analysis, so in this case, prpD expression could be assessed only in the presence of vitamin B12 supplementation. Levels of prpD transcript were determined by real-time qRT-PCR and normalized against the values obtained from bacteria grown in Middlebrook 7H9 medium supplemented with 0.2% glycerol, oleic acid-albumin-dextrose-catalase enrichment, and 0.05% Tween 80 (7H9) to assess any differential regulation of prpD as a function of the carbon source. Significant differences in the expression of prpD in fatty acid carbon sources relative to that in the 7H9 control are indicated by an asterisk (P < 0.0001).
DISCUSSION
The metabolic capacity of pathogens during infection is a key factor in defining the interaction with the host. Where pathogenesis is obligate, as is the case with M. tuberculosis, metabolism is integral to the ability of the organism to infect, survive, and be transmitted and therefore cannot be separated from concepts of virulence. Propionyl-CoA, as a precursor in several lipid biosynthetic pathways, including those for the production of the virulence factors sulfolipid 1 and phthiocerol dimycocerosate as well as the terminal product of the β-oxidation cycle, effectively encapsulates this notion, providing a natural intersection for virulence and central carbon metabolism. In this study, we established the capacity of the vitamin B12-dependent methylmalonyl pathway to fulfill a key role in propionate metabolism during the growth of the organism on fatty acids of odd chain length. Specifically, we presented genetic evidence that mutAB encodes a functional vitamin B12-dependent MCM and that an active methylmalonyl pathway enables the utilization of propionate as the sole carbon source in the absence of both the glyoxylate and methylcitrate cycles. The differential growth kinetics on propionate of a prpDC mutant strain versus H37Rv in the presence of the ICL inhibitor 3NP illustrated the effect that toxic intermediates of the methylcitrate cycle may have on the growth phenotype of M. tuberculosis in the absence of ICL function. The marked upregulation of prpD observed in propionate (this study) and of prpDC in macrophages (34, 48) and mouse lung (48) suggests the presence of high levels of propionate in M. tuberculosis in vivo (50). The oxidation of propionyl-CoA via the methylcitrate cycle would lead to toxic levels of 2-methylcitrate, which may account, at least in part, for the attenuated phenotypes of ICL and PrpD mutants in vivo (34, 36, 50), even if vitamin B12 were available.
β-Oxidation of odd-chain fatty acids comprising five carbons or more yields an acetyl-CoA subunit(s) in addition to propionyl-CoA. In the absence of vitamin B12, the wild-type strain displayed relatively poor growth on the C5 substrate valerate, whereas the prpDC mutant did not appear to grow at all. However, in both cases, growth was improved by the addition of vitamin B12. Therefore, in the absence of vitamin B12, the methylcitrate cycle is able to process the propionyl-CoA derived from this carbon source, albeit poorly. It is likely that competition between isocitrate (Km = 180 μM) and 2-methylisocitrate (Km = 718 μM) (18) for binding to Icl1 results in the toxic accumulation of the less-favored 2-methylisocitrate substrate. However, since the methylcitrate cycle of M. tuberculosis is not upregulated to any significant extent in valerate (this study), the presence of vitamin B12 allows the inhibitory effects of compromised methylcitrate cycle function to be circumvented by the processing of propionyl-CoA predominantly through the methylmalonyl pathway. In addition, by treating cells with the ICL inhibitor 3NP in the presence of vitamin B12, we demonstrated the capacity of the methylmalonyl pathway to perform an anaplerotic function during growth on valerate. The failure to elucidate differential responses to 3NP in parental strain H37Rv and the prpDC mutant strain further underscored the anaplerotic contribution of the methylmalonyl pathway to the growth of M. tuberculosis on this substrate.
