Abstract
Currently, respiratory syncytial virus (RSV) infection is identified in epidemiological studies by virus antigen or nucleic acid detection in combination with serology. Oral-fluid specimens may provide a noninvasive alternative to blood, and oral fluid is more suitable for sampling outside of the clinic setting. We evaluated an indirect enzyme-linked immunosorbent assay for the detection of RSV-specific immunoglobulin G (IgG) and IgA by using oral-fluid samples collected from individuals with RSV infections confirmed by an immunofluorescent antibody test. For five children sampled repeatedly from birth, antibody profiles in oral fluid quite consistently tracked those in paired sera, and RSV infections were detected by rising titers of antibodies of at least one Ig class. Specific IgG responses were generally more reliable than IgA responses, except in early infancy, where the reverse was sometimes true. For a further five young children from whom oral fluid was collected weekly following RSV infection, boosted antibody responses, frequently of a transient nature, lasting a few weeks, were observed; specific IgG responses were of longer duration and more pronounced than specific IgA responses. Our data show significant promise for the use of oral fluid alone in RSV infection surveillance. The observed rapid dynamics of the antibody responses are informative in defining study sampling intervals.
Serological determination of infection with respiratory syncytial virus (RSV) has provided an important addition to virus detection methods in epidemiological and disease burden studies (3-5). However, the collection of blood presents difficulties in certain situations, for example, with infants and young children, in settings outside of the clinic, and where repeated sampling is required, irrespective of signs of disease. Such obstacles are frequently encountered in community-based studies and are a factor contributing to the limited data available on RSV transmission in the family and school settings. For example, information about community transmission is of interest in identifying who infects whom within the household and in determining the role of school children in annual forcing of RSV epidemics; thus, such information is potentially important in devising immunization strategies for the control of RSV.
The use of oral-fluid samples as a noninvasive alternative to blood for determining the occurrence of a recent infection (through specific immunoglobulin M [IgM]) and the prevalence of immunity (inferred from specific IgG) has become well established for childhood monoserotypic viral infections such as measles and rubella (9, 10, 16). Oral fluid has also been used successfully to determine current human immunodeficiency virus infection status (18). In the case of RSV, one study utilized repeated oral-fluid samples for the detection of specific IgG boosting in order to estimate incidence among schoolchildren in the United Kingdom (21). However, the assay was not evaluated against paired serum specimens, and there was no confirmation of infection by a virus detection test. The human RSV-specific IgG antibody response is known to be poor in a high proportion of early infant infections, particularly in the presence of significant maternally derived specific antibodies (7), and may be transient following primary infection (2). Knowledge of the specific antibody dynamics in oral fluid is practically nonexistent but would have value in defining the optimal interval between successive samplings.
We undertook a study to evaluate an anti-RSV indirect enzyme-linked immunosorbent assay for IgG and IgA using paired blood and oral-fluid samples from individuals with clinically identified, antigen detection-confirmed RSV infections, prior to implementation of this assay in screening of a sample set from a large community cohort. Our objectives were to appraise the use of oral-fluid samples to recognize early-age RSV infection and later reinfections and to define the dynamics of the boosting response in order to aid data interpretation and determine the optimal sampling interval for estimating infection rates.
MATERIALS AND METHODS
Various sample sets were available for this study from community-based epidemiological studies in a rural Kenyan community (12, 13, 15). Briefly, a birth cohort of 635 children was intensively monitored through active and passive surveillance for acute respiratory infections (ARI) over three RSV epidemics. Active surveillance took the form of weekly household visits during the RSV season and monthly visits at other times. Passive surveillance ran throughout the study period: mothers were encouraged to bring their children to the research outpatient clinic based at the District Hospital in Kilifi town if they identified any respiratory symptoms. Nasal washings were collected at every episode of ARI. The presence of RSV-infected cells in nasal specimens was determined by immunofluorescence (RSV DFA; Chemicon International, Temecula, CA). A subsample of 70 households of birth cohort children (i.e., those cohort children with siblings) enrolled in a family study was monitored for ARI with RSV in the manner described above. For each RSV episode identified, acute- and convalescent-phase blood and oral-fluid specimen pairs were obtained in order to assess short-term antibody changes. Furthermore, for all participants, oral fluid was collected at roughly 3-month intervals, and for birth cohort children, collection of oral fluid was paired with collection of a venous blood sample. Separately, for a subsample of the household recruits, upon antigen-confirmed RSV infection, oral fluids were collected at weekly intervals for 3 months (13 weekly specimens) in order to quantify longer-term RSV-specific antibody dynamics following infection. Oral fluid was collected using a well-evaluated (10, 19) sponge device (Oracol; Malvern Medical Development, Worcester, United Kingdom) that was rubbed firmly along the gums for 1 min. Extraction and storage methods are detailed elsewhere (11, 21). Ethical approval for this study was provided by the Kenya National Ethical Review Committee and the Coventry Research Ethics Committee, Coventry, United Kingdom.
