Abstract
We report the case of a 3-year-old girl with lymphadenopathy caused by the recently described species Mycobacterium colombiense. M. colombiense is a nonpigmented slow grower that is included in the Mycobacterium avium complex. Partial sequencing of the 16S rRNA gene was used for species identification.
CASE REPORT
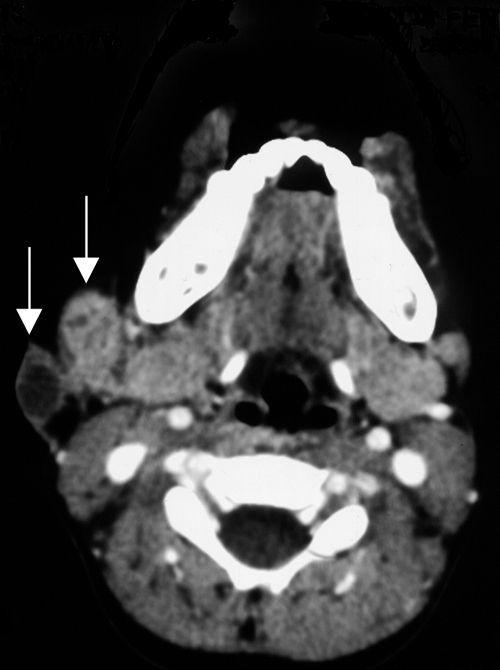
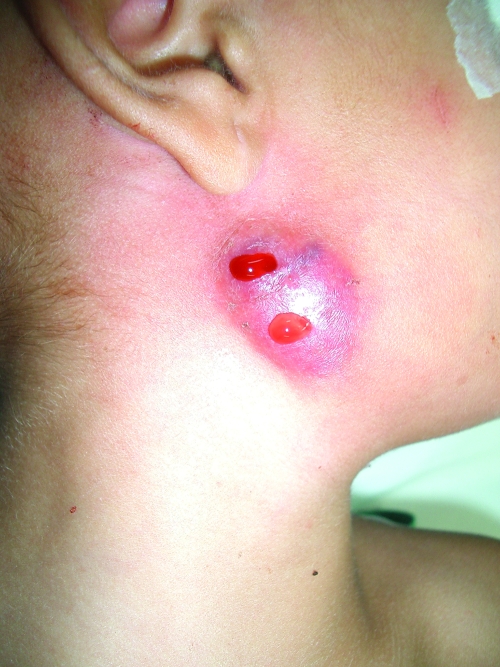
In July 2006, a 3-year-old girl presented to our hospital for evaluation of an isolated right cervical unilateral lymphadenopathy of 1 month's duration. Anti-inflammatory treatment resulted in no improvement. Her body temperature was normal, and systemic examination revealed no significant abnormality. The lymphadenopathy was 3 cm in diameter, hard, nonmobile, and without signs of fistulization. All hematological parameters were within normal limits, with the exception of alkaline phosphatase level (187 IU/liter) and globular sedimentation rate (19 mm/h). An X-ray of the chest was normal. There were no indications of immune deficiency in her medical history. The tuberculin skin test was performed twice, with negative results. Because infectious pathology was suspected, a fine-needle aspiration was performed and submitted for microbiological and histopathological analysis. Auramine and Ziehl-Nielsen staining of the aspirate demonstrated few acid-fast bacilli (AFB), and inflammation was nonspecific. A PCR-specific test for Mycobacterium tuberculosis complex (MTD; Gen-Probe Inc., San Diego, CA) was negative. With the probable diagnosis of atypical mycobacterial infection, antimicrobial treatment with clarithromycin, rifampin, and pyrazinamide was started. Fifteen days later, a computerized tomography exam with contrast enhancement showed an enlarged right submaxillar gland with two masses, of 1.8 and 1.5 cm in diameter, on the external face (Fig. 1). One mass showed enhancement with only small areas of internal necrosis, while the other showed no enhancement. Both were suggestive of pathological adenopathies. Twenty days later, one of the adenopathies developed suppuration (Fig. 2). One week later, antibiotic treatment was suspended due to digestive tract intolerance. The patient was admitted for surgical excision by cervicotomy of pathological lymph nodes of the submandibular and subdigastric regions, including the affected skin. A 6- by 4-cm tissue specimen was sent for histopathological analysis, but microbiological analysis was not performed. Ten nodules with diameters between 0.4 and 1.5 cm were dissected from this tissue specimen. Histopathological examination showed granulomatous adenitis with necrosis, compatible with mycobacterial infection. No AFB were seen with Kinyoun stain. The postsurgical period was uneventful, and the patient was discharged 3 days after surgery. She is presently healthy, without signs of infection.
