Abstract
In Neisseria gonorrhoeae, the mosaic structure of the penA gene (encoding penicillin-binding protein 2 [PBP 2]), which is composed of fragments of the penA genes from Neisseria cinerea and Neisseria perflava, has been significantly associated with decreased susceptibility to cephalosporins, particularly oral cephalosporins. The aim of this study was to develop a rapid assay for the detection of mosaic PBP 2 of N. gonorrhoeae by real-time PCR. This assay successfully detected the mosaic penA gene of N. gonorrhoeae, and its sensitivity was ≥101 copies/reaction. Six hundred twenty-one clinical strains were examined by this assay for the presence of mosaic PBP 2, which was detected in 85 (39.4%) of 216 strains from 2002, 69 (40.6%) of 170 strains from 2003, 71 (44.4%) of 160 strains from 2004, and 31 (41.3%) of 75 strains from 2005. The MICs of cephalosporins for strains with the mosaic PBP 2 detected by the assay were statistically higher than those for strains without the mosaic PBP 2. One hundred sixty-six (64.8%) of 256 strains with the mosaic PBP 2 exhibited cefixime MICs of ≥0.5 μg/ml. The emergence and spread of strains with mosaic PBP 2 could be a threat to the cefixime treatment of gonorrhea. This real-time PCR assay for the detection of mosaic PBP 2 of N. gonorrhoeae is thus useful in the prediction of decreased susceptibilities to oral cephalosporins.
Since the emergence and spread of fluoroquinolone-resistant Neisseria gonorrhoeae, cephalosporins have been used as the primary treatment for gonococcal infections. In Japan, however, clinical isolates of N. gonorrhoeae have been found to be less susceptible to oral cephalosporins, including cefixime (1, 7, 11). A mosaic penicillin-binding protein 2 (PBP 2) in clinical strains of N. gonorrhoeae, composed of fragments of PBP 2 from Neisseria cinerea and Neisseria perflava, has been reported to be associated with decreased susceptibility to oral cephalosporins (8). We have suggested that the decreased affinity of mosaic-structure recombinant PBP 2 for oral cephalosporins might contribute to the decreased susceptibility of N. gonorrhoeae to these antibiotics (12). In addition, recent studies have suggested that three amino acid substitutions in the mosaic PBP 2 might reduce the susceptibility of N. gonorrhoeae to cefixime (14) and that the penA mosaic structure, in conjunction with genetic polymorphisms in mtrR, porB1b, and ponA, might reduce the susceptibility to cefixime and ceftriaxone (9). The emergence and spread of strains with such a mosaic PBP 2 could threaten the possibility of treating gonorrhea with cephalosporins. Therefore, the detection of the N. gonorrhoeae penA gene encoding the mosaic PBP 2 associated with decreased susceptibilities to oral cephalosporins could be useful for identifying cephalosporin resistance in clinical strains of N. gonorrhoeae. However, to the best of our knowledge, no studies of the detection of the mosaic penA gene have yet been reported. In the present study, we present our newly developed real-time PCR assay for the mosaic penA gene-specific amplification and detection of N. gonorrhoeae associated with decreased susceptibilities to cephalosporins.
MATERIALS AND METHODS
Bacteria.
To develop a real-time PCR assay, we examined the type strains of N. gonorrhoeae (ATCC 19424), N. cinerea (ATCC 14685), and N. perflava (ATCC 10555) and four clinical strains of N. gonorrhoeae (GU01-12, GU01-29, GU01-89, and GU01-185) isolated from men with gonorrhea who were treated at Gifu University Hospital or its affiliated hospitals in central Japan in 2001. The clinical strains were included in our previous study, in which susceptibilities to penicillin, cephalosporins, and PBP 2 isoforms were analyzed (8). Compared to PBP 2 from ATCC 19424, GU01-12 and GU01-29 PBP 2 had the Asp-345a insertion (2). PBP 2 from GU01-89 and GU01-185 possessed a mosaic structure composed of fragments of PBP 2 from N. cinerea and N. perflava. To detect the mosaic PBP 2 in clinical strains, we examined 70 previously described clinical strains (8) and 621 clinical strains of N. gonorrhoeae isolated from men treated for gonorrhea at Gifu University Hospital or its affiliated hospitals in central Japan between 2002 and 2005. The MICs of cefozopran (Takeda, Osaka, Japan), cefdinir (Astellas Pharma, Inc., Tokyo, Japan), cefixime (Astellas Pharma), and ceftriaxone (Roche Diagnostics, Tokyo, Japan) for each strain were tested using the Clinical and Laboratory Standards Institute performance standards for antimicrobial susceptibility testing (3).