Oral-fluid and serum specimens were analyzed for anti-RSV IgG and IgA antibodies by using an indirect enzyme-linked immunosorbent assay based on one described by Wilson et al. (21), with further modifications detailed by Scott et al. (17). Briefly, harvested RSV (strain A2) lysate was vortexed before wells of Nunc-Immuno (Fisher Scientific, Leicestershire, United Kingdom) 96-well MaxiSorp plates were coated with 50 μl of lysate/well at a 1/32 dilution in phosphate-buffered saline (PBS) for serum or a 1/16 dilution for oral-fluid analysis. The plates were incubated overnight at 37°C and 120 rpm in a rotating incubator. They were then blocked with 200 μl/well of 5% dry milk (Marvel) in PBS and incubated for 1 h at 37°C. Oral-fluid samples and controls were diluted 1/2 in dry milk in PBS. Serum samples and controls were similarly diluted but at a 1/100 dilution. The remainder of the specific-antibody assay was carried out as previously described (21). Samples were tested for both IgG and IgA antibodies using horseradish peroxidase-conjugated rabbit anti-human IgG and horseradish peroxidase-conjugated rabbit anti-human IgA (Dako) (1/1,000), respectively. Optimal operational dilutions of the lysate and specimen samples (oral fluid or serum) were determined by checkerboard titration. All samples from one individual were tested within a single microtiter plate in order to overcome possible interplate variability. Results were generated as adjusted optical densities (OD) at 492 nm; for this purpose, the background OD for a mock-antigen lysate was subtracted from the OD for the infected lysate. Serum-specific IgG antibody levels were quantified in arbitrary units (AU) by using a log-linear regression line derived from double dilutions of a pooled adult serum standard (1,000 to 31.25 AU per μl) in each plate. While a cutoff point for seropositivity is not specified, the low levels of specific IgA around the time of birth and those of specific IgG antibody attained at the age of 6 to 9 months (once maternal specific antibodies have decayed) in the absence of infection are suggestive of the antibody levels in naïve susceptible infants. We present the results of sample sets for five birth cohort children and for five siblings from families of birth cohort children (note that the two groups of children were unrelated in this instance). These represent the first children tested for whom confirmation of infection by an immunofluorescent antibody test (IFAT) was available and who had sufficiently complete sample sets for depiction of antibody dynamics and comparison of antibody isotypes.