In contrast, the methylmalonyl pathway was entirely dispensable for the growth of H37Rv on heptadecanoate, the β-oxidation of which yields seven molecules of acetyl-CoA for each molecule of propionyl-CoA. Although vitamin B12 supplementation improved the growth of the prpDC mutant on heptadecanoate, it is important to note that this strain was nonetheless able to grow on this carbon source in the absence of vitamin B12. This suggests that the relative abundance of acetyl-CoA available to support cell growth and division allows small amounts of derivative propionyl-CoA to be assimilated into cellular lipids, thus reducing the toxic buildup of propionyl-CoA in the methylcitrate cycle-defective prpDC mutant.
Previously, in demonstrating the functionality of the vitamin B12-dependent methionine synthase MetH, we established the inability of M. tuberculosis to produce vitamin B12 in vitro in medium containing dextrose as the carbon source (56). Here, we have extended that observation to include fatty acids of odd chain length (C3, C5, and C17), reinforcing the need to supplement growth media with vitamin B12 if the contribution of vitamin B12-dependent pathways to M. tuberculosis metabolism is to be assessed in vitro. Importantly, the demonstrated functionality of MCM reiterates the potential relevance of vitamin B12 to mycobacterial pathogenesis. In this regard, it is interesting that the genome of the related mycobacterial pathogen Mycobacterium leprae encodes homologs of both MetH and MCM as well as MeaB (11). The M. leprae genome is the product of reductive evolution to an extent that it is considered to approximate a minimal mycobacterial gene set (11). The conservation of two vitamin B12-dependent enzymes therefore strongly implies a selective advantage associated with the retention of vitamin B12-dependent pathways (11). It also suggests that vitamin B12 is available in vivo, as unlike M. tuberculosis, the M. leprae genome has undergone wholesale decay in vitamin B12 biosynthetic genes but has retained intact vitamin B12 riboswitch regulatory motifs (46). Importantly, the possibility that M. tuberculosis is able to synthesize and/or access vitamin B12 in vivo could inform the apparently paradoxical dispensability of the methylcitrate cycle for the growth and persistence of M. tuberculosis Erdman in mice (37); that is, a functional methylmalonyl pathway might compensate for the loss of methylcitrate cycle activity, thereby enabling the replication (and persistence) of the prpDC mutant. This possibility, in turn, suggests that mutant strains might be profitably applied as bioprobes to establish the availability of vitamin B12 in vivo. These issues are currently under investigation in our laboratories.
The metabolic capacity of M. tuberculosis is a function of the environments encountered during parasitism of the human host (16) and therefore represents evolution from an environmental ancestor to a well-adapted intracellular pathogen. It is likely, therefore, that the conservation of vitamin B12-dependent enzymes, in some cases, in addition to corresponding vitamin B12-independent isoforms (12, 56), is indicative of the differential enzyme and cofactors required in heterogeneous in vivo environments. Although M. tuberculosis resides primarily within macrophages, accumulating evidence suggests that the number of cellular environments serving as potential habitats is probably diverse (19, 39, 53). ICL activity has been shown to be essential to the establishment of infection in the acute stage of tuberculosis in a murine infection model (36). Our finding that the methylmalonyl pathway can provide an anaplerotic feed to the TCA cycle raises the possibility that in addition to the carbon sources utilized, this essentiality may be dictated by the availability of vitamin B12 in the initial stages of infection. The extents to which metabolic pathway and substrate utilizations are defined by the stage of infection, the tissue-specific distribution of nutrients, and the ability of the bacillus to access those nutrients therefore constitute fundamental aspects of mycobacterial pathogenesis that continue to demand elucidation. Is it significant, for example, that transcriptional profiling of end-stage human granulomas has revealed the downregulation of icl1 and the concomitant upregulation of meaB (45), thereby potentially implicating the methylmalonyl pathway in this stage of M. tuberculosis infection? Recent advances in the use of conditional gene silencing to elucidate M. tuberculosis gene function at various stages of infection (15) suggest that it may now be possible to determine whether the methylmalonyl pathway is indeed able to provide an anaplerotic function at late but not early stages of infection, thereby rendering ICL essential for early-stage growth but not late-stage persistence.