DNA extraction.
For the N. gonorrhoeae, N. cinerea, and N. perflava strains and the four clinical strains of N. gonorrhoeae, DNAs were extracted by the phenol-chloroform method (10). The 70 previously described clinical strains and the 621 clinical strains of N. gonorrhoeae were dissolved in TE buffer (10 mM Tris, 1 mM EDTA; pH 8) and boiled for 10 min in a water bath, after which they were used directly as template DNA, without further purification of the DNA.
Preparation of standard DNA for real-time PCR.
The 1,760-bp fragment including the penA gene was amplified with a pair of primers, penA-F2 (5′-GAATTCTTGATTAAAAGCGAATATAAGCCC-3′) and penA-R3 (5′-GCGGCCGCTTAAGACGGTGTTTTGACGGCT-3′), as previously reported (12). The amplified 1,760-bp fragment of the mosaic structure of the GU01-89 penA gene was inserted into pAcHLT-A (BD Biosciences, San Jose, CA) to generate penA/pAcHLT-A in TOP10 (Invitrogen, San Diego, CA) as previously reported (12). Next, penA/pAcHLT-A was purified with a QIAprep Spin Miniprep kit (Qiagen, Hilden, Germany). The penA/pAcHLT-A was stored at −80°C; its copy number was calculated by measuring the optical density at 260 nm.
Detection of the mosaic structure of the N. gonorrhoeae penA gene.
Specific amplification of the mosaic structure of the penA gene was performed by TaqMan PCR with the primers NG89-F2 (5′-GTTGGATGCCCGTACTGGG-3′), which was the same as a sequence region in N. cinerea, and NG89-R1 (5′-ACCGATTTTGTAAGGCAGGG-3′), which was the same as a sequence region in N. perflava. These were designed to specifically amplify the regions between nucleotides 801 and 1020 of the mosaic penA gene in N. gonorrhoeae and yielded a 220-bp fragment (Fig. 1). The probe NG89-P1 (5′-6-carboxyfluorescein-CGGCAAAGTGGATGCAACCGA-3′-6-carboxytetramethylrhodamine) was designed to detect mutations between nucleotides 968 and 989 of the mosaic penA gene (Fig. 1). The primers and probe were determined to be specific for the mosaic structure of the N. gonorrhoeae penA gene except in the Neisseria meningitidis penA gene (accession no. AY 294556) by online BLAST analysis on the National Center for Biotechnology Information website. The components of PCR in a final volume of 50 μl included TaqMan 1000 Reaction PCR core reagents (Applied Biosystems Japan, Tokyo, Japan) containing AmpliTaq Gold, AmpErase uracil N-glycosylase, 10× TaqMan Buffer A, deoxynucleoside triphosphate mix, and 25 mM MgCl2. The AmpliTaq Gold and AmpErase uracil N-glycosylase were added to final concentrations of 1.25 and 0.5 U/reaction, respectively. The primers, probe, and MgCl2 were added to final concentrations of 1 μM, 250 nM, and 5 mM, respectively. Finally, 5 μl of template DNA was added to each reaction mixture. An ABI PRISM 7900 HT sequence detection system (Applied Biosystems Japan) was used for amplification and detection (2 min at 50°C, 10 min at 95°C, and 40 cycles of 15 s at 95°C and 60 s at 60°C).
FIG. 1.
Locations of the primers and probe in the penA gene and the sequence of the penA genes from bases 801 to 1020. The light-gray arrows indicate the positions of the primers; the dark-gray shading indicates the position of the probe.
Sequencing of the penA gene.
The nucleotide sequence of the full-length penA genes from the N. gonorrhoeae clinical strains was determined as reported previously (8). The penA genes were sequenced by the dye terminator method and with an automatic sequencer (model 3100; Applied Biosystems, Inc., Foster City, CA).
Statistical analysis.
Mann-Whitney U tests were used to compare the MIC distributions. P values of less than 0.05 were considered significant.
RESULTS
Susceptibility to cephalosporins.
The cephalosporin susceptibilities of the 621 strains analyzed in the present study are summarized in Table 1. There were no significant changes in the MIC distributions of cephalosporins for the clinical strains over 4 years.
