Abstract
The molecular characterization of a Bartonella vinsonii subsp. berkhoffii genotype III strain (NCSU strain 06-CO1) isolated from the blood of a military working dog diagnosed with endocarditis is reported in this study. Several genes were amplified and sequenced for comparative sequence similarity with other strains.
Bartonellae are fastidious hemotropic gram-negative bacteria which have been recently identified in a wide range of domestic and wild animals (2, 6). Based upon sequence differences within the 16S-23S intergenic spacer region (ITS) and the bacteriophage-associated heme-binding protein Pap31 gene (pap31), four Bartonella vinsonii subsp. berkhoffii genotypes have been characterized (3, 5, 11, 14). B. vinsonii subsp. berkhoffii genotype I has been isolated from dog, coyote, and human blood and from dog saliva (1, 3, 5, 10, 14); genotype II has been isolated from dog, coyote, and human blood and dog saliva (3, 10, 14); genotype III was isolated from gray foxes in the United States and was sequenced from a human endocarditis patient in Europe (14, 17); and genotype IV has only been amplified from two dogs with endocarditis, one from Canada and one from Colorado (7, 14; E. B. Breitschwerdt and R. G. Maggi, unpublished data). In this study, we report the molecular characterization of a B. vinsonii subsp. berkhoffii genotype III strain which was obtained by blood culture from a military working dog that originated from Germany and developed endocarditis while stationed in the United States.
Case report.
A 3-year-old, 28.6-kg, spayed female German shepherd obtained from a breeder in Germany at 18 months of age was subsequently stationed in Texas. At procurement, the dog was healthy and serologically negative for Dirofilaria immitis, Babesia canis, Ehrlichia canis, Borrelia burgdorferi, and Rickettsia rickettsii. No clinical abnormalities were noted at her semiannual examination; however, a complete blood count revealed thrombocytopenia (platelet count, 168,000/μl; reference range, 200,000 to 500,000/μl). Three months later the left hock became significantly swollen, with mild crepitus and possible joint effusion. Overnight, the right hock also became painful and swollen; the dog was obtunded, febrile (rectal temperature 103.4°F), and had enlarged popliteal lymph nodes and bilateral pitting edema distal to the tarsus. Lymph node cytology identified lymphadenitis and plasmacytosis, with no etiologic agents visualized. The dog was thrombocytopenic (platelet count, 105,000/μl) and hyperglobulinemic (globulin, 4.7 g/dl). Ehrlichiosis was suspected, and doxycycline was administered (6.7 mg/kg of body weight every 12 h for 3 weeks). Within 9 days, the dog was clinically normal, with complete resolution of the hind-limb swelling and lameness; however, thrombocytopenia persisted on serial hemograms (range, 53,000 to 176,000 platelets/μl). Subsequently, by immunofluorescent antibody testing at the Vector Borne Diseases Diagnostic Laboratory at North Carolina State University, the reciprocal antibody titers were 64 for Babesia canis, 2,048 for Bartonella henselae, 8,192 for B. vinsonii subsp. berkhoffii, 64 for Ehrlichia canis, and 32 for Rickettsia rickettsii, and Bartonella vinsonii subsp. berkhoffii was isolated by Bartonella-Alphaproteobacteria growth medium (BAPGM) blood culture (13). Echocardiography identified marked thickening and irregularity of the aortic valve. Because of the poor prognosis associated with Bartonella endocarditis, the dog was humanely euthanized. Histopathology confirmed aortic-valve endocarditis, with intralesional gram-negative coccobacilli, diffuse neutrophilic and histiocytic inflammation accompanied by hemorrhage, mineralization, marked granulation tissue, and fibrosis of the aortic valve. There was also multifocal lymphocytic plasmacytic myocarditis.