RESULTS AND DISCUSSION
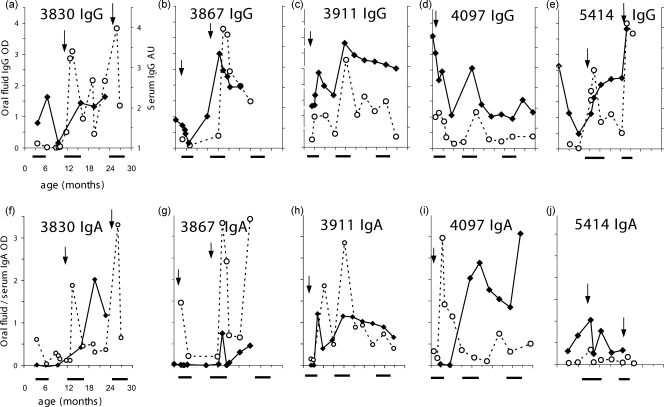
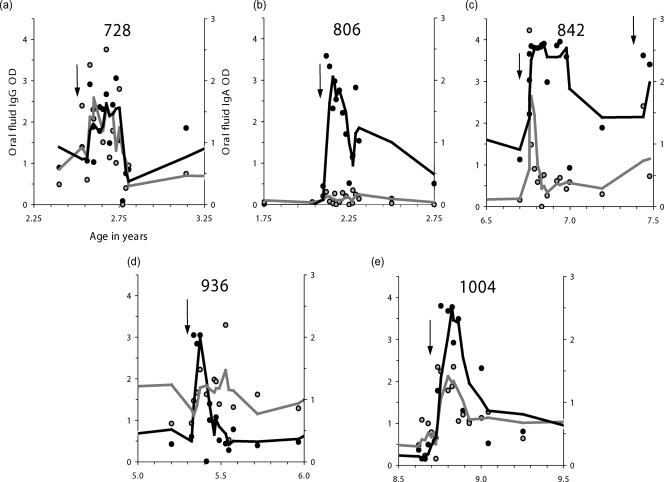
Comparative results of oral-fluid and serum assays are shown in Fig. 1. These data compare the profiles of RSV-specific IgG (Fig. 1a to e) and IgA (Fig. 1f to j) antibody responses in paired serum and oral-fluid samples from five cohort children from birth to the age of 30 months. For each of the five children, there was at least one documented antigen-positive RSV infection, sometimes more than one. Figure 2 shows profiles of specific IgG and IgA antibodies over a 1-year period for oral-fluid samples from five family cohort children who were 2 to 8 years old at the time of infection. In each case, the specific-antibody dynamics over a 13-week period following laboratory-confirmed RSV infection is shown.
FIG. 1.
Evaluation of oral fluid for the detection of RSV infection in birth cohort children from rural Kenya, 2002 to 2005. Shown are specific IgG (a to e) and corresponding IgA (f to j) levels in serum (filled diamonds) and oral-fluid (open circles) samples from five individuals followed from birth over three epidemics (solid horizontal bars below graphs). Arrows indicate time (age) points of IFAT-positive clinical episodes. The labeling and scales for the x and y axes in graph a apply to graphs a to e, and those in graph f apply to graphs f to j.
FIG. 2.
Evaluation of oral fluid for the detection of RSV infection in children from a family study in rural Kenya. Shown are specific IgG (solid symbols and lines) and IgA (shaded symbols and lines) levels in oral fluids from five children in a family cohort study who were sampled every 3 months routinely and every week for 13 weeks following each IFAT-positive clinical episode (arrows identify time points of infection). Lines through data are 3-point moving averages. The labeling and scales for the y axes of graph a apply to graphs a through e. For each graph, the age in years is given along the x axis.
The data for both the birth cohort (Fig. 1) and family (Fig. 2) samples indicate that individually the oral-fluid IgG and IgA tests provided good indications of infection identified by IFAT and that together they proved at least as reliable as (if not better than) serum for identifying infections. Comparison of the profiles reveals a high degree of concordance between serum and oral fluid in detecting seroconversion and early-age reinfection in the presence of preexisting antibodies (Fig. 1) as well as presumed reinfection boosting in older children (Fig. 2).
Although responses were detected for both IgG and IgA antibodies, IgG appears to be more reliable than IgA overall, with much higher signal-to-noise (background variability) ratios and more-prolonged high titers (see, in particular, Fig. 2). Furthermore, in some instances, oral fluid showed more-obvious detection of infection than serum (see, e.g., Fig. 1a and f). This may be attributed to the low levels of antibody in oral fluid (and the resultant lower assay sensitivity), whence such samples are more likely to show a conversion from nondetectable to highly detectable antibodies than serum samples. In agreement with earlier studies (1, 14, 17, 20), infections in the presence of high levels of maternally derived specific IgG (children 3867, 3911, and 4097, with infections at 2.4, 2.2, and 0.8 months, respectively [Fig. 1b to d]) were not always clearly discernible by the IgG assay. However, in each case the IgA OD level or change was suggestive of a response to infection (Fig. 1g to i, respectively). The results also show, in combination with the occurrence of epidemics (Fig. 1), that the serum and oral-fluid results have identified infections not seen by clinical surveillance (e.g., child 4097 at the ages of 12 and 26 months [Fig. 1d and i] and child 3911 at 12 months [Fig. 1c and h]).