TABLE 1.
Cephalosporin susceptibilities of clinical strains of N. gonorrhoeae isolated in central Japan from 2002 to 2005
| Cephalosporin and yr in which strains were isolated (no. of strains) | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | MIC50 | MIC90 | |
| Cefozopran | |||
| 2002 (216) | 0.015-32 | 0.5 | 16 |
| 2003 (170) | 0.015-64 | 0.5 | 16 |
| 2004 (160) | 0.008-32 | 0.5 | 16 |
| 2005 (75) | 0.03-32 | 1 | 16 |
| Cefdinir | |||
| 2002 (216) | 0.004-2 | 0.06 | 1 |
| 2003 (170) | 0.004-4 | 0.125 | 2 |
| 2004 (160) | 0.004-2 | 0.06 | 1 |
| 2005 (75) | 0.008-2 | 0.125 | 1 |
| Cefixime | |||
| 2002 (216) | 0.001-2 | 0.06 | 0.5 |
| 2003 (170) | 0.002-2 | 0.06 | 1 |
| 2004 (160) | 0.004-1 | 0.06 | 0.5 |
| 2005 (75) | 0.004-1 | 0.125 | 0.5 |
| Ceftriaxone | |||
| 2002 (216) | 0.001-1 | 0.06 | 0.125 |
| 2003 (170) | <0.001-0.5 | 0.06 | 0.25 |
| 2004 (160) | <0.001-0.25 | 0.06 | 0.125 |
| 2005 (75) | 0.002-2 | 0.03 | 0.125 |
Sensitivity.
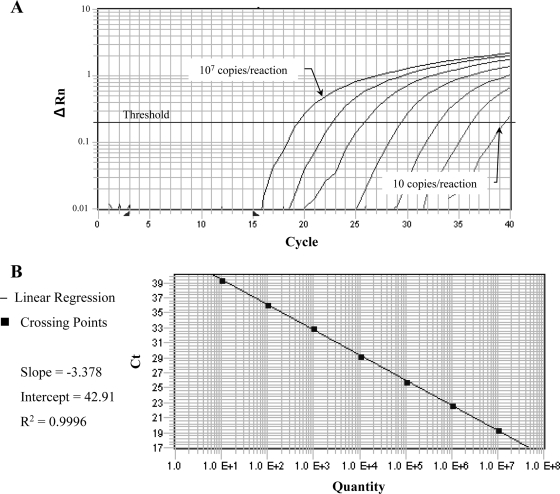
The 10-fold serially diluted plasmid DNA, penA/pAcHLT-A, was amplified by real-time PCR, and the 220-bp fragment was identified by ethidium bromide staining when the sample contained more than 1 × 101 copies/reaction. When the threshold cycles were plotted against the log10 of the copy number of the penA/pAcHLT-A, linearity was observed over the range from 1 × 101 to 1 × 107 copies/reaction (Fig. 2). In one out of three reactions, 1 × 101 copies were detectable.
FIG. 2.
(A) Fluorescence profiles of 10-fold serially diluted penA/pAcHLT-A DNA from 1 × 101 to 1 × 107 copies/reaction analyzed by real-time PCR using an NG89-P1 probe. ΔRn was calculated by subtracting the baseline fluorescence from the reporter fluorescence, which was normalized by an internal reference. (B) A standard curve plot of the 10-fold serial dilution of penA/pAcHLT-A. Linearity is observed throughout the range from 1 × 101 to 1 × 107 copies/reaction.
Specificity.
The specificity of the assay to detect the mosaic penA gene was determined by testing DNAs from N. gonorrhoeae (ATCC 19424), N. cinerea (ATCC 14685), and N. perflava (ATCC 10555), as well as from the two clinical strains (GU01-12 and GU01-29) with the nonmosaic penA gene and the two clinical strains (GU01-89 and GU01-185) with the mosaic penA gene. Signals in real-time PCR were detected only in GU01-89 and GU01-185.
Detection of the mosaic structure of the N. gonorrhoeae penA gene in clinical strains.
