Abstract
Campylobacter infection is one of the major causes of ovine abortions worldwide. Historically, Campylobacter fetus subsp. fetus was the major cause of Campylobacter-associated abortion in sheep; however, Campylobacter jejuni is increasingly associated with sheep abortions. We examined the species distribution, genotypes, and antimicrobial susceptibilities of abortion-associated Campylobacter isolates obtained from multiple lambing seasons on different farms in Iowa, Idaho, South Dakota, and California. We found that C. jejuni has replaced C. fetus as the predominant Campylobacter species causing sheep abortion in the United States. Most strikingly, the vast majority (66 of 71) of the C. jejuni isolates associated with sheep abortion belong to a single genetic clone, as determined by pulsed-field gel electrophoresis, multilocus sequence typing, and cmp gene (encoding the major outer membrane protein) sequence typing. The in vitro antimicrobial susceptibilities of these isolates to the antibiotics that are routinely used in food animal production were determined using the agar dilution test. All of the 74 isolates were susceptible to tilmicosin, florfenicol, tulathromycin, and enrofloxacin, and 97% were sensitive to tylosin. However, all were resistant to tetracyclines, the only antibiotics currently approved in the United States for the treatment of Campylobacter abortion in sheep. This finding suggests that feeding tetracycline for the prevention of Campylobacter abortions is ineffective and that other antibiotics should be used for the treatment of sheep abortions in the United States. Together, these results indicate that a single tetracycline-resistant C. jejuni clone has emerged as the major cause of Campylobacter-associated sheep abortion in the United States.
Campylobacter infection is one of the most prevalent causes of ovine abortion in the United States and worldwide, with an overall abortion rate of 5 to 50% (average, 23.2%) in affected flocks (39). A recent national study conducted by the USDA/APHIS/Veterinary Services in collaboration with the American Sheep Industry Association investigated the causes of sheep abortion (43). This study involved 22 participating states and covered 87.4% of the U.S. sheep inventory and 72.3% of the U.S. sheep producers. The study revealed that Campylobacter species ranked first among infectious causes of abortion within the last 3 years of the study. The highest percentage of producers (8.8%) reported that the cause was Campylobacter species, and the diagnosis was confirmed by a veterinarian or laboratory at 53.7% of these operations (43).
Campylobacter species can be carried in the intestines and gall bladder of healthy sheep without causing clinical diseases (1, 28, 41). In susceptible pregnant ewes, initial exposure may be followed by bacteremia with subsequent placentitis, fetal infection, and abortion, which usually occurs in the last trimester of pregnancy (39). Once an abortion storm starts, healthy ewes can be exposed to high levels of Campylobacter organisms through contact with the aborted fetus, placenta, and uterine discharges. During the initial period of infection, ewes usually do not show clinical signs of disease; however, occasionally ewes die due to uterine sepsis and septicemia if the fetus dies and is retained in utero (39). Gross lesions in aborted ewes include thickened uterine walls with edema, swollen caruncles covered with exudate, and placentas with mottled swollen cotyledons (17). Aborted fetuses can be mildly to severely autolyzed and commonly have serosanguinous fluid in the abdomen and thorax and, less often, focal liver necrosis (17, 20, 39). Histologically, the aborted placentas often have the signs of acute suppurative placentitis with congestion and necrosis of cotyledons, and the trophoblasts can be distended with intracytoplasmic organisms that can be stained with Giemsa stain (17, 39). A mild to moderate suppurative fetal bronchopneumonia typically is identified. Less consistently, the livers of aborted fetuses have multifocal areas of necrosis surrounded by a thin to moderate infiltrate of neutrophils. Campylobacter organisms can readily be cultured in large numbers from aborted placentas, fetal stomach contents, and, to a lesser extent, lung and liver.
Historically, C. fetus subsp. fetus accounted for the majority of the Campylobacter species associated with ovine abortion; however, recent studies indicated that C. jejuni is increasingly associated with ovine abortions (7, 20, 24, 31, 39, 44). A 1993 report of 1,784 ovine abortions presented to the Veterinary Diagnostic Laboratory (VDL) in South Dakota attributed 184 (10.3%) of the cases to Campylobacter species, second only to Toxoplasma gondii (190 cases [10.7%]) among the infectious causes (20). A closer examination of species distribution revealed that although C. fetus subsp. fetus accounted for the majority of the isolates for the first 8 years (1980 to 1988) of the study, the proportion of C. jejuni isolates steadily increased and outnumbered C. fetus subsp. fetus isolates during the final years of the study (20). Another study by Delong et al. (7) examined abortion cases from Idaho, Oregon, and Wyoming during one lambing season from 27 separate farms and reported the isolation of Campylobacter from 14 flocks. An analysis of the 15 isolates obtained from these flocks identified 14 of the isolates as C. jejuni and only one as C. fetus subsp. fetus. The extent and reason for this shift in the species distribution of Campylobacter isolates associated with ovine abortion in the United States and the impact of this shift on the control of the disease are unknown.
The serotyping/genotyping of Campylobacter isolates from ovine abortions in different parts of the world indicates that although heterogeneity within a flock usually is limited, high genetic diversity exists in Campylobacter spp. cultured from sheep abortions occurring on different farms during different lambing seasons (11, 23-25, 44). Studies from New Zealand (11, 25) reported the existence of multiple genotypes of both C. jejuni and C. fetus subsp. fetus isolated from ovine abortions from different farms and regions, although the presence of a predominant C. fetus subsp. fetus strain that caused the majority of abortions in certain flocks also was observed (23, 24). In the United States, apart from the two aforementioned reports indicating the regional species distribution and subtypes of Campylobacter isolates from sheep abortions (7, 20), a detailed investigation of the species distribution and genetic variability of Campylobacter isolates associated with sheep abortions at the national level is lacking. To date, the genetic typing of Campylobacter isolates from sheep abortions in the United States is limited to only one study (7), in which DNA restriction enzyme analysis indicated the presence of marked genetic heterogeneity among C. jejuni isolates during a single lambing season on different farms. The genetic characterization of Campylobacter isolates from ovine abortions is needed to enhance our understanding of the epidemiology and ecology of this important pathogen in sheep flocks.