Supplementary Material
Acknowledgments
This work was supported by grants from the Medical Research Council of South Africa (to V.M.), the National Research Foundation (to S.S.D. and V.M.), and the NHLS Trust (to S.S.D.) and by an International Research Scholar's grant from the Howard Hughes Medical Institute (to V.M.).
We thank Bhavna Gordhan for technical assistance and members of the Mizrahi Laboratory for helpful discussions.
Footnotes
Published ahead of print on 28 March 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bobik, T. A., and M. E. Rasche. 2004. Purification and partial characterization of the Pyrococcus horikoshii methylmalonyl-CoA epimerase. Appl. Microbiol. Biotechnol. 63682-685. [DOI] [PubMed] [Google Scholar]
- 3.Boshoff, H. I., and C. E. Barry III. 2005. Tuberculosis—metabolism and respiration in the absence of growth. Nat. Rev. Microbiol. 370-80. [DOI] [PubMed] [Google Scholar]
- 4.Brämer, C. O., and A. Steinbüchel. 2001. The methylcitric acid pathway in Ralstonia eutropha: new genes identified involved in propionate metabolism. Microbiology 1472203-2214. [DOI] [PubMed] [Google Scholar]
- 5.Brock, M., and W. Buckel. 2004. On the mechanism of action of the antifungal agent propionate. Eur. J. Biochem. 2713227-3241. [DOI] [PubMed] [Google Scholar]
- 6.Brock, M., C. Maerker, A. Schütz, U. Volker, and W. Buckel. 2002. Oxidation of propionate to pyruvate in Escherichia coli. Involvement of methylcitrate dehydratase and aconitase. Eur. J. Biochem. 2696184-6194. [DOI] [PubMed] [Google Scholar]
- 7.Brosch, R., S. V. Gordon, A. Billault, T. Garnier, K. Eiglmeier, C. Soravito, B. G. Barrell, and S. T. Cole. 1998. Use of a Mycobacterium tuberculosis H37Rv bacterial artificial chromosome library for genome mapping, sequencing, and comparative genomics. Infect. Immun. 662221-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerniglia, C. E., and J. J. Perry. 1975. Metabolism of n-propylamine, isopropylamine, and 1,3-propane diamine by Mycobacterium convolutum. J. Bacteriol. 124285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, J. C., N. S. Harik, R. P. Liao, and D. R. Sherman. 2007. Identification of mycobacterial genes that alter growth and pathology in macrophages and in mice. J. Infect. Dis. 196788-795. [DOI] [PubMed] [Google Scholar]
- 10.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393537-544. [DOI] [PubMed] [Google Scholar]
- 11.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 4091007-1011. [DOI] [PubMed] [Google Scholar]
- 12.Dawes, S. S., D. F. Warner, L. Tsenova, J. Timm, J. D. McKinney, G. Kaplan, H. Rubin, and V. Mizrahi. 2003. Ribonucleotide reduction in Mycobacterium tuberculosis: function and expression of genes encoding class Ib and class II ribonucleotide reductases. Infect. Immun. 716124-6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downing, K. J., J. C. Betts, D. I. Young, R. A. McAdam, F. Kelly, M. Young, and V. Mizrahi. 2004. Global expression profiling of strains harbouring null mutations reveals that the five rpf-like genes of Mycobacterium tuberculosis show functional redundancy. Tuberculosis (Edinburgh) 84167-179. [DOI] [PubMed] [Google Scholar]
- 14.Gago, G., D. Kurth, L. Diacovich, S. C. Tsai, and H. Gramajo. 2006. Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. J. Bacteriol. 188477-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandotra, S., D. Schnappinger, M. Monteleone, W. Hillen, and S. Ehrt. 2007. In vivo gene silencing identifies the Mycobacterium tuberculosis proteasome as essential for the bacteria to persist in mice. Nat. Med. 131515-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glickman, M. S., and W. R. Jacobs, Jr. 2001. Microbial pathogenesis of Mycobacterium tuberculosis: dawn of a discipline. Cell 104477-485. [DOI] [PubMed] [Google Scholar]