The findings on the dynamics of the boosting response following clinical infection (Fig. 2) suggest that although boosting of antibodies does take place (in all children for specific IgG), the responses are often short-lived for both antibody classes, and both IgG and IgA antibodies may decline to preboost levels within a few weeks (Fig. 2b, d, and e) and usually within 3 months. This would imply that a sampling interval of less than 3 months, preferably of 1 month, would be necessary to identify all RSV infections. As with birth cohort children, specific IgG responses were more pronounced (see, e.g., Fig. 2b) and of longer duration (see, e.g., Fig. 2c) than specific IgA responses in these older sibling children.
Our results show a reasonable correlation between oral fluid and serum for both the anti-RSV IgG and IgA assays. These data are the first documented results of a study evaluating the use of oral-fluid samples to identify antigen-confirmed early-age RSV infection and later reinfections and comparing the responses with those detected by serum. The results reveal a high potential for oral fluid as a replacement for serum in identifying RSV infection, a strategy that will be particularly useful in settings such as the home or schools, where repeated invasive sampling may not be possible. Moreover, in some instances, subclinical infections were identified serologically. This would suggest that oral fluid can be used as a means of estimating incidence in the community, especially among older populations, where infection is frequently mild, with low-level viral shedding, so that clinical identification and antigen detection are less reliable. However, the transient nature of the boosted response in older children is important for defining the sampling interval in surveillance studies; it suggests that an interval of more than 1 month may fall short of identifying all infections occurring within a study population.
More often than not, IgG responses were observed to be more pronounced and of longer duration than IgA responses (Fig. 1 and 2); hence, IgG would be the preferred class of antibody for identifying RSV infection. However, as shown here as well as in previous reports (1, 6-8), the infant RSV-specific IgG serological response to infection is not always detectable, particularly in the presence of maternally derived antibodies (Fig. 1). The detection of early RSV infection by specific IgA responses in some children suggests a possible complementary role for IgA class surveillance for infection in early life. Notwithstanding these positive findings, the degree of variability in specific antibody levels suggests that further work to assess the effects of scaling these class-specific responses to the total IgG and IgA levels present within specimens would be useful.
Oral-fluid sampling is noninvasive and painless, and little expertise is required to collect the samples. This method is thus more acceptable than blood sampling, and oral-fluid samples can be collected more frequently (11, 19). Our findings, therefore, provide an encouraging indication of significant potential for large-scale and community-based studies of RSV infection using oral-fluid sampling.
Acknowledgments
We are indebted to all the enrolled children and their guardians and to all staff of the RSV team (field, ward, and lab) and the research outpatient clinic. This study is published with the permission of the Director of KEMRI.
Support for this study was provided by The Wellcome Trust (061584, 076278).
Footnotes
Published ahead of print on 27 February 2008.
REFERENCES
- 1.Brandenburg, A. H., J. Groen, H. A. van Steensel-Moll, E. C. Claas, P. H. Rothbarth, H. J. Neijens, and A. D. Osterhaus. 1997. Respiratory syncytial virus specific serum antibodies in infants under six months of age: limited serological response upon infection. J. Med. Virol. 5297-104. [DOI] [PubMed] [Google Scholar]
- 2.Collins, P. L., R. M. Chanock, and B. R. Murphy. 2001. Respiratory syncytial virus, p. 1443-1485. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 3.Glezen, W., L. Taber, A. Frank, and J. Kasel. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 140543-546. [DOI] [PubMed] [Google Scholar]
- 4.Hall, C., J. Geiman, R. Biggar, D. Kotok, P. Hogan, and R. J. Douglas. 1976. Respiratory syncytial virus infections within families. N. Engl. J. Med. 294414-419. [DOI] [PubMed] [Google Scholar]