In the United States, the treatment or prevention of abortion storms associated with Campylobacter traditionally has relied on the use of chlortetracycline or tetracycline in the feed, which is an approved nonprescription use at 80 mg/head/day. However, recently there have been multiple anecdotal reports of the clinical failure of tetracyclines to prevent or control Campylobacter-associated abortion storms (http://www.aasrp.org/; http://www.pipevet.com/articles/Practioners_Approach_to_Ovine_Abortion.htm; http://www.u-s-s-a.org/Abortions.htm). To our knowledge, no reports have been published on the actual susceptibility of Campylobacter isolates from sheep abortions to these antibiotics in the United States. A detailed analysis of the species/strain diversity and antimicrobial resistance profiling of Campylobacter spp. associated with sheep abortion is needed to facilitate the effective control of the disease on sheep farms.
To address these concerns from the sheep industry, we initiated a project to characterize the clinical isolates associated with sheep abortions. An analysis of 46 Campylobacter isolates cultured from sheep abortion cases submitted to the VDL at Iowa State University (VDL-ISU) indicated that 41 of the isolates were C. jejuni. The genetic fingerprinting of 33 of the C. jejuni isolates using pulsed-field gel electrophoresis (PFGE) showed that 32 of them appeared to be clonal, although the isolates were obtained from abortion cases that occurred during multiple lambing seasons on different farms over several years. Considering the high level of genetic diversity of Campylobacter isolates from animal reservoirs, including sheep, this finding was quite surprising and prompted us to further evaluate C. jejuni isolates associated with sheep abortion cases that occurred in different regions of the United States. This work reports the findings from the genetic fingerprinting and antimicrobial susceptibility tests.
MATERIALS AND METHODS
Collection of Campylobacter isolates from sheep abortions.
Campylobacter isolates analyzed in this study included 46 isolates from Iowa and 38 isolates from other states. The isolates from Iowa were cultured from ovine abortion cases submitted to the VDL-ISU during 2003 to 2007. The isolates came from 33 different farms located in Iowa. Aborted fetuses and placentas were necropsied and evaluated for gross pathological lesions, and tissue sections were fixed in formalin, embedded in paraffin, and stained with hematoxylin and eosin for histopathology by light microscopy. For Campylobacter species isolation, fetal stomach contents, placenta, and occasionally fetal lung and liver were cultured according to the established protocols (7, 17). A drop of stomach contents also was examined under dark-field microscopy for characteristic darting motility. Campylobacter spp. were identified to the species level by using standard biochemical and molecular methods (36).
For comparison, 38 C. jejuni isolates derived from ovine abortion cases were obtained from the VDLs in several states in the United States. These included 19 isolates from Idaho (isolated in 2004 and 2005 from multiple farms), 11 from South Dakota (all isolated in 2005 from different farms), and 8 from California (isolated during 2003 to 2007 from different farms).
PFGE typing.
PFGE analysis of the macrorestriction fragment patterns of C. jejuni genomic DNA was performed as described elsewhere (18). Briefly, fresh cultures of Campylobacter grown in Mueller-Hinton broth were embedded in chromosomal-grade agarose (Bio-Rad Laboratories, Inc., Hercules, CA) and treated with lysis buffer (10 mM Tris, 100 mM EDTA, pH 8.0, 1% N-lauroylsarcosine, 0.5 mg/ml proteinase K) overnight at 55°C in a shaker. After being washed, the gel plugs were digested with KpnI and SmaI separately under conditions optimal for the restriction enzymes. The digested plugs were embedded into a 1% agarose gel. DNA fragments were separated using the CHEF Mapper system (Bio-Rad) for 20 h, stained with ethidium bromide, and photographed with a digital imaging system (ChemiImager 5500; Alpha Innotech Corp., San Leandro, CA).
cmp sequence typing.
In addition to the PFGE typing of C. jejuni, the sequencing of the cmp gene (which codes for the major outer membrane protein [MOMP]) in eight representative C. jejuni isolates also was conducted using the method described previously (48). The PCR-amplified products were purified using the QIAquick PCR purification kit (Qiagen, Valencia, CA) and subsequently were sequenced at the DNA facility at Iowa State University. Sequence analysis and multiple-sequence alignments were performed by the Clustal W method with Lasergene 7.2 software (DNASTAR Inc., Madison, WI).
MLST.
The multilocus sequence typing (MLST) method for C. jejuni originally developed by Dingle et al. (9) was performed on four representative C. jejuni abortion isolates. The primer sets for the amplification and sequencing of the seven housekeeping genes were used and the PCRs were performed as described on the C. jejuni MLST website (http://pubmlst.org/campylobacter/), developed by Keith Jolley and Man-Suen Chan at the University of Oxford (19). Allelic numbers were assigned to the isolates by performing BLAST searches for the assembled sequences using the single-locus query function, whereas sequence types were assigned using the allelic profile query function in the MLST database. Sequences that were identical to existing alleles in the MLST database were assigned the corresponding allele numbers.
Antimicrobial susceptibility testing.
MICs of various antibiotics were determined using the standard agar dilution method as recommended by the CLSI (5). The antibiotics evaluated were penicillin, ceftiofur, oxytetracycline, tilmicosin, florfenicol, tulathromycin, tylosin, and enrofloxacin. These antibiotics are commonly used in food animal production medicine, but not all are labeled for sheep. The antibiotics were purchased from Sigma Chemical Co., St. Louis, MO (penicillin, ceftiofur, oxytetracycline, tilmicosin, and florfenicol); ICN Biomedicals Inc., Aurora, OH (enrofloxacin); Elanco Animal Health (tylosin); and Pfizer Inc., New York, NY (tulathromycin). C. jejuni ATCC 33560 was used as the quality control organism. The results were read after the samples were incubated in a microaerobic environment produced by CampyPack plus (BBL Microbiology Systems, Cockeysville, MD) at 42°C for 24 h. Although quality control ranges currently are not available for any of these antibiotics with Campylobacter, the MICs of these drugs for C. jejuni ATCC 33560 were consistent, falling within a two-dilution range throughout the study (data not shown). The MICs of these antibiotics for C. jejuni ATCC 33560 also matched very closely the MICs of their corresponding members within the same classes, such as ciprofloxacin, tetracycline, and erythromycin, for which the quality control ranges have been established for Campylobacter (5, 26). The MIC breakpoints of ≥4, ≥16, and ≥32 μg/ml established by the CLSI for ciprofloxacin, tetracycline, and chloramphenicol (5) were used for enrofloxacin, oxytetracycline, and florfenicol, respectively (Table 1). Similarly, the resistance breakpoints used for tylosin, tilmicosin, and tulathromycin (all macrolides) were based on the breakpoint indicated by the CLSI for erythromycin (a macrolide), 32 μg/ml (5). The antimicrobial resistance breakpoints for ceftiofur and penicillin (≥8 and ≥16 μg/ml, respectively) were chosen according to the interpretive standards established by the CLSI for bacteria isolated from animals (29, 30).