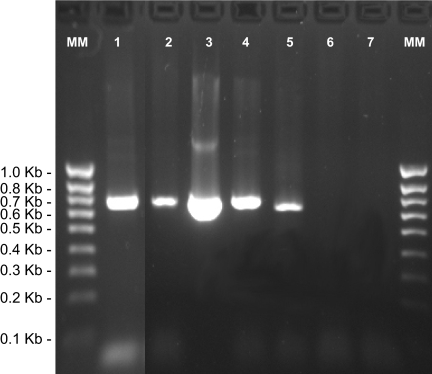
A blood culture isolate (NCSU strain 06-CO1) was obtained after BAPGM preenrichment culture and subinoculation onto blood agar plates (9, 13). Using Bartonella genus ITS primers, amplicons were obtained from blood, tissues, and the isolate (Fig. 1) (15). After cloning, DNA sequences differed by only one base among the various sample sources (14, 15). Based upon alignment of GenBank Bartonella ITS sequences, the dog was infected with B. vinsonii subsp. berkhoffii genotype III. As this was the first B. vinsonii subsp. berkhoffii genotype III isolate from the United States, the partial 16S rRNA gene (4, 15, 18), 16S-23S ITS region (14), pap31 gene (14), RNA polymerase enzyme subunit B gene (rpoB) (8), glucose-6-phosphate 1-dehydrogenase gene (gdh1), and invasion-associated protein B gene (ialB) were sequenced and compared to GenBank sequences using BLAST. Primers, alignment results, and sequence comparisons are shown in Tables 1 and 2. NCSU strain 06-CO1 had higher percent identities with two B. vinsonii subsp. berkhoffii ITS sequences recently deposited from dog blood culture isolates (GenBank accession no. DQ360834 and DQ360835) obtained in China (12) and a sequence from a human endocarditis case from Europe (GenBank accession no. AF143446) (17) and a less-similar identity to a gray fox sequence from California (GenBank accession no. DQ059764). NCSU strain 06-COI is available upon request for research purposes.
FIG. 1.
Bartonella genus ITS PCR from blood, aortic valve, and left ventricular myocardium. ITS PCR amplicon products were resolved through 2% agarose gel electrophoresis, stained with ethidium bromide, and visualized under UV light. Lane 1, preantibiotic blood sample; lane 2, postantibiotic blood sample; lane 3, aortic valve; lane 4, left ventricle; lane 5, Bartonella henselae at 0.001 pg/μl; lane 6, blood from a healthy dog; lane 7, water control. MM, 1-kb molecular marker ladder.
TABLE 1.
List of primers used in PCRs
| Region or gene namea | Primer name | Primer sequence | Reference |
|---|---|---|---|
| 16S rRNA | 8 f | 5′-AGAGTTTGATCCTGGCTCAG-3′ | 20 |
| 325 r | 5′-CGCCAGAAGGCTTGGGATCATCATCTGAAG-3′ | 17 | |
| ITS | 325 f | 5′-TTCAGATGATGATCCCAAGCCTTTTGGCG-3′ | 17 |
| 1100 r | 5′-GAACCGACGACCCCCTGCTTGCAAAGCA-3′ | 17 | |
| pap31 | 1 f | 5′-GACTTCTGTTATCGCTTTGATTT-3′ | 16 |
| 688 r | 5′-CACCACCAGCAAMATAAGGCAT-3′ | 16 | |
| rpoB | 1615 f | 5′-GACTTCTGTTATCGCTTTGATTT-3′ | 9 |
| 2267 r | 5′-CACCACCAGCAAMATAAGGCAT-3′ | 9 | |
| gdh1 | 8 f | 5′-GTAAAATTATTCCAGTCTCTCCCTTT-3′ | |
| 408 f | 5′-TGTCCCAATCTCATCGCAATATCACC-3′ | ||
| ialB | 49 f | 5′-TTGAGTATTTCYTCTGYRTTTGC-3′ | |
| 530 r | 5′-TGCAAAGMARTYAAACGMTTAAGWGC-3′ |
TABLE 2.