FIG. 1.
Computerized tomography scan of the neck showing two masses on the right submaxillar gland (arrows).
FIG. 2.
Photograph of adenopathy showing suppuration.
The fine-needle aspiration specimen was cultured using the following two methods: the BACTEC automatic radiometric method (Becton-Dickinson Instrument Systems, Sparks, MD) and solid Löwenstein-Jensen medium. The liquid culture turned positive for AFB after 22 days of incubation. Based on microscopic features upon Ziehl-Nielsen staining, the AccuProbe Mycobacterium avium complex (MAC) identification test (Gen-Probe Inc., San Diego, CA) was performed and was positive. Subsequently, the AccuProbe M. avium and Mycobacterium intracellulare identification tests (Gen-Probe Inc., San Diego, CA) were performed but were negative. In an attempt to obtain a specific etiologic diagnosis, DNA was extracted using an InstaGene matrix following the manufacturer's instructions (Bio-Rad, Glattbrugg, Switzerland) and subjected to PCR amplification of an ∼1,000-bp fragment of the 16S rRNA gene. PCR was conducted with two universal primers, i.e., 27 F (5′-AGA GTT TGA TC[AC] TGG CTC AG-3′) and 907 R (5′-CCG TCA ATT C[AC]T TT[GA] AGT TT-3′). Partial sequencing of the 16S rRNA gene was done as previously described (3). The sequence obtained was compared against the NCBI (GenBank) database. The 469-bp sequence showed zero mismatches with the sequence of the type strain of M. colombiense (GenBank accession number AM062764). The sequence showed four mismatches with both M. avium 104 (GenBank accession number CP000479) and M. intracellulare CIP104243 (GenBank accession number AF547939) sequences. Two Löwenstein-Jensen samples, subcultured from BACTEC medium at the same time that molecular identification was carried out, showed a nonpigmented slow grower. No biochemical tests were performed.
Most sources of nontuberculous mycobacterial (NTM) infection in humans are found in the environment. Infection is acquired through exposure to aerosolized bacilli via inhalation, but also through ingestion or direct invasion (15). In childhood, cervicofacial lymphadenitis is the most common manifestation of infection with NTM, whereas pulmonary disease is a relatively rare occurrence. Most cases occur in children between 1 and 5 years of age. In considering the diagnosis of NTM cervical adenitis in a child, one should first determine whether M. tuberculosis is the causative agent because anti-tuberculous mycobacterial chemotherapy is required even if surgery is undertaken, and the public health implications if the child has tuberculosis are significant. Adenitis due to NTM has a typical radiographic appearance, characterized by ring-enhancing lesions with a minimal inflammatory stranding of the subcutaneous fat, such as in this case. NTM lymphadenitis is associated with a subacute or chronic, but benign, course that may be complicated by the development of fistula if no surgery is undertaken (9). Surgical excision has been the treatment of choice for decades, and most experts continue to recommend this approach (8, 10, 16). Such surgery can be difficult, but complications are uncommon. Incision and drainage of a lymph node with suspected mycobacterial infection should be avoided because such practice commonly leads to chronic drainage and/or sinus tract formation (10). NTM cervical adenitis can be locally destructive, causing chronic or recurring infection associated with significant cosmetic defects (11). Several case reports and small series have reported successful treatment with chemotherapy alone or combined with surgery. Moreover, chemotherapy may render the lesions more amenable to surgery. Macrolides should not be used as monotherapy because resistance occurs rapidly. Rifamycins, either rifampin or rifabutin, are commonly used as a second drug. There is currently no consensus concerning the optimal duration of therapy, but several authors recommend approximately 6 months of therapy (1, 4).