TABLE 1.
Antimicrobial susceptibility of 74 Campylobacter jejuni isolates as determined by the agar dilution test
| Antibiotic | Range of concn (μg/ml) tested | MIC (μg/ml)
|
Resistance breakpoint (μg/ml) | No. (%) of resistant isolates | ||
|---|---|---|---|---|---|---|
| Range | 50% | 90% | ||||
| Ceftiofur | 0.5-64 | >64 | >64 | >64 | ≥8a | 74 (100) |
| Enrofloxacin | 0.13-64 | <0.13 | <0.13 | <0.13 | ≥4 | 0 |
| Florfenicol | 0.5-64 | 1-2 | 1 | 2 | ≥32 | 0 |
| Oxytetracycline | 0.25-128 | 16-128 | 64 | 64 | ≥16 | 74 (100) |
| Penicillin | 0.13-64 | 8-64 | 16 | 16 | ≥16a | 40 (54) |
| Tilmicosin | 0.13-64 | 1-4 | 1 | 2 | ≥32 | 0 |
| Tulatromycin | 0.13-64 | 0.25-0.5 | 0.25 | 0.5 | ≥32 | 0 |
| Tylosin | 0.5-64 | 8-64 | 8 | 8 | ≥32 | 2 (2.7) |
PCR screening for tet(O).
PCR was employed to determine whether the resistance to tetracyclines was linked to the presence of the tet(O) gene. For this purpose, primers tet(O)-F (5′-GGCGTTTTGTTTATGTGCG-3′) and tet(O)-R (5′-ATGGACAACCCGACAGAAGC-3′) were used to amplify a 559-bp region of the tet(O) gene as described elsewhere (13). Total genomic DNA and/or boiled whole-cell preparations were used as the template in a master mix (Promega, Madison, WI) PCR mixture with the following conditions: an initial denaturation at 95°C for 5 min; 30 cycles of 95°C for 30 s, 57°C for 30 s, and 72°C for 1 min; and a final extension of 72°C for 10 min. Genomic DNAs of C. jejuni strains 81-176 [which carries the tet(O) gene on the pTet plasmid] (22) and 11168 [which does not have the tet(O) gene] (32) were used as the positive and negative controls, respectively. PCR products were separated by gel electrophoresis, stained with ethidium bromide, and visualized with a digital imaging system (ChemiImager 5500).
RESULTS
Association of C. jejuni with sheep abortions in Iowa.
Campylobacter spp. were isolated from aborted sheep fetuses/placentas from 33 different farms located in Iowa between 2003 and 2007. From a total of 182 abortion cases submitted to the VDL during this period, Campylobacter spp. were the only pathogenic organisms detected in 46 (25.2%) of the cases. Of these 46 isolates, 41 (89%) were identified as C. jejuni, and the remaining 5 (11%) were classified as C. fetus subsp. fetus. A definitive diagnosis was not made for 88 (48.3%) cases. Among the infectious causes, Campylobacter spp. ranked first, followed by Toxoplasma gondii (23 cases; 12.6%), Salmonella spp. (7 cases; 3.8%), and a few cases of Escherichia coli, Coxiella burnetii, Chlamydia psittaci, and uncharacterized bacteria.
Aborted fetuses and placentas were submitted to the VDL-ISU by field veterinarians. The clinical conditions for aborting ewes were not sought in this study. Most fetuses from which Campylobacter spp. were isolated were in the last trimester of gestation and showed mild to severe autolysis. Gross placentitis was evident in the majority of the cases. Areas of necrosis in the fetal liver and lungs of the cases were seen occasionally. Histopathologically, placentitis was encountered in almost all cases, followed by occasional fetal bronchopneumonia (Fig. 1A) and hepatitis (Fig. 1B). A microscopic examination of placenta tissue indicated areas of necrosis and trophoblasts with distended cytoplasm due to the presence of large numbers of Campylobacter-like organisms within these cells (Fig. 1C). These findings are in agreement with the observations previously published by other investigators (17, 20, 39) and further indicate that Campylobacter is highly pathogenic to ovine fetuses.
FIG. 1.
Histopathology findings in aborted fetuses from which C. jejuni was the only agent isolated. Scale bars are shown at the lower right of each picture. (A) Purulent, multifocal bronchopneumonia. The infiltration of neutrophils and macrophages within the alveolar septae and in the alveolar spaces is seen (arrows). B, bronchiole; V, vein. (B) Necropurulent, multifocal hepatitis. The focally extensive replacement of hepatocytes with neutrophils, fibrin, and necrotic cellular debris is seen (arrows). (C) Hematoxylin and eosin stain of placenta tissue with the trophoblasts distended by large numbers of intracytoplasmic bacteria typical of Campylobacter (arrows). The chorionic epithelium (trophoblasts) is sloughed.
PFGE analysis of C. jejuni isolates from ovine abortion.
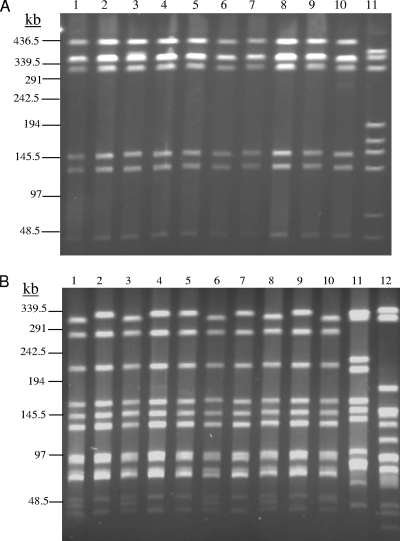
PFGE typing of the C. jejuni isolates from Iowa sheep abortions showed that 32 of 33 Iowa isolates were genetically indistinguishable by SmaI fingerprinting and showed only two minor differences by KpnI fingerprinting (representative isolates are shown in Fig. 2). This observation suggested that these clinical C. jejuni isolates were clonally related. This finding was quite surprising considering the facts that C. jejuni isolates are genetically diverse and that the isolates were obtained from abortion cases that occurred on different farms during different lambing seasons. To determine if this clone also was involved in sheep abortions in other regions, C. jejuni isolates associated with sheep abortions were obtained from three other states. Strikingly, 18 of the 19 Idaho isolates, 9 of the 11 South Dakota isolates, and 7 of the 8 California isolates obtained from different farms during different lambing seasons had SmaI fingerprints identical to that of the Iowa isolates (partially shown in Fig. 2A). Their KpnI patterns showed two closely related subtypes, which exactly matched those of the Iowa isolates (Fig. 2B). For clarity, the isolates with the KpnI banding patterns shown in lanes 1, 3, 6, 8, and 10 were classified as subtype I, whereas those having the patterns shown in lanes 2, 4, 5, 7, and 9 were classified as subtype II. The only detectable differences between the two subtypes were the presence of an extra low-molecular-weight band (second from the bottom) for subtype I and a slight downward shift of the very top band for subtype I compared to the position of the bands for subtype II (Fig. 2B). It is very likely that the subtle banding differences were due to a single KpnI site change, because gaining an extra KpnI site in the top band of subtype II would convert subtype II to subtype I. The fact that these isolates were not distinguishable by SmaI fingerprinting suggests that KpnI is a more discriminatory restriction enzyme for Campylobacter typing, as observed in previous studies (33, 48). A total of three isolates from Iowa, Idaho, and California (one from each state) had macrorestriction patterns that were distinct from each other and from the PFGE types described above, while two of the South Dakota isolates were untypeable by PFGE (data not shown). These results strongly suggest that the vast majority of the C. jejuni isolates associated with sheep abortions belong to a single genetic clone of two subtypes.