- 17.Gordhan, B. G., S. J. Andersen, A. R. De Meyer, and V. Mizrahi. 1996. Construction by homologous recombination and phenotypic characterization of a DNA polymerase domain polA mutant of Mycobacterium smegmatis. Gene 178125-130. [DOI] [PubMed] [Google Scholar]
- 18.Gould, T. A., H. van de Langemheen, E. J. Muñoz-Elías, J. D. McKinney, and J. C. Sacchettini. 2006. Dual role of isocitrate lyase 1 in the glyoxylate and methylcitrate cycles in Mycobacterium tuberculosis. Mol. Microbiol. 61940-947. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Pando, R., M. Jeyanathan, G. Mengistu, D. Aguilar, H. Orozco, M. Harboe, G. A. Rook, and G. Bjune. 2000. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet 3562133-2138. [DOI] [PubMed] [Google Scholar]
- 20.Höner Zu Bentrup, K., A. Miczak, D. L. Swenson, and D. G. Russell. 1999. Characterization of activity and expression of isocitrate lyase in Mycobacterium avium and Mycobacterium tuberculosis. J. Bacteriol. 1817161-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horswill, A. R., A. R. Dudding, and J. C. Escalante-Semerena. 2001. Studies of propionate toxicity in Salmonella enterica identify 2-methylcitrate as a potent inhibitor of cell growth. J. Biol. Chem. 27619094-19101. [DOI] [PubMed] [Google Scholar]
- 22.Hubbard, P. A., D. Padovani, T. Labunska, S. A. Mahlstedt, R. Banerjee, and C. L. Drennan. 2007. Crystal structure and mutagenesis of the metallochaperone MeaB: insight into the causes of methylmalonic aciduria. J. Biol. Chem. 28231308-31316. [DOI] [PubMed] [Google Scholar]
- 23.Hüser, A. T., A. Becker, I. Brune, M. Dondrup, J. Kalinowski, J. Plassmeier, A. Pühler, I. Wiegrabe, and A. Tauch. 2003. Development of a Corynebacterium glutamicum DNA microarray and validation by genome-wide expression profiling during growth with propionate as carbon source. J. Biotechnol. 106269-286. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim-Granet, O., M. Dubourdeau, J. P. Latge, P. Ave, M. Huerre, A. A. Brakhage, and M. Brock. 2008. Methylcitrate synthase from Aspergillus fumigatus is essential for manifestation of invasive aspergillosis. Cell. Microbiol. 10134-148. [DOI] [PubMed] [Google Scholar]
- 25.Jain, M., C. J. Petzold, M. W. Schelle, M. D. Leavell, J. D. Mougous, C. R. Bertozzi, J. A. Leary, and J. S. Cox. 2007. Lipidomics reveals control of Mycobacterium tuberculosis virulence lipids via metabolic coupling. Proc. Natl. Acad. Sci. USA 1045133-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kana, B. D., B. G. Gordhan, K. J. Downing, N. Sung, G. Vostroktunova, E. E. Machowski, L. Tsenova, M. Young, A. Kaprelyants, G. Kaplan, and V. Mizrahi. 2008. The resuscitation-promoting factors of Mycobacterium tuberculosis are required for virulence and resuscitation from dormancy but are collectively dispensable for growth in vitro. Mol. Microbiol. 67672-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kendall, S. L., M. Withers, C. N. Soffair, N. J. Moreland, S. Gurcha, B. Sidders, R. Frita, A. Ten Bokum, G. S. Besra, J. S. Lott, and N. G. Stoker. 2007. A highly conserved transcriptional repressor controls a large regulon involved in lipid degradation in Mycobacterium smegmatis and Mycobacterium tuberculosis. Mol. Microbiol. 65684-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolattukudy, P. E., N. D. Fernandes, A. K. Azad, A. M. Fitzmaurice, and T. D. Sirakova. 1997. Biochemistry and molecular genetics of cell-wall lipid biosynthesis in mycobacteria. Mol. Microbiol. 24263-270. [DOI] [PubMed] [Google Scholar]