- 5.Henderson, F. W., A. M. Collier, W. A. Clyde, Jr., and F. W. Denny. 1979. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N. Engl. J. Med. 300530-534. [DOI] [PubMed] [Google Scholar]
- 6.McIntosh, K., H. Masters, I. Orr, R. Chao, and R. Barkin. 1978. The immunologic response to infection with respiratory syncytial virus in infants. J. Infect. Dis. 13824-32. [DOI] [PubMed] [Google Scholar]
- 7.Murphy, B. R., D. W. Alling, M. H. Snyder, E. E. Walsh, G. A. Prince, R. M. Chanock, V. G. Hemming, W. J. Rodriguez, H. W. Kim, B. S. Graham, and P. F. Wright. 1986. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J. Clin. Microbiol. 24894-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy, B. R., B. S. Graham, G. A. Prince, E. E. Walsh, R. M. Chanock, D. T. Karzon, and P. F. Wright. 1986. Serum and nasal-wash immunoglobulin G and A antibody response of infants and children to respiratory syncytial virus F and G glycoproteins following primary infection. J. Clin. Microbiol. 231009-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigatu, W., D. J. Nokes, A. Afework, D. W. Brown, F. T. Cutts, and L. Jin. 2006. Serological and molecular epidemiology of measles virus outbreaks reported in Ethiopia during 2000-2004. J. Med. Virol. 781648-1655. [DOI] [PubMed] [Google Scholar]
- 10.Nokes, D. J., F. Enquselassie, W. Nigatu, A. J. Vyse, B. J. Cohen, D. W. Brown, and F. T. Cutts. 2001. Has oral fluid the potential to replace serum for the evaluation of population immunity levels? A study of measles, rubella and hepatitis B in rural Ethiopia. Bull. W. H. O. 79588-595. [PMC free article] [PubMed] [Google Scholar]
- 11.Nokes, D. J., F. Enquselassie, A. Vyse, W. Nigatu, F. T. Cutts, and D. W. Brown. 1998. An evaluation of oral-fluid collection devices for the determination of rubella antibody status in a rural Ethiopian community. Trans. R. Soc. Trop. Med. Hyg. 92679-685. [DOI] [PubMed] [Google Scholar]
- 12.Nokes, D. J., E. A. Okiro, M. Ngama, R. Ochola, L. J. White, P. D. Scott, M. English, P. A. Cane, and G. F. Medley. 2008. Respiratory syncytial virus infection and disease in infants and young children observed from birth in Kilifi District, Kenya. Clin. Infect. Dis. 4650-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nokes, D. J., E. A. Okiro, M. Ngama, L. J. White, R. Ochola, P. D. Scott, P. A. Cane, and G. F. Medley. 2004. Respiratory syncytial virus epidemiology in a birth cohort from Kilifi District, Kenya: infection during the first year of life. J. Infect. Dis. 1901828-1832. [DOI] [PubMed] [Google Scholar]
- 14.Ogilvie, M. M., A. S. Vathenen, M. Radford, J. Codd, and S. Key. 1981. Maternal antibody and respiratory syncytial virus infection in infancy. J. Med. Virol. 7263-271. [DOI] [PubMed] [Google Scholar]
- 15.Okiro, E. A. 2007. Transmission dynamics of respiratory syncytial virus within the household and in the community. Open University, Milton Keynes, United Kingdom.
- 16.Ramsay, M. E., L. Jin, J. White, P. Litton, B. Cohen, and D. Brown. 2003. The elimination of indigenous measles transmission in England and Wales. J. Infect. Dis. 187(Suppl. 1)S198-S207. [DOI] [PubMed] [Google Scholar]
- 17.Scott, P. D., R. Ochola, C. Sande, M. Ngama, E. A. Okiro, G. F. Medley, D. J. Nokes, and P. A. Cane. 2007. Comparison of strain-specific antibody responses during primary and secondary infections with respiratory syncytial virus. J. Med. Virol. 791943-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamashiro, H., and N. T. Constantine. 1994. Serological diagnosis of HIV infection using oral fluid samples. Bull. W. H. O. 72135-143. [PMC free article] [PubMed] [Google Scholar]
- 19.Vyse, A. J., B. J. Cohen, and M. E. Ramsay. 2001. A comparison of oral fluid collection devices for use in the surveillance of virus diseases in children. Public Health 115201-207. [DOI] [PubMed] [Google Scholar]
- 20.Welliver, R. C., T. N. Kaul, T. I. Putnam, M. Sun, K. Riddlesberger, and P. L. Ogra. 1980. The antibody response to primary and secondary infection with respiratory syncytial virus: kinetics of class-specific responses. J. Pediatr. 96808-813. [DOI] [PubMed] [Google Scholar]
- 21.Wilson, S., K. Roberts, K. Hammond, J. Ayres, and P. Cane. 2000. Estimation of incidence of respiratory syncytial virus infections in school children using salivary antibodies. J. Med. Virol. 6181-84. [DOI] [PubMed] [Google Scholar]