FIG. 2.
PFGE analysis of representative C. jejuni isolates from sheep abortions using SmaI (A) and KpnI (B). Abortion isolates from Iowa (lanes l to 3), Idaho (lanes 4 to 6), South Dakota (lanes 7 to 9), and California (lane 10) are represented in both panels. As controls, C. jejuni NCTC 11168 (lane 11 in panel A and lane 12 in panel B) and C. jejuni 81-176 (lane 11 in panel B) were included. The size markers based on the lambda DNA ladder (Bio-Rad) are indicated on the left of each panel. The actual identities of the abortion isolates are as follows: lane 1, Iowa 5908; lane 2, Iowa 3842; lane 3, Iowa 975; lane 4, Idaho 04-181 B077-6; lane 5, Idaho 04-174 B090-A; lane 6, Idaho 04-0030 B006-8; lane 7, South Dakota 3629; lane 8, South Dakota 4165; lane 9, South Dakota 3634; and lane 10, California T0403562B.
cmp typing of representative C. jejuni isolates.
To confirm the results of PFGE macrorestriction typing, another molecular typing method established for C. jejuni also was included in this study (4, 18). For this purpose, sequences of the cmp gene (encoding the MOMP protein) of representative Campylobacter abortion isolates, including PFGE subtypes I (IA-2, IA-3, SD-2, ID-2, and CA-1) and II (IA-1, SD-1, and ID-1), were determined (Fig. 3). The sequence alignment of the deduced amino acids of cmp alleles indicated an overall high degree of identity (99.2 to 100%) among the Campylobacter abortion isolates of different locations (Fig. 3). The lowest identity (99.2%) was among a California isolate and one Iowa isolate (IA-1), two South Dakota isolates, and two Idaho isolates. Interestingly, the isolates IA-1, SD-1, SD-2, ID-1, and ID-2 were 100% identical in their cmp gene sequences, although they had different PFGE subtypes. A comparison of the deduced amino acid sequences to the known protein sequences using the BLAST program indicated that the sheep abortion isolates had 99 to 100% identity to a few C. jejuni isolates from diverse sources, including humans and sheep (4, 48). Considering our previous observations that MOMP sequences could diverge up to 36% among different C. jejuni strains and that this method was a highly discriminatory one that is comparable to PFGE (18, 48), the clonality of C. jejuni sheep abortion isolates examined in this study is further evidenced by the results of cmp sequence typing.
FIG. 3.
Multiple alignment of deduced MOMP sequences of C. jejuni isolates from sheep abortion cases from different states in the United States. Dots and dashes represent matches and gaps, respectively, and question marks indicate undetermined sequences. Isolate names are shown on the left. Numbers on the right denote amino acid positions. The identification numbers of the isolates are as follows: IA-1, Iowa 3902; IA-2, Iowa 35; IA-3, Iowa 698; SD-1, South Dakota 3629; SD-2, South Dakota 4165; ID-1, Idaho 04-215 B100-A; ID-2, Idaho 04-0130 B054-C; and CA-1, California T04035.
MLST of C. jejuni isolates.
To further confirm the PFGE and cmp sequencing results and to determine if the sheep isolates matched any sequences deposited in the MLST database, MLST was performed on four representative isolates, two from each PFGE subtype (IA 975 and CA T0501095B for subtype I and IA 3902 and ID 04-218 B099 for subtype II). The sequence results showed that all four isolates had identical allelic sequences for each of the seven loci tested regardless of their PFGE subtypes (results not shown). BLAST results using the MLST database (http://pubmlst.org/campylobacter/) indicated that the allelic sequences for all seven loci had exact matches to some other sequences previously deposited in the database; thus, the preexisting allelic numbers were assigned for each locus of the sheep isolates. This gave an allelic profile of 2, 1, 1, 3, 2, 1, and 6 (for aspA, glnA, gltA, glyA, pgm, tkt, and uncA, respectively), which then was assigned to sequence type 8 (ST-8). There are 10 C. jejuni isolates belonging to ST-8 that have been deposited in the Campylobacter MLST database so far. The source, country of origin, year of isolation, clinical data, and other information concerning these isolates are given in Table 2. Nine of these isolates were from humans, and one was from cattle. All of the isolates from the United States (n = 8) were from human stool, and all but one were associated with two outbreaks, which occurred in different regions (Oklahoma and Maine) in 1988 and 1989 (38). The identical sequence types among the four examined sheep isolates further confirmed the clonal nature of these abortion-associated isolates. Their exact sequence type match to that of the human isolates in ST-8 suggests that the sheep clone also is present in human populations.
TABLE 2.