Over 130 species and subspecies of mycobacteria have been established and validated (J. P. Euzéby, List of Prokaryotic Names with Standing in Nomenclature [http://www.bacterio.citc.fr]). Many species of NTM have been associated with cervical adenitis. The MAC is presently responsible for 70% to 90% of cases (16). Members of the MAC are currently identified on the basis of positive results with the commercial AccuProbe MAC-specific probe (Gen-Probe, San Diego, CA). Two species, M. avium and M. intracellulare, were initially identified within the MAC. However, recent studies have drawn attention to the widely diverse population of isolates that can be detected in the complex (5, 6, 12). The recently described species Mycobacterium palustre (11) and Mycobacterium saskatchewanense (13) may be confused with the MAC due to their positive reactions with the AccuProbe MAC test, but they are otherwise genetically distant from the MAC (14).
In 2006, the isolation of Mycobacterium colombiense was described for one sputum and six blood samples from human immunodeficiency virus-positive patients who died as a consequence of their underlying immunocompromised status (7). The authors described the type strain as a nonpigmented slow grower. Urease activity was the main biochemical characteristic that enabled the new species to be distinguished from other members of the complex. Analysis of mycolic acids revealed the three-cluster pattern typical of species belonging to the MAC. M. colombiense tested positive with the AccuProbe MAC identification test (Gen-Probe, San Diego, CA) and tested negative with the M. intracellulare species-specific test. Some of the strains studied by Murcia et al. (7) tested positive with the M. avium species-specific test. The hsp65 gene restriction enzyme analysis pattern was identical to that of M. avium variant I, and sequencing of the internal transcribed space showed a unique sequence, named MAC-X (7). 16S rRNA gene sequencing is considered the gold standard in molecular methodology for the identification of mycobacteria (2). This methodology has also been used to identify rare bacterial species and to establish new species. The type strain of M. colombiense showed a unique sequence of the 16S rRNA gene (7).
Correct identification of clinically relevant mycobacteria is important for proper antimicrobial treatment and the establishment of a detailed taxonomic system. Our laboratory routinely uses sequencing analysis of a portion of the 16S rRNA gene to provide quick and timely identification of mycobacterial isolates that are not identified at the species level with AccuProbe tests.
In conclusion, we report, to our knowledge, the first description of a lymphadenopathy in an immunocompetent child caused by M. colombiense, a recently described species of Mycobacterium. The introduction of more advanced molecular diagnostic methods will dramatically improve the ability to identify less common species that may occasionally cause disease.
Footnotes
Published ahead of print on 27 February 2008.