Real-time PCR amplification was carried out on DNA isolated from the 70 previously described clinical strains and the 621 clinical strains isolated from 2002 to 2005. Real-time PCR identified the presence of the mosaic penA allele in all 47 of the 70 previously described clinical isolates that were shown by sequencing to contain the mosaic gene and did not detect the mosaic penA gene in the remaining 23 strains that were known not to contain the mosaic allele. The mosaic penA gene was detected in 256 strains, including 85 (39.4%) of 216 strains isolated in 2002, 69 (40.6%) of 170 strains isolated in 2003, 71 (44.4%) of 160 strains isolated in 2004, and 31 (41.3%) of 75 strains isolated in 2005.
Association of mosaic PBP 2 with MICs of cephalosporins.
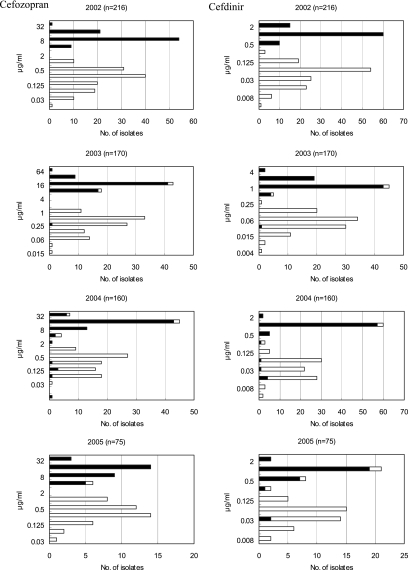
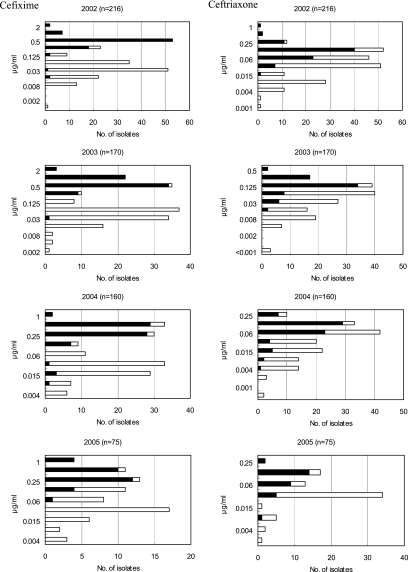
The strains with the mosaic PBP 2 detected by the present assay had statistically higher MICs than the nonmosaic strains for cefozopran, cefdinir, cefixime, and ceftriaxone from 2002 to 2005 (Fig. 3), with P values of less than 0.01. Two hundred forty-eight (96.9%) and 245 (95.7%) of the 256 strains with the mosaic PBP 2 exhibited cefozopran MICs in the range of 4 to 64 μg/ml and cefdinir MICs in the range of 0.5 to 2 μg/ml, respectively, over the 4 years. Two hundred thirty-three (91.0%) of the 256 strains with the mosaic PBP 2 exhibited cefixime MICs in the range of 0.25 to 2 μg/ml. One hundred sixty-six (64.8%) exhibited cefixime MICs of 0.5 μg/ml or greater than 0.5 μg/ml. The distributions of ceftriaxone MICs for the strains with the mosaic PBP 2 and those without the mosaic PBP 2 overlapped. However, the ceftriaxone MICs for strains with the mosaic PBP 2, ranging from 0.004 to 2 μg/ml, were significantly greater than those for strains without the mosaic PBP 2, which ranged from <0.001 to 0.5 μg/ml.
FIG. 3.
Distributions of the MICs of cefozopran, cefdinir, cefixime, and ceftriaxone for the clinical strains of N. gonorrhoeae from 2002 to 2005. The black bars indicate the number of strains with the mosaic PBP 2; the white bars indicate the number of strains without the mosaic PBP 2. Mann-Whitney U tests were used to compare the MICs for the strains with the mosaic PBP 2 with those for the strains without the mosaic PBP 2. The MICs of each cephalosporin for the strains with the mosaic PBP 2 were statistically higher than those for the strains without the mosaic PBP 2.
Sequencing of the penA gene.
There were nine strains of N. gonorrhoeae detected by the real-time PCR assay that showed susceptibility to cephalosporins (cefozopran, MICs of <0.5 μg/ml; cefdinir, cefixime, or ceftriaxone, MICs of <0.25 μg/ml). Of these nine clinical strains, eight strains showed the mosaic structure and one strain showed a nonmosaic structure (Table 2). Compared to PBP 2 from GU01-12, this strain had an alteration of Asn-542→His. On the other hand, there were 12 strains of N. gonorrhoeae without the mosaic PBP 2 that showed decreased susceptibility to cephalosporins (cefozopran, MICs of ≥1 μg/ml; cefdinir, cefixime, or ceftriaxone, MICs of ≥0.5 μg/ml). In these clinical strains, the penA gene did not show the mosaic structure (Table 2).