List of C. jejuni isolates belonging to the ST-8 allelic profile in the MLST database
| Name | Location | Yr | Source | Disease status | Epidemiology | Clonal complex |
|---|---|---|---|---|---|---|
| 85/7729a | United Kingdom | 1985 | Human | Systemic | Inpatient | ST-21 |
| C26a | United Kingdom | 1999 | Cattle | Carrier | Carrier | ST-21 |
| D5490a | Kansas | 1998 | Human | Enteritis | Sporadic | ST-21 |
| D2643a | Oklahoma | 1988 | Human | Enteritis | Outbreak | ST-21 |
| D2642a | United States | 1988 | Human | Enteritis | Outbreak | ST-21 |
| D2641a | Oklahoma | 1988 | Human | Enteritis | Outbreak | ST-21 |
| D2651a | Oklahoma | 1988 | Human | Enteritis | Outbreak | ST-21 |
| D2763a | Maine | 1989 | Human | Enteritis | Outbreak | ST-21 |
| D2770a | Maine | 1989 | Human | Enteritis | Outbreak | ST-21 |
| D2769a,b | Maine | 1989 | Human | Enteritis | Outbreak | ST-21 |
| IA3902c | Iowa | 2006 | Sheep | Abortion | Outbreak | ST-21 |
| IA975c | Iowa | 2003 | Sheep | Abortion | Outbreak | ST-21 |
| ID04-218 B099Bc | Idaho | 2004 | Sheep | Abortion | Outbreak | ST-21 |
| CAT0501095Bc | California | 2005 | Sheep | Abortion | Outbreak | ST-21 |
These were isolates deposited in the Campylobacter MLST database (http://pubmlst.org/Campylobacter) by other investigators (8, 12, 38).
The patient from whom this isolate was taken developed Guillain-Barré syndrome (38).
New isolates from this study.
Antimicrobial susceptibility of the C. jejuni isolates.
To facilitate the selection of antibiotics for the treatment of sheep abortions, the susceptibilities of C. jejuni isolates to commonly used antibiotics in animal productions were measured. The MIC ranges, the MICs at which the growth of 50% and 90% of C. jejuni isolates (n = 74 total) was inhibited, and the percentages of resistance to each antimicrobial drug are summarized in Table 1. The results indicated that all of the isolates were susceptible to tilmicosin, tulathromycin, florfenicol, and enrofloxacin; however, 100, 54, and 2.7% of the isolates were resistant to ceftiofur, penicillin, and tylosin, respectively. It should be pointed out that thermophilic Campylobacter spp. are intrinsically resistant to cephalosporins, including ceftiofur (6, 27, 42), and these compounds often are included in the selective medium for the isolation of Campylobacter (6). Regardless, we included ceftiofur in this panel, since it is frequently used in food animal production for the treatment of diseases caused by other bacterial pathogens. Strikingly, 100% of the tested C. jejuni isolates were resistant to oxytetracycline. The MICs of this antibiotic ranged from 16 to 128 μg/ml, with the MIC at which the growth of 90% of the C. jejuni isolates was inhibited being 64 μg/ml (Table 1).
Characterization of tetracycline resistance in C. jejuni isolates.
Of the 48 oxytetracycline-resistant C. jejuni isolates tested, 47 (98%) of them had the tet(O) gene as determined by PCR (results not shown), which is in accordance with findings from other investigators (13, 35). Interestingly, the only isolate without the tet(O) gene was the only isolate with a different PFGE pattern among the Iowa collection, and it also was one of the few having a relatively low level of resistance (MIC = 16 μg/ml). Although several attempts by PCR to detect the tet(O) gene in this C. jejuni isolate were unsuccessful, other molecular methods are needed to determine if the tetracycline resistance in this isolate is mediated by tet(O) or by other mechanisms. The antimicrobial susceptibility testing result strongly indicates that these clinical sheep isolates have acquired resistance to oxytetracycline, a key antibiotic currently used for treating sheep abortions.
DISCUSSION
Historically, most of the ovine Campylobacter abortions have been caused by C. fetus subsp. fetus in different regions of the world (15, 20, 23, 39). However, several studies from the United States during the late 1980s and early 1990s indicated an increasing role for C. jejuni in sheep abortions (7, 20). In contrast, C. fetus subsp. fetus still remains the major cause of Campylobacter-associated ovine abortion in New Zealand (11, 23-25, 34). In this work, a detailed analysis of the species distribution, genetic diversity, and antimicrobial susceptibility of Campylobacter-associated ovine abortion in the United States was conducted. The study revealed several important and unique features of ovine abortions currently occurring in the United States. Firstly, the predominant Campylobacter species causing sheep abortions in the United States has shifted from C. fetus to C. jejuni. Among 46 cases of Campylobacter sheep abortions from different farms submitted to the VDL-ISU between 2003 and 2007, 41 of them were caused by C. jejuni. Similarly, most Campylobacter isolates (19 of 20) obtained by the Idaho VDL (the samples were from Oregon, Idaho, and Nevada) and all of the isolates obtained by the California VDL also were identified as C. jejuni (personal communications with the staff of the participating VDLs). Secondly, the vast majority of the recent cases of Campylobacter-associated abortions in the United States were associated with a single clone of C. jejuni. This finding is in contrast to those of previous findings (20, 23, 24, 44), in which multiple Campylobacter species and strains were found to be the cause of sheep abortions. Thirdly, it was determined that all recent C. jejuni isolates associated with ovine abortion were resistant to tetracyclines, the only class of antibiotics currently approved for the treatment of Campylobacter abortion in sheep. These findings provide useful information for the control of sheep abortion in the United States.
Multiple molecular typing methods have been developed for Campylobacter (21, 46) that have greatly improved our understanding of the genetic relationships between Campylobacter isolates from different sources. PFGE and MLST are the two most frequently used methods for the molecular typing of Campylobacter. Recently, the cmp-based method also is increasingly used for typing, because although the cmp locus is highly conserved, its sequence varies among different C. jejuni strains (4, 18, 48). Using PFGE, we found that most of the C. jejuni isolates (66 of 71) from sheep abortions occurred at different lambing seasons, in different years (2003 to 2007), and in different states (Iowa, Idaho, South Dakota, Oregon, Nevada, and California) and were clonally related (Fig. 2). This conclusion was further confirmed by typing representative isolates using the cmp-based method and MLST. PFGE and cmp-based typing are considered more discriminatory than MLST (4, 37, 38). Thus, it is not surprising that the MLST method showed identical profiles, while PFGE and the cmp-based method showed some subtle differences among the sheep isolates examined in this study. The differences between the two PFGE subtypes are likely due to the gain or loss of a single KpnI site in the top band of the PFGE pattern. Other investigators also reported the occurrence of different PFGE variants from a single clone of C. jejuni due to genetic variation caused by recombinations, mutations, or insertions/deletions (3, 16, 45). Despite these subtle differences as determined by PFGE and cmp sequences, we conclude that the examined sheep isolates are clonally related. Considering the high genetic variability among different Campylobacter isolates from animals, that many different Campylobacter strains are present in sheep (1, 8, 44), and that genetically diverse strains of Campylobacter were associated with sheep abortions (7, 24, 25), our findings are quite surprising and strongly suggest that this unique clone of C. jejuni has become entrenched in the sheep production system in the United States.