- 29.Korotkova, N., and M. E. Lidstrom. 2004. MeaB is a component of the methylmalonyl-CoA mutase complex required for protection of the enzyme from inactivation. J. Biol. Chem. 27913652-13658. [DOI] [PubMed] [Google Scholar]
- 30.Leadlay, P. F. 1981. Purification and characterization of methylmalonyl-CoA epimerase from Propionibacterium shermanii. Biochem. J. 197413-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, S. K., and J. D. Keasling. 2006. A Salmonella-based, propionate-inducible, expression system for Salmonella enterica. Gene 3776-11. [DOI] [PubMed] [Google Scholar]
- 32.Lin, T. W., M. M. Melgar, D. Kurth, S. J. Swamidass, J. Purdon, T. Tseng, G. Gago, P. Baldi, H. Gramajo, and S. C. Tsai. 2006. Structure-based inhibitor design of AccD5, an essential acyl-CoA carboxylase carboxyltransferase domain of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 1033072-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancia, F., N. H. Keep, A. Nakagawa, P. F. Leadlay, S. McSweeney, B. Rasmussen, P. Bosecke, O. Diat, and P. R. Evans. 1996. How coenzyme B12 radicals are generated: the crystal structure of methylmalonyl-coenzyme A mutase at 2 Å resolution. Structure 4339-350. [DOI] [PubMed] [Google Scholar]
- 34.Mattow, J., F. Siejak, K. Hagens, D. Becher, D. Albrecht, A. Krah, F. Schmidt, P. R. Jungblut, S. H. Kaufmann, and U. E. Schaible. 2006. Proteins unique to intraphagosomally grown Mycobacterium tuberculosis. Proteomics 62485-2494. [DOI] [PubMed] [Google Scholar]
- 35.Muñoz-Elías, E. J., and J. D. McKinney. 2006. Carbon metabolism of intracellular bacteria. Cell. Microbiol. 810-22. [DOI] [PubMed] [Google Scholar]
- 36.Muñoz-Elías, E. J., and J. D. McKinney. 2005. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 11638-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muñoz-Elías, E. J., A. M. Upton, J. Cherian, and J. D. McKinney. 2006. Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol. Microbiol. 601109-1122. [DOI] [PubMed] [Google Scholar]
- 38.Narumi, K., J. M. Keller, and C. E. Ballou. 1973. Biosynthesis of a mycobacterial lipopolysaccharide. Incorporation of (14C)-acyl groups by whole cells in vivo. Biochem. J. 132329-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neyrolles, O., R. Hernandez-Pando, F. Pietri-Rouxel, P. Fornes, L. Tailleux, J. A. Barrios Payan, E. Pivert, Y. Bordat, D. Aguilar, M. C. Prevost, C. Petit, and B. Gicquel. 2006. Is adipose tissue a place for Mycobacterium tuberculosis persistence? PLoS ONE 1e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padovani, D., and R. Banerjee. 2006. Assembly and protection of the radical enzyme, methylmalonyl-CoA mutase, by its chaperone. Biochemistry 459300-9306. [DOI] [PubMed] [Google Scholar]
- 41.Palacios, S., and J. C. Escalante-Semerena. 2004. 2-Methylcitrate-dependent activation of the propionate catabolic operon (prpBCDE) of Salmonella enterica by the PrpR protein. Microbiology 1503877-3887. [DOI] [PubMed] [Google Scholar]
- 42.Pandey, D. P., and K. Gerdes. 2005. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 33966-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parish, T., and N. G. Stoker. 2000. Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology 1461969-1975. [DOI] [PubMed] [Google Scholar]