TABLE 2.
Sequence patterns of the strains for which the MIC and the real-time PCR results were in disagreement
| Strain | Real-time PCR result (CT)a | Sequence pattern | MIC (μg/ml) of drug:
|
|||
|---|---|---|---|---|---|---|
| Cefozopran | Cefdinir | Cefixime | Ceftriaxone | |||
| GU03-252 | + (24.3396) | Nonmosaic | 0.25 | 0.03 | 0.03 | 0.015 |
| GU04-001 | + (18.5181) | Mosaic | 0.125 | 0.015 | 0.015 | 0.008 |
| GU04-061 | + (21.0147) | Mosaic | 0.125 | 0.015 | 0.015 | 0.015 |
| GU04-063 | + (20.7398) | Mosaic | 0.06 | 0.015 | 0.008 | 0.004 |
| GU04-068 | + (20.4905) | Mosaic | 0.25 | 0.06 | 0.03 | 0.06 |
| GU04-091 | + (20.3814) | Mosaic | 0.125 | 0.015 | 0.015 | 0.008 |
| GU04-207 | + (19.5516) | Mosaic | 0.008 | 0.03 | 0.125 | 0.015 |
| GU05-012 | + (19.4374) | Mosaic | 16 | 0.03 | 0.25 | 0.06 |
| GU05-016 | + (21.9816) | Mosaic | 4 | 0.03 | 0.25 | 0.03 |
| GU03-065 | − | Nonmosaic | 8 | 1 | 0.5 | 0.125 |
| GU03-201 | − | Nonmosaic | 16 | 1 | 0.06 | 0.06 |
| GU03-204 | − | Nonmosaic | 16 | 0.5 | 0.03 | 0.06 |
| GU04-052 | − | Nonmosaic | 16 | 1 | 0.5 | 0.125 |
| GU04-060 | − | Nonmosaic | 32 | 1 | 0.5 | 0.25 |
| GU04-062 | − | Nonmosaic | 16 | 1 | 0.5 | 0.125 |
| GU04-244 | − | Nonmosaic | 4 | 0.25 | 0.5 | 0.25 |
| GU04-312 | − | Nonmosaic | 4 | 0.25 | 0.25 | 0.25 |
| GU05-011 | − | Nonmosaic | 0.25 | 1 | 0.06 | 0.03 |
| GU05-013 | − | Nonmosaic | 0.25 | 1 | 0.03 | 0.03 |
| GU05-018 | − | Nonmosaic | 0.25 | 0.5 | 0.5 | 0.03 |
| GU05-062 | − | Nonmosaic | 4 | 0.25 | 0.25 | 0.125 |
CT, threshold cycle; +, positive; −, negative.
DISCUSSION
We developed a real-time PCR assay for the detection of the mosaic structure of the N. gonorrhoeae penA gene, associated with decreased susceptibilities to cephalosporins. The real-time PCR assay targeting the mosaic structure of the N. gonorrhoeae penA gene was both highly specific and highly sensitive and could detect the mosaic PBP 2 in 256 of 621 clinical strains. A large number of clinical strains of N. gonorrheae could be examined for the presence of the mosaic PBP 2 without labor-intensive and time-consuming procedures, including sequencing of DNAs.
In our previous study, we sequenced the penA gene in 70 clinical strains of N. gonorrhoeae, finding this type of mosaic PBP 2 in 47 strains (8). In this previous study, all of the strains with the mosaic PBP 2 exhibited cefdinir MICs in the range of 0.25 to 2 μg/ml, while the remaining 23 strains, all possessing PBP 2 with Asp-345a or Asp-345a and additional amino acid alterations of one to six substitutions, showed cefdinir MICs in the range of 0.015 to 0.125 μg/ml. With respect to cefixime, 45 of 47 strains with the mosaic PBP 2 showed MICs in the range of 0.25 to 1 μg/ml and two showed MICs of 0.125 μg/ml. The remaining 23 strains with nonmosaic PBP 2 showed cefixime MICs in the range of 0.015 to 0.125 μg/ml. The clinical strains were distinctly categorized into oral-cephalosporin-resistant and -susceptible types based on the presence or absence of mosaic PBP 2. In the present study, we examined 621 clinical strains for the presence of the mosaic PBP 2 by means of our developed assay. The MICs of cephalosporins for 256 strains with the mosaic PBP 2 detected by the assay were statistically higher than those for strains without the mosaic PBP 2. This assay could thus be a relevant tool for detecting the mosaic PBP 2 associated with decreased susceptibility to cephalosporins.