How this unique C. jejuni clone has emerged as a predominant strain causing sheep abortion in the United States is unknown. One possibility is that this strain has evolved unique virulence factors that increase the tropism of Campylobacter to the reproductive system of ewes. Another possibility is that selection pressure in the sheep production environment has facilitated the expansion and transmission of this unique strain. For example, oxytetracycline is commonly used in sheep production for disease prevention and control (14), and all of the abortion-associated isolates were resistant to this antibiotic, suggesting that the use of oxytetracycline promoted the spread of this virulent strain. However, carrying tetracycline resistance alone cannot fully explain the high prevalence of this clone in clinical sheep abortions, because tetracycline resistance can be associated with multiple C. jejuni strains (13, 35). Thus, it is possible that the combined effect of enhanced virulence and tetracycline resistance is responsible for the spread of this strain in sheep. To satisfy Koch's postulates, this C. jejuni strain needs to be inoculated into sheep to reproduce abortion, which will be pursued in future studies. Currently we are sequencing the genome of this abortion-causing clone (isolate Iowa 3902, PFGE subtype 2) to determine if unique virulence traits are present in this strain and if the tet(O) element is associated with any virulence factors. In addition, we are currently using multidisciplinary approaches to understand the epidemiology of this strain in sheep production and the pathogenic mechanisms of Campylobacter-associated sheep abortion.
The tetracycline class of antibiotics (tetracycline, oxytetracycline, and chlortetracycline) is commonly used in the prevention and control of abortion storms associated with Campylobacter spp. in the United States (14); however, the anecdotal clinical experience of veterinary practitioners has suggested an increasing ineffectiveness of these drugs in treating ovine abortions due to Campylobacter. To help producers choose effective antibiotics for the treatment and control of Campylobacter-associated sheep abortions, in this study we also determined the antimicrobial susceptibility patterns of C. jejuni isolates to drugs that are commonly used in food animal production. Strikingly, the results demonstrated that 100% of the C. jejuni isolates associated with sheep abortions across the United States were resistant to oxytetracycline (Table 1). Although MIC tests cannot predict the outcomes of clinical treatment, our results suggest that tetracyclines are no longer effective in the treatment of abortion storms caused by Campylobacter in the United States. Based on the susceptibility results (Table 1), other potential alternatives could include using tilmicosin (approved for use in sheep), tylosin, florfenicol, and tulathromycin in an extralabel fashion. The use of these drugs in an extralabel fashion requires the adherence to the standards of the Animal Medicinal Drug Use Clarification Act (http://www.avma.org/reference/amduca/amduca1.asp). It should be pointed out that enrofloxacin is not permitted for use in sheep for any reason in the United States, although all of the Campylobacter isolates from sheep abortions were highly susceptible to this drug. A further investigation of the mechanism of tetracycline resistance in C. jejuni isolates from sheep abortions showed that the resistance was encoded by the tet(O) gene (results not shown), which is in accordance with previous studies (13, 35). At this stage, it is unknown whether the tet(O) gene in the abortion-associated isolates is encoded in the chromosome or on a plasmid; this remains to be determined in future studies using specialized techniques that allow the differentiation of plasmids from genomic DNA.
Vaccination against Campylobacter has been used to control sheep abortion, but Campylobacter still remains one of the major causes of ovine abortion worldwide. The great genetic and antigenic variations among Campylobacter strains as well as insufficient cross-protection conferred by the vaccine strain(s) against the field isolates could contribute to vaccination failures. This is illustrated by a New Zealand study in which isolates from aborted fetuses were compared to the strain used to vaccinate the ewes. Abortions in vaccinated ewes were due to a C. fetus subsp. fetus isolate that was different from the vaccine strain (11). As a result, the authorities in New Zealand have developed a newer vaccine incorporating three serotypes of C. fetus subsp. fetus plus a single strain of C. jejuni, and it has shown better protection in challenge studies (CampyVax4 technical manual [http://www.intervet.co.nz/binaries/90_109204.pdf]). The efficacies of vaccines against Campylobacter-associated sheep abortions in the United States vary widely. Abortions in flocks vaccinated with bivalent vaccines containing C. fetus subsp. fetus and C. jejuni antigens have been reported (7, 47), and there are numerous anecdotal reports describing vaccine failures. Currently, there are at least two vaccines available in the United States against sheep abortions caused by Campylobacter, and it is likely that the strains incorporated in these formulations were those that were the most common species/serotypes at the time the vaccine was prepared. Since results from this study demonstrated the occurrence of a predominant clone of C. jejuni in sheep abortions across the United States, and since there is only a limited degree of cross-protection between different Campylobacter serotypes (7, 11, 47), a new vaccine incorporating this predominant C. jejuni type or the inclusion of this strain in the current vaccines merits further investigations.
In addition to being a leading cause of food-borne bacterial gastroenteritis (2), C. jejuni also is a rarely reported organism associated with abortion, stillbirth, and neonatal death in humans (40). Although its pathogenesis is not well understood, C. jejuni can infect placental and fetal tissues via bacteremia after the ingestion of the organism and the invasion of the intestines in susceptible pregnant women (40). To date, there is no evidence linking campylobacteriosis in pregnant women, fetuses, or newborns to Campylobacter abortions in ewes. However, animal caretakers can acquire the organism from aborting ewes and develop enteritis (10). Since the intestinal carriage of Campylobacter by healthy sheep occurs frequently (41) and Campylobacter spp. are well-established placental pathogens in many animals and humans, female owners or caretakers of reproductive age should be aware of the risk of handling aborted sheep fetuses and fluids. At this point, the zoonotic involvement and extent (if any) of the C. jejuni clone from sheep abortion described here in human cases (whether in enteritis or more serious problems, such as bacteremia and abortion) are unknown. However, MLST results indicate that this C. jejuni clone not only is associated with the sheep host but also is distributed among different isolation sources, including humans. In addition, the cmp sequence typing indicated that the sheep isolates had 99% identity to a human C. jejuni strain, which also was shown to have a KpnI PFGE restriction pattern almost identical to that of the C. jejuni clone associated with sheep abortions (18). These pieces of circumstantial evidence suggest that the abortion clone is pathogenic to the human host, but this possibility remains to be examined in future studies.