- 44.Plassmeier, J., A. Barsch, M. Persicke, K. Niehaus, and J. Kalinowski. 2007. Investigation of central carbon metabolism and the 2-methylcitrate cycle in Corynebacterium glutamicum by metabolic profiling using gas chromatography-mass spectrometry. J. Biotechnol. 130354-363. [DOI] [PubMed] [Google Scholar]
- 45.Rachman, H., M. Strong, T. Ulrichs, L. Grode, J. Schuchhardt, H. Mollenkopf, G. A. Kosmiadi, D. Eisenberg, and S. H. Kaufmann. 2006. Unique transcriptome signature of Mycobacterium tuberculosis in pulmonary tuberculosis. Infect. Immun. 741233-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodionov, D. A., A. G. Vitreschak, A. A. Mironov, and M. S. Gelfand. 2003. Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes. J. Biol. Chem. 27841148-41159. [DOI] [PubMed] [Google Scholar]
- 47.Sassetti, C. M., D. H. Boyd, and E. J. Rubin. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 4877-84. [DOI] [PubMed] [Google Scholar]
- 48.Schnappinger, D., S. Ehrt, M. I. Voskuil, Y. Liu, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stjernholm, R. L., R. E. Noble, and D. Koch-Weser. 1962. Formation of methylmalonyl-CoA and succinyl-CoA by extracts of Mycobacterium smegmatis. Biochim. Biophys. Acta 64174-177. [DOI] [PubMed] [Google Scholar]
- 50.Upton, A. M., and J. D. McKinney. 2007. Role of the methylcitrate cycle in propionate metabolism and detoxification in Mycobacterium smegmatis. Microbiology 1533972-3983. [DOI] [PubMed] [Google Scholar]
- 51.Valentin, H. F., and D. Dennis. 1996. Metabolic pathway for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) formation in Nocardia corallina: inactivation of mutB by chromosomal integration of a kanamycin resistance gene. Appl. Environ. Microbiol. 62372-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van der Geize, R., K. Yam, T. Heuser, M. H. Wilbrink, H. Hara, M. C. Anderton, E. Sim, L. Dijkhuizen, J. E. Davies, W. W. Mohn, and L. D. Eltis. 2007. A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc. Natl. Acad. Sci. USA 1041947-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Wel, N., D. Hava, D. Houben, D. Fluitsma, M. van Zon, J. Pierson, M. Brenner, and P. J. Peters. 2007. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 1291287-1298. [DOI] [PubMed] [Google Scholar]
- 54.Vestal, J. R., and J. J. Perry. 1969. Divergent metabolic pathways for propane and propionate utilization by a soil isolate. J. Bacteriol. 99216-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vrijbloed, J. W., K. Zerbe-Burkhardt, A. Ratnatilleke, A. Grubelnik-Leiser, and J. A. Robinson. 1999. Insertional inactivation of methylmalonyl coenzyme A (CoA) mutase and isobutyryl-CoA mutase genes in Streptomyces cinnamonensis: influence on polyketide antibiotic biosynthesis. J. Bacteriol. 1815600-5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warner, D. F., S. Savvi, V. Mizrahi, and S. S. Dawes. 2007. A riboswitch regulates expression of the coenzyme B12-independent methionine synthase in Mycobacterium tuberculosis: implications for differential methionine synthase function in strains H37Rv and CDC1551. J. Bacteriol. 1893655-3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu, L., Y. Zhang, J. S. Teh, J. Zhang, N. Connell, H. Rubin, and M. Inouye. 2006. Characterization of mRNA interferases from Mycobacterium tuberculosis. J. Biol. Chem. 28118638-18643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.