In this study, 64.8% of clinical strains with mosaic PBP 2 exhibited cefixime MICs of 0.5 μg/ml or greater. We also reported that the emergence and spread of N. gonorrhoeae with the mosaic PBP 2 showing decreased susceptibility to cefixime could be a threat to the cefixime treatment for gonorrhea. In particular, therefore, this assay, which is able to detect the mosaic PBP 2 in N. gonorrhoeae, could be useful in screening for resistance to cefixime in clinical strains of N. gonorrhoeae.
With respect to ceftriaxone, in both our previous and present studies, the ceftriaxone MICs for strains with mosaic PBP 2 were significantly greater than those for strains with nonmosaic PBP 2. However, the distribution of the former MICs overlapped that of the latter MICs, and some strains with mosaic PBP 2 showed lower ceftriaxone MICs than did those with nonmosaic PBP 2. Recent studies suggest that the ceftriaxone-resistant type is not based on the presence of mosaic PBP 2 (15, 16). We also measured the affinity of mosaic-structure and non-mosaic-structure recombinant PBP 2 for cefdinir, cefixime, and ceftriaxone and found that the decreased affinity of mosaic-structure recombinant PBP 2 for oral cephalosporins might contribute to decreased susceptibility to these antibiotics in N. gonorrhoeae (12). Furthermore, we showed that mosaic-structure recombinant PBP 2 showed the same affinity for ceftriaxone as non-mosaic-structure recombinant PBP 2, suggesting that the susceptibility of ceftriaxone may be determined not only by alterations in PBP 2 but also by other mechanisms. Therefore, the detection of the mosaic structure of the N. gonorrhoeae penA gene in clinical strains could not be effective enough to predict resistance to ceftriaxone.
The present real-time PCR assay was significantly useful for detecting the mosaic PBP 2 in clinical strains of N. gonorrhoeae, which is indicative of decreased susceptibility to oral cephalosporins such as cefixime. However, this assay has several limitations. There were 12 strains of N. gonorrhoeae without the mosaic PBP 2 that showed decreased susceptibility to cephalosporins (cefozopran, MICs of ≥1 μg/ml; cefdinir, cefixime, or ceftriaxone, MICs of ≥0.5 μg/ml). In these clinical strains, the penA gene did not show the mosaic structure (Table 2), and susceptibility to cephalosporins appeared to be determined by other mechanisms (4-6, 13). On the other hand, nine strains of N. gonorrhoeae were detected by the real-time PCR assay that showed susceptibility to cephalosporins (cefozopran, MICs of <0.5 μg/ml; cefdinir, cefixime, or ceftriaxone, MICs of <0.25 μg/ml). All but one of these clinical strains had the mosaic-structure penA gene (Table 2). In these eight strains with the mosaic structure, the mosaic PBP 2 could not decrease susceptibility to cephalosporins. These nine strains, which include one strain for which the sequence pattern result was different from the real-time PCR, require further investigation. The decreased susceptibility to cephalosporins in these strains could not be screened by the assay. Another limitation of the present assay was that the assay could not distinguish the mosaic penA gene of N. gonorrhoeae from the N. meningitidis penA gene (accession no. AY 294556), because the homology of AY 294556 to our primers (NG89-F2 and NG89-R1) and probe (NG89-P1) was 100% in all cases. When the assay is used to detect mosaic PBP 2 of N. gonorrhoeae from clinical specimens, the discrimination between N. gonorrhoeae and N. meningitidis must be carried out by other tests. This real-time PCR assay is a specific amplification and detection of the mosaic penA gene of N. gonorrhoeae; therefore, alterations in areas other than the amplification and detection region are undetectable. Nevertheless, the mosaic PBP 2 in clinical strains of N. gonorrhoeae, composed of fragments of PBP 2 from N. cinerea and N. perflava, was relevant to the decreased susceptibility to oral cephalosporins; the present real-time PCR assay was effective in predicting resistance to oral cephalosporins such as cefixime in clinical strains of N. gonorrhoeae. The amplification and detection region in this assay may contribute to the activity of transpeptidase. This assay is therefore useful as a rapid and simple screening tool in the surveillance of N. gonorrhoeae with decreased susceptibility to cephalosporins, particularly cefixime.