Acknowledgments
This work was funded by the Iowa Livestock Health Advisory Council and the Iowa Sheep & Wool Promotion Board. K.S. was supported by the Fulbright Scholar Program, and L.W. was supported by a scholarship from the China Scholarship Council.
We thank the personnel at the VDLs in the participating states, including Idaho (Beth Mamer and Greta Anderson), South Dakota, and California, for providing isolates from sheep abortion cases.
Footnotes
Published ahead of print on 5 March 2008.
REFERENCES
- 1.Açik, M. N., and B. Cetinkaya. 2006. Heterogeneity of Campylobacter jejuni and Campylobacter coli strains from healthy sheep. Vet. Microbiol. 115370-375. [DOI] [PubMed] [Google Scholar]
- 2.Allos, B. M. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 321201-1206. [DOI] [PubMed] [Google Scholar]
- 3.Barton, C., L. K. Ng, S. D. Tyler, and C. G. Clark. 2007. Temperate bacteriophages affect pulsed-field gel electrophoresis patterns of Campylobacter jejuni. J. Clin. Microbiol. 45386-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark, C. G., A. Beeston, L. Bryden, G. Wang, C. Barton, W. Cuff, M. W. Gilmour, and L. K. Ng. 2007. Phylogenetic relationships of Campylobacter jejuni based on porA sequences. Can. J. Microbiol. 5327-38. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing. Supplement M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Corry, J. E., D. E. Post, P. Colin, and M. J. Laisney. 1995. Culture media for the isolation of campylobacters. Int. J. Food Microbiol. 2643-76. [DOI] [PubMed] [Google Scholar]
- 7.Delong, W. J., M. D. Jaworski, and A. C. Ward. 1996. Antigenic and restriction enzyme analysis of Campylobacter spp. associated with abortion in sheep. Am. J. Vet. Res. 57163-167. [PubMed] [Google Scholar]
- 8.Dingle, K. E., F. M. Colles, R. Ure, J. A. Wagenaar, B. Duim, F. J. Bolton, A. J. Fox, D. R. A. Wareing, and M. C. J. Maiden. 2002. Molecular characterization of Campylobacter jejuni clones: a basis for epidemiologic investigation. Emerg. Infect. Dis. 8949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dingle, K. E., F. M. Colles, D. R. Wareing, R. Ure, A. J. Fox, F. E. Bolton, H. J. Bootsma, R. J. Willems, R. Urwin, and M. C. Maiden. 2001. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 3914-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffell, S. J., and M. B. Skirrow. 1978. Shepherd's scours and ovine Campylobacter abortion-a “new” zoonosis? Vet. Rec. 103144. [DOI] [PubMed] [Google Scholar]
- 11.Fenwick, S. G., D. M. West, J. E. Hunter, N. D. Sargison, F. Ahmed, J. S. Lumsden, and M. G. Collett. 2000. Campylobacter fetus fetus abortions in vaccinated ewes. N. Z. Vet. J. 48155-157. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald, C., L. O. Helsel, M. A. Nicholson, S. J. Olsen, D. L. Swerdlow, R. Flahart, J. Sexton, and P. I. Fields. 2001. Evaluation of methods for subtyping Campylobacter jejuni during an outbreak involving a food handler. J. Clin. Microbiol. 392386-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibreel, A., D. M. Tracz, L. Nonaka, T. M. Ngo, S. R. Connell, and D. E. Taylor. 2004. Incidence of antibiotic resistance in Campylobacter jejuni isolated in Alberta, Canada, from 1999 to 2002, with special reference to tet(O)-mediated tetracycline resistance. Antimicrob. Agents Chemother. 483442-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giguere, S., J. F. Prescott, J. D. Baggot, R. D. Walker, and P. M. Dowling. 2006. Antimicrobial therapy in veterinary medicine, 4th ed. Blackwell Publishing, Ames, IA.
- 15.Grogono-Thomas, R., M. J. Blaser, M. Ahmadi, and D. G. Newell. 2003. Role of S-layer protein antigenic diversity in the immune responses of sheep experimentally challenged with Campylobacter fetus subsp. fetus. Infect. Immun. 71147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hänninen, M. L., M. Hakkinen, and H. Rautelin. 1999. Stability of related human and chicken Campylobacter jejuni genotypes after passage through chick intestine studied by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 652272-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedstrom, O. R., R. J. Sonn, E. D. Lassen, B. D. Hultgren, R. O. Crisman, B. B. Smith, and S. P. Snyder. 1987. Pathology of Campylobacter jejuni abortion in sheep. Vet. Pathol. 24419-426. [DOI] [PubMed] [Google Scholar]
- 18.Huang, S., T. Luangtongkum, T. Y. Morishita, and Q. Zhang. 2005. Molecular typing of Campylobacter strains using the cmp gene encoding the major outer membrane protein. Foodborne Pathog. Dis. 212-23. [DOI] [PubMed] [Google Scholar]
- 19.Jolley, K. A., M. S. Chan, and M. C. J. Maiden. 2004. mlstdbNet—distributed multi-locus sequence typing (MLST) databases. BMC Bioinformatics 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirkbride, C. A. 1993. Diagnoses in 1,784 ovine abortions and stillbirths. J. Vet. Diagn. Investig. 5398-402. [DOI] [PubMed] [Google Scholar]
- 21.Lukinmaa, S., U. M. Nakari, M. Eklund, and A. Siitonen. 2004. Application of molecular genetic methods in diagnostics and epidemiology of food-borne bacterial pathogens. APMIS 112908-929. [DOI] [PubMed] [Google Scholar]
- 22.Manavathu, E. K., K. Hiratsuka, and D. E. Taylor. 1988. Nucleotide sequence analysis and expression of a tetracycline resistance gene from Campylobacter jejuni. Gene 6217-26. [DOI] [PubMed] [Google Scholar]
- 23.Mannering, S. A., R. M. Marchant, A. Middelberg, N. R. Perkins, D. M. West, and S. G. Fenwick. 2003. Pulsed-field gel electrophoresis typing of Campylobacter fetus subsp. fetus from sheep abortions in the Hawke's Bay region of New Zealand. N. Z. Vet. J. 5133-37. [DOI] [PubMed] [Google Scholar]
- 24.Mannering, S. A., D. M. West, S. G. Fenwick, R. M. Marchant, and K. O'Connell. 2006. Pulsed-field gel electrophoresis of Campylobacter jejuni sheep abortion isolates. Vet. Microbiol. 115237-242. [DOI] [PubMed] [Google Scholar]