Bartonella vinsonii subsp. berkhoffii genotype III (NCSU strain 06-CO1) partial gene sequence identities with other Bartonella strain sequences
| Region or gene | % Identity | GenBank accession no. | Organism designation | Origin |
|---|---|---|---|---|
| ITS region | 99.9 | AF143446 | B. vinsonii subsp. berkhoffii type III | Amplified from a human aortic valve |
| 99.7 | DQ360834 | B. vinsonii subsp. berkhoffii type III strain Q52SHD | Blood culture isolate from a dog | |
| 99.7 | DQ360833 | B. vinsonii subsp. berkhoffii type III strain Q64SHD | Blood culture isolate from a dog | |
| 98.9 | DQ059764 | B. vinsonii subsp. berkhoffii type III | Blood culture isolate from a gray fox | |
| pap31 | 99.3 | DQ059762 | B. vinsonii subsp. berkhoffii type II | Blood culture isolate from a dog |
| 99.1 | AY663045 | B. vinsonii subsp. berkhoffii type I 93-COI | Blood culture isolate from a dog | |
| 98.7 | DQ112677 | B. vinsonii subsp. berkhoffii type IV | Amplified from a dog's aortic valve | |
| 98.4 | DQ071677 | B. vinsonii subsp. berkhoffii type III | Blood culture isolate from a gray fox | |
| 16S rRNA | 100 | DQ228134 | B. vinsonii subsp. berkhoffii type III strain Q52SHD | Blood culture isolate from a dog |
| 100 | DQ228135 | B. vinsonii subsp. berkhoffii type III strain Q64SHD | Blood culture isolate from a dog | |
| 100 | AF143446 | B. vinsonii subsp. berkhoffii type III | Amplified from a human aortic valve | |
| 99.7 | L35052 | B. vinsonii subsp. berkhoffii type I strain 93-COI | Blood culture isolate from a dog | |
| 99.7 | none | B. vinsonii subsp. berkhoffii type II | Blood culture isolate from a dog | |
| rpoB | 99.8 | AF165989 | B. vinsonii subsp. berkhoffii type I strain 93-COI | Blood culture isolate from a dog |
| 99.8 | EF196805 | B. vinsonii subsp. berkhoffii type I by ITS | Blood culture isolate from a dog | |
| 99.8 | none | B. vinsonii subsp. berkhoffii type II by ITS | Blood culture isolate from a dog | |
| gdh1 | 87 | AY074765 | B. henselae strain Houston 1 | Database of complete genome |
| 87 | BX897699 | B. henselae strain Houston 1 | Complete genome from a human isolate | |
| ialB | 88 | BX897700 | Bartonella quintana strain Toulouse | Complete genome from a human isolate |
We report the isolation of B. vinsonii subsp. berkhoffii genotype III from a military working dog that originated in Germany and was stationed in Texas for 18 months prior to the diagnosis of bartonella endocarditis. When the ITS sequence was compared to an ITS sequence from a California gray fox (GenBank accession no. DQ059764) (14), the percent identity was lower (98.9%) than comparable identities (99.9 to 99.7%) with sequences from Europe or China. As persistent intravascular infection for 14 months with a B. vinsonii subsp. berkhoffii genotype II strain in a healthy dog from North Carolina was reported (11), it is possible, based upon ITS sequence similarities, that the dog was infected in Germany prior to shipment to the United States. Alternatively, the dog may have been coinfected with E. canis and Bartonella vinsonii subsp. berkhoffii. Previous studies have reported a seroepidemiological association between exposure to Bartonella vinsonii subsp. berkhoffii and to E. canis, suggesting potential cotransmission by Rhipicephalus sanguineus (16). Whether ITS sequence differences among dog and fox strains reflect host adaptation, strain variation due to geographic origin, or random events remains unanswered. Among the Bartonella species, B. vinsonii subsp. berkhoffii appears to have evolved to preferentially infect canines, including coyotes, dogs, and gray foxes. Those factors that result in disease expression in dogs, including but not limited to endocarditis, are most likely multifactorial and are not well understood. Obtaining additional B. vinsonii subsp. berkhoffii genotype data should better define the reservoir potential, carrier patterns, modes of transmission, and geographic distribution of these zoonotic organisms in nature.
Nucleotide sequence accession numbers.
GenBank accession numbers include EU295657, 16S rRNA gene and 16S-23S intergenic spacer region partial sequence; EU295660, pap31 partial sequence; EU295661, rpoB partial sequence; EU295658, gdh1 partial sequence; and EU295659, ialB partial sequence.
Acknowledgments
We thank those military veterinarians, internists, pathologists, radiologists, and technicians that cared for this dog, provided history, necropsy and histopathology results, and provided blood and tissue samples to the NCSU-VBDDL for testing. Our appreciation is extended to Natalie Cherry for technical assistance and Tonya Lee for editorial assistance.
This research was funded in part by Bayer Animal Health, IDEXX Laboratories, the Sigmon Trust, and the State of North Carolina.
Footnotes
Published ahead of print on 26 March 2008.