Footnotes
Published ahead of print on 26 March 2008.
REFERENCES
- 1.Akasaka, S., T. Muratani, Y. Yamada, H. Inatomi, K. Takahashi, and T. Matsumoto. 2001. Emergence of cephem- and aztreonam-high-resistant Neisseria gonorrhoeae that does not produce beta-lactamase. J. Infect. Chemother. 749-50. [DOI] [PubMed] [Google Scholar]
- 2.Brannigan, J. A., I. A. Tirodimos, Q. Y. Zhang, C. G. Dowson, and B. G. Spratt. 1990. Insertion of an extra amino acid is the main cause of the low affinity of penicillin-binding protein 2 in penicillin-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 4913-919. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Faruki, H., and P. F. Sparling. 1986. Genetics of resistance in a non-beta-lactamase-producing gonococcus with relatively high-level penicillin resistance. Antimicrob. Agents Chemother. 30856-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill, M. J., S. Simjee, K. Al-Hattawi, B. D. Robertson, C. S. Easmon, and C. A. Ison. 1998. Gonococcal resistance to beta-lactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antimicrob. Agents Chemother. 422799-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagman, K. E., W. Pan, B. G. Spratt, J. T. Balthazar, R. C. Judd, and W. M. Shafer. 1995. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141611-622. [DOI] [PubMed] [Google Scholar]
- 7.Ito, M., M. Yasuda, S. Yokoi, S. Ito, Y. Takahashi, S. Ishihara, S. Maeda, and T. Deguchi. 2004. Remarkable increase in central Japan in 2001-2002 of Neisseria gonorrhoeae isolates with decreased susceptibility to penicillin, tetracycline, oral cephalosporins, and fluoroquinolones. Antimicrob. Agents Chemother. 483185-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito, M., T. Deguchi, K. S. Mizutani, M. Yasuda, S. Yokoi, S. Ito, Y. Takahashi, S. Ishihara, Y. Kawamura, and T. Ezaki. 2005. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in Central Japan. Antimicrob. Agents Chemother. 49137-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindberg, R., H. Fredlund, R. Nicholas, and M. Unemo. 2007. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: association with genetic polymorphisms in penA, mtrR, porB1b, and ponA. Antimicrob. Agents Chemother. 512117-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maniatis, T., J. Sambrook, and E. F. Fritsch. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 11.Muratani, T., S. Akasaka, T. Kobayashi, Y. Yamada, H. Inatomi, K. Takahashi, and T. Matsumoto. 2001. Outbreak of cefozopran (penicillin, oral cephems, and aztreonam)-resistant Neisseria gonorrhoeae in Japan. Antimicrob. Agents Chemother. 453603-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ochiai, S., S. Sekiguchi, A. Hayashi, M. Shimadzu, H. Ishiko, R. Matsushima-Nishiwaki, O. Kozawa, M. Yasuda, and T. Deguchi. 2007. Decreased affinity of mosaic-structure recombinant penicillin-binding protein 2 for oral cephalosporins in Neisseria gonorrhoeae. J. Antimicrob. Chemother. 6054-60. [DOI] [PubMed] [Google Scholar]
- 13.Ropp, P. A., M. Hu, M. Olesky, and R. A. Nicholas. 2002. Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahata, S., N. Senju, Y. Osaki, T. Yoshida, and T. Ida. 2006. Amino acid substitutions in mosaic penicillin-binding protein 2 associated with reduced susceptibility to cefixime in clinical isolates of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 503638-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiley, D. M., E. A. Limnios, S. Ray, T. P. Sloots, and J. W. Tapsall. 2007. Further questions regarding the role of mosaic penA sequences in conferring reduced susceptibility to ceftriaxone in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 51802-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiley, D. M., E. A. Limnios, S. Ray, T. P. Sloots, and J. W. Tapsall. 2007. Diversity of penA alterations and subtypes in Neisseria gonorrhoeae strains from Sydney, Australia, that are less susceptible to ceftriaxone. Antimicrob. Agents Chemother. 513111-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]