REFERENCES
- 1.Berger, C., G. E. Pfyffer, and D. Nadal. 1996. Treatment of nontuberculous mycobacterial lymphadenitis with clarithromycin plus rifabutin. J. Pediatr. 128383-386. [DOI] [PubMed] [Google Scholar]
- 2.Clarridge, J. E., III. 2004. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 17840-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cloud, J. L., P. S. Conville, A. Croft, D. Harmsen, F. G. Witebsky, and K. C. Carroll. 2004. Evaluation of partial 16S ribosomal DNA sequencing for identification of Nocardia species by using the MicroSeq 500 system with an expanded database. J. Clin. Microbiol. 42578-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green, P. A., C. F. von Reyn, and R. P. Smith. 1993. Mycobacterium avium complex parotid lymphadenitis: successful therapy with clarithromycin and ethambutol. Pediatr. Infect. Dis. J. 12615-617. [DOI] [PubMed] [Google Scholar]
- 5.Lebrun, L., F. X. Weill, L. Lafendi, F. Houriez, F. Casanova, M. C. Gutierrez, D. Ingrand, P. Lagrange, V. Vincent, and J. L. Herrmann. 2005. Use of the INNO-LiPA-Mycobacteria assay (version 2) for identification of Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum complex isolates. J. Clin. Microbiol. 432567-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mijs, W., P. de Haas, R. Rossau, T. Van der Laan, L. Rigouts, F. Portaels, and D. Van Soolingen. 2002. Molecular evidence to support a proposal to reserve the designation Mycobacterium avium subsp. avium for bird-type isolates and “M. avium subsp. hominissuis” for the human/porcine type of M. avium. Int. J. Syst. Evol. Microbiol. 521505-1518. [DOI] [PubMed] [Google Scholar]
- 7.Murcia, M. I., E. Tortoli, M. C. Menendez, E. Palenque, and M. J. Garcia. 2006. Mycobacterium colombiense sp. nov., a novel member of the Mycobacterium avium complex and description of MAC-X as a new ITS genetic variant. Int. J. Syst. Evol. Microbiol. 562049-2054. [DOI] [PubMed] [Google Scholar]
- 8.Starke, J. R. 1992. Nontuberculous mycobacterial infections in children. Adv. Pediatr. Infect. Dis. 7123-159. [PubMed] [Google Scholar]
- 9.Starke, J. R., and A. G. Correa. 1995. Management of mycobacterial infection and disease in children. Pediatr. Infect. Dis. J. 14455-469. [DOI] [PubMed] [Google Scholar]
- 10.Stewart, M. G., J. R. Starke, and N. J. Coker. 1994. Nontuberculous mycobacterial infections of the head and neck. Arch. Otolaryngol. Head Neck Surg. 120873-876. [DOI] [PubMed] [Google Scholar]
- 11.Torkko, P., S. Suomalainen, E. Livanainen, E. Tortoli, M. Suutari, J. Seppanen, L. Paulin, and M. L. Katila. 2002. Mycobacterium palustre sp. nov., a potentially pathogenic, slowly growing mycobacterium isolated from clinical and veterinary specimens and from Finnish stream waters. Int. J. Syst. Evol. Microbiol. 521519-1525. [DOI] [PubMed] [Google Scholar]
- 12.Tortoli, E., L. Rindi, M. J. Garcia, P. Chiaradonna, R. Dei, C. Garzelli, R. M. Kroppenstedt, N. Lari, R. Mattei, A. Mariottini, G. Mazzarelli, M. I. Murcia, A. Nanetti, P. Piccoli, and C. Scarparo. 2004. Proposal to elevate the genetic variant MAC-A, included in the Mycobacterium avium complex, to species rank as Mycobacterium chimaera sp. nov. Int. J. Syst. Evol. Microbiol. 541277-1285. [DOI] [PubMed] [Google Scholar]
- 13.Turenne, C. Y., L. Thibert, K. Williams, T. V. Burdz, V. J. Cook, J. N. Wolfe, D. W. Cockcroft, and A. Kabani. 2004. Mycobacterium saskatchewanense sp. nov., a novel slowly growing scotochromogenic species from human clinical isolates related to Mycobacterium interjectum and Accuprobe-positive for Mycobacterium avium complex. Int. J. Syst. Evol. Microbiol. 54659-667. [DOI] [PubMed] [Google Scholar]
- 14.Turenne, C. Y., R. Wallace, Jr., and M. A. Behr. 2007. Mycobacterium avium in the postgenomic era. Clin. Microbiol. Rev. 20205-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolinsky, E. 1979. Nontuberculous mycobacteria and associated diseases. Am. Rev. Respir. Dis. 119107-159. [DOI] [PubMed] [Google Scholar]
- 16.Wolinsky, E. 1995. Mycobacterial lymphadenitis in children: a prospective study of 105 nontuberculous cases with long-term follow-up. Clin. Infect. Dis. 20954-963. [DOI] [PubMed] [Google Scholar]