- 25.Mannering, S. A., D. M. West, S. G. Fenwick, R. M. Marchant, N. R. Perkins, and K. O'Connell. 2004. Pulsed-field gel electrophoresis typing of Campylobacter fetus subsp. fetus isolated from sheep abortions in New Zealand. N. Z. Vet. J. 52358-363. [DOI] [PubMed] [Google Scholar]
- 26.McDermott, P. F., S. M. Bodeis, F. M. Aarestrup, S. Brown, M. Traczewski, P. Fedorka-Cray, M. Wallace, I. A. Critchley, C. Thornsberry, S. Graff, R. Flamm, J. Beyer, D. Shortridge, L. J. Piddock, V. Ricci, M. M. Johnson, R. N. Jones, B. Reller, S. Mirrett, J. Aldrobi, R. Rennie, C. Brosnikoff, L. Turnbull, G. Stein, S. Schooley, R. A. Hanson, and R. D. Walker. 2004. Development of a standardized susceptibility test for Campylobacter with quality-control ranges for ciprofloxacin, doxycycline, erythromycin, gentamicin, and meropenem. Microb. Drug Resist. 10124-131. [DOI] [PubMed] [Google Scholar]
- 27.McGill, K., D. Cowley, L. Moran, P. Scates, A. O'Leary, R. H. Madden, C. Carroll, E. McNamara, J. E. Moore, S. Fanning, J. D. Collins, and P. Whyte. 2006. Antibiotic resistance of retail food and human Campylobacter isolates on the island of Ireland from 2001-2002. Epidemiol. Infect. 1341282-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milnes, A. S., I. Stewart, F. A. Clifton-Hadley, R. H. Davies, D. G. Newell, A. R. Sayers, T. Cheasty, C. Cassar, A. Ridley, A. J. Cook, S. J. Evans, C. J. Teale, R. P. Smith, A. McNally, M. Toszeghy, R. Futter, A. Kay, and G. A. Paiba. 2007. Intestinal carriage of verocytotoxigenic Escherichia coli O157, Salmonella, thermophilic Campylobacter and Yersinia enterocolitica, in cattle, sheep and pigs at slaughter in Great Britain during 2003. Epidemiol. Infect. 20071-13. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing. Supplement M100-S12. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 30.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. Approved standard M31-A2, 2nd ed. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 31.Orr, M. 1991. Abortions of sheep in 1990. Surveillance 2727-28. [Google Scholar]
- 32.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403665-668. [DOI] [PubMed] [Google Scholar]
- 33.Petersen, L., and S. L. On. 2000. Efficacy of flagellin gene typing for epidemiological studies of Campylobacter jejuni in poultry estimated by comparison with macrorestriction profiling. Lett. Appl. Microbiol. 3114-19. [DOI] [PubMed] [Google Scholar]
- 34.Poland, R. 2004. Animal disease surveillance. NZ Ministry Agric. Forest. Surveill. 319-11. [Google Scholar]
- 35.Pratt, A., and V. Korolik. 2005. Tetracycline resistance of Australian Campylobacter jejuni and Campylobacter coli isolates. J. Antimicrob. Chemother. 55452-460. [DOI] [PubMed] [Google Scholar]
- 36.Sahin, O., Q. Zhang, and T. Y. Morishita. 2003. Detection of Campylobacter, p. 183-193. In M. E. Torrence and R. E. Isaacson (ed.), Microbial food safety in animal agriculture. Iowa State Press, Ames.
- 37.Sails, A. D., B. Swaminathan, and P. I. Fields. 2003. Clonal complexes of Campylobacter jejuni identified by multilocus sequence typing correlate with strain associations identified by multilocus enzyme electrophoresis. J. Clin. Microbiol. 414058-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sails, A. D., B. Swaminathan, and P. I. Fields. 2003. Utility of multilocus sequence typing as an epidemiological tool for investigation of outbreaks of gastroenteritis caused by Campylobacter jejuni. J. Clin. Microbiol. 414733-4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skirrow, M. B. 1994. Diseases due to Campylobacter, Helicobacter and related bacteria. J. Comp. Pathol. 111113-149. [DOI] [PubMed] [Google Scholar]
- 40.Smith, J. L. 2002. Campylobacter jejuni infection during pregnancy: long-term consequences of associated bacteremia, Guillain-Barre syndrome, and reactive arthritist. J. Food Prot. 65696-708. [DOI] [PubMed] [Google Scholar]
- 41.Stanley, K., and K. Jones. 2003. Cattle and sheep farms as reservoirs of Campylobacter. J. Appl. Microbiol. 94(Suppl.)104S-113S. [DOI] [PubMed] [Google Scholar]
- 42.Taylor, D. E., and P. Courvalin. 1988. Mechanisms of antibiotic-resistance in Campylobacter species. Antimicrob. Agents Chemother. 321107-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.USDA. 2001. Part II: reference of sheep health in the United States. USDA, APHIS, VS, CEAH, National Animal Health Monitoring System, Fort Collins, CO.
- 44.Varga, J., B. Mezes, L. Fodor, and I. Hajtos. 1990. Serogroups of Campylobacter fetus and Campylobacter jejuni isolated in cases of ovine abortion. J. Vet. Med. B 37148-152. [DOI] [PubMed] [Google Scholar]
- 45.Wassenaar, T. M., B. Geilhausen, and D. G. Newell. 1998. Evidence of genomic instability in Campylobacter jejuni isolated from poultry. Appl. Environ. Microbiol. 641816-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wassenaar, T. M., and D. G. Newell. 2000. Genotyping of Campylobacter spp. Appl. Environ. Microbiol. 661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams, C. E., H. W. Renshaw, W. A. Meinershagen, D. O. Everson, R. K. Chamberlain, R. F. Hall, and D. G. Waldhalm. 1976. Ovine campylobacterosis: preliminary studies of the efficacy of the in vitro serum bactericidal test as an assay for the potency of Campylobacter (Vibrio) fetus subsp. intestinalis bacterins. Am. J. Vet. Res. 37409-415. [PubMed] [Google Scholar]
- 48.Zhang, Q., J. C. Meitzler, S. Huang, and T. Morishita. 2000. Sequence polymorphism, predicted secondary structures, and surface-exposed conformational epitopes of Campylobacter major outer membrane protein. Infect. Immun. 685679-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]