REFERENCES
- 1.Breitschwerdt, E. B., D. L. Kordick, D. E. Malarkey, B. Keene, T. L. Hadfield, and K. Wilson. 1995. Endocarditis in a dog due to infection with a novel Bartonella subspecies. J. Clin. Microbiol. 33154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breitschwerdt, E. B., and D. L. Kordick. 2000. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin. Microbiol. Rev. 13428-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breitschwerdt, E. B., R. G. Maggi, A. W. Duncan, W. L. Nicholson, B. C. Hegarty, and C. W. Woods. 2007. Bartonella species in blood of immunocompetent persons with animal and arthropod contact. Emerg. Infect. Dis. 13938-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadenas, M. B., R. G. Maggi, P. P. V. P. Diniz, K. T. Breitschwerdt, S. Sontakke, and E. B. Breitschwerdt. 2007. Identification of bacteria from clinical samples using Bartonella-alpha proteobacteria growth medium. J. Microbiol. Methods 71147-155. [DOI] [PubMed] [Google Scholar]
- 5.Chang, C. C., R. W. Kasten, B. B. Chomel, D. C. Simpson, C. M. Hew, D. L. Kordick, R. Heller, Y. Piemont, and E. B. Breitschwerdt. 2000. Coyotes (Canis latrans) as the reservoir for a human pathogenic Bartonella sp.: molecular epidemiology of Bartonella vinsonii subsp. berkhoffii infection in coyotes from central coastal California. J. Clin. Microbiol. 384193-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chomel, B. B., H.-J. Boulouis, S. Maruyama, and E. B. Breitschwerdt. 2006. Bartonella spp in pets and effect on human health. Emerg. Infect. Dis. 12389-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cockwill, K. R., S. M. Taylor, H. M. Philibert, E. B. Breitschwerdt, and R. G. Maggi. 2007. Bartonella vinsonii subsp. berkhoffii endocarditis in a dog from Saskatchewan. Can. Vet. J. 48839-844. [PMC free article] [PubMed] [Google Scholar]
- 8.Diniz, P. P. V. D. P., R. G. Maggi, D. S. Schwartz, M. B. Cadenas, J. M. Bradley, B. Hegarty, and E. B. Breitschwerdt. 2007. Canine bartonellosis: serological and molecular prevalence in Brazil and evidence of co-infection with Bartonella henselae and Bartonella vinsonii subsp. berkhoffii. Vet. Res. 38697-710. [DOI] [PubMed] [Google Scholar]
- 9.Duncan, A. W., R. G. Maggi, and E. B. Breitschwerdt. 2007. A combined approach for the enhanced detection and isolation of Bartonella species in dog blood samples: pre-enrichment culture followed by PCR and subculture onto agar plates. J. Microbiol. Methods 69273-281. [DOI] [PubMed] [Google Scholar]
- 10.Duncan, A. W., R. G. Maggi, and E. B. Breitschwerdt. 2007. Bartonella DNA detected in saliva collected from dogs. Emerg. Infect. Dis. 131948-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kordick, D. L., and E. B. Breitschwerdt. 1998. Persistent infection of pets within a household with three Bartonella species. Emerg. Infect. Dis. 4325-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, D. M., F. X. Meng, X. P. Song, Z. J. Qin, X. R. Yang, H. X. Wu, D. S. Ren, and Q. Y. Liu. 2006. Study on Bartonella vinsonii berkhoffii isolated from blood of native dogs in China. Zhonghua Liu Xing Bing Xue Za Zhi 27333-338. (In Chinese.) [PubMed] [Google Scholar]
- 13.Maggi, R. G., A. W. Duncan, and E. B. Breitschwerdt. 2005. Novel chemically modified liquid medium that will support the growth of seven Bartonella species. J. Clin. Microbiol. 432651-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maggi, R. G., B. B. Chomel, B. C. Hegarty, J. Henn, and E. B. Breitschwerdt. 2006. A Bartonella vinsonii berkhoffii typing scheme based upon 16S-23S ITS and Pap31 sequences from dog, coyote, gray fox, and human isolates. Mol. Cell. Probes 20128-134. [DOI] [PubMed] [Google Scholar]
- 15.Maggi, R. G., P. P. Diniz, M. B. Cadenas, and E. B. Breitschwerdt. 2006. The use of molecular diagnostic techniques to detect Anaplasma, Bartonella and Ehrlichia species in arthropods or patients. Proc. First Int. Canine Vector-Borne Dis. Symp., p. 9-13. Bayer, Birmingham, United Kingdom.
- 16.Pappalardo, B. L., M. T. Correa, C. C. York, C. Y. Peat, and E. B. Breitschwerdt. 1997. Epidemiologic evaluation of the risk factors associated with exposure and seroreactivity to Bartonella vinsonii in dogs. Am. J. Vet. Res. 58467-471. [PubMed] [Google Scholar]
- 17.Roux, V., S. J. Eykyn, S. Wyllie, and D. Raoult. 2000. Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture-negative endocarditis in a human. J. Clin. Microbiol. 381698-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacchi, C. T., A. M. Whitney, L. W. Mayer, R. Morey, A. Steigerwalt, A. Boras, R. S. Weyant, and T. Popovic. 2002. Sequencing of 16S rRNA gene: a rapid tool for identification of Bacillus anthracis. Emerg. Infect. Dis. 81117-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]