Abstract
Respiratory syncytial virus (RSV) and human metapneumovirus (hMPV) are two important viral pathogens that cause respiratory tract infections in the pediatric population. The rapid detection of these agents allows the prompt isolation and treatment of infected patients. In the present prospective study, we evaluated the performances of four rapid antigen detection assays, including a rapid chromatographic immunoassay (CIA) for RSV (Directigen EZ RSV; Becton Dickinson, Sparks, MD), a direct fluorescent-antibody assay (DFA) for RSV (Bartels; Trinity Biotech, Carlsbad, CA), and two DFAs for hMPV manufactured by Diagnostic Hybrids Inc. (DHI; Athens, OH) and Imagen (Oxoid Ltd., Basingstoke, Hampshire, United Kingdom). The clinical specimens tested comprised 515 nasopharyngeal aspirates submitted to the Clinical Microbiology Laboratory at Hartford Hospital from 1 November 2006 to 21 April 2007. Compared to the results of real-time reverse transcription-PCR (RT-PCR), the CIA had a sensitivity of 79.8% and a specificity of 89.5%. The RSV DFA with Bartels reagents showed a sensitivity of 94.1% and a specificity of 96.8%. For hMPV, the sensitivity and specificity were 62.5% and 99.8%, respectively, for the DHI DFA and 63.2% and 100%, respectively, for the Imagen DFA. The hands-on and test turnaround times for CIA were 10 and 30 to 60 min, respectively, and the hands-on and test turnaround times for the RSV and hMPV DFAs were 30 and 105 min, respectively. We conclude that while the RSV CIA is user-friendly, it lacks sensitivity and specificity, especially during off-peak months. In contrast, the RSV DFA is more sensitive and specific, but interpretation of its results is subjective and it demands technical time and expertise. Similarly, both hMPV DFAs are highly specific in comparison to the results of RT-PCR, but their sensitivities await further improvements.
Respiratory syncytial virus (RSV) is the single most important cause of respiratory tract infections in children. It is estimated that each year in the United States, 100,000 hospitalizations and 4,500 deaths are attributed to RSV infection (20). Similar to RSV, human metapneumovirus (hMPV), identified in The Netherlands in 2001, is thought to cause upper and lower respiratory tract infections in children (23). Both RSV and hMPV are members of the family Paramyxoviridae (20). They are enveloped, single-stranded, negative-sense RNA viruses. Epidemiological studies indicate that, like RSV, hMPV is a significant human respiratory pathogen with a worldwide distribution (6, 16, 23, 24). Indeed, hMPV appears to affect many of the same subpopulations and cause clinical manifestations, including upper respiratory tract infections, bronchiolitis, and pneumonia, similar to those caused by RSV, although they are of lesser severity (24). Both RSV and hMPV have been shown to infect the majority of children by the age of 5 years. Moreover, reinfections have been observed in all age groups (4).
The laboratory diagnosis of RSV and hMPV infections can be made by virus isolation, detection of viral antigens, amplification of viral RNA by molecular techniques, demonstration of a rise in serum antibody levels, or a combination of these approaches (7, 9, 13, 15, 21, 26). The use of rapid tests for the diagnosis of RSV and hMPV infections allows implementation of appropriate infection control measures, thus reducing nosocomial spread, and is useful for consideration of timely treatment with antiviral agents (8, 12). The clinical and financial benefits of the rapid detection of RSV in respiratory specimens have been demonstrated in several studies, indicating a direct correlation between a rapid turnaround time and decreased mortality, a decreased length of stay, overall costs, and better antibiotic stewardship (1, 8, 12, 25). On the other hand, few rapid antigen assays for hMPV detection with hMPV-specific monoclonal antibodies have been reported (3, 9, 18). While enzyme immunoassay, chromatographic immunoassay (CIA), and direct fluorescent-antibody assay (DFA) have been adapted to RSV rapid antigen testing (13, 26), DFA remains the only format being used for hMPV rapid antigen testing (5, 15).
The goal of this study was to prospectively evaluate the performances of four commercially available rapid diagnostic assays (one CIA and three DFAs) for the detection of these two viruses in respiratory samples during a respiratory virus season.
(This study was presented in part at the 107th General Meeting of the American Society for Microbiology, Toronto, Ontario, Canada, 21 to 25 May 2007.)
MATERIALS AND METHODS
Clinical specimens.
Nasopharyngeal samples submitted to the Clinical Microbiology Laboratory at Hartford Hospital for RSV testing from 1 November 2006 to 21 April 2007 were included in this study. A nasopharyngeal aspirate or wash received in a cup or a French feeding tube was suspended in 2 ml of sterile saline and was mixed with a sterile disposable pipette. A 0.5-ml aliquot from each sample suspension was placed in a Sarstedt screw-cap microcentrifuge tube and stored at −70°C until it was tested for RSV and hMPV by reverse transcription-PCR (RT-PCR). The remainder of the specimen was immediately used to perform a CIA for RSV and DFAs for RSV and hMPV.
RSV CIA.
The Directigen EZ RSV (Becton Dickinson, Sparks, MD) test was performed according to the manufacturer's recommendations (13, 26). Briefly, 250 μl of each sample was extracted and added to an individual CIA device. RSV antigen, if it was present, was allowed to bind to the antibody-colloidal gold conjugate in the test strip to form an antigen-antibody complex. The complex was allowed to migrate across the test strip to the reaction area, where it was captured by the line of a second RSV antibody on the membrane. Excess conjugate in the strip also migrates along the strip and binds to a second line consisting of inactivated RSV antigen, which serves as an internal control.
RSV and hMPV DFAs.
Samples were first centrifuged at 1,500 rpm (400 ×g) for 10 min at 4°C to remove excess mucus. The supernatant was aspirated, and the pellet was washed twice in 4 to 6 ml of phosphate-buffered saline (PBS) to break up and to remove excess mucus. The final pellet was resuspended in PBS to attain a turbidity of about 1 McFarland standard. Two smears from the cell suspension were made on a two-well fluorescence slide. The smears were allowed to air dry thoroughly at room temperature and were fixed in cold acetone for 10 min. Twenty microliters of RSV-specific fluorescein isothiocyanate (FITC)-antibody (Trinity Biotech, Carlsbad, CA) (17) was placed in one well, and 20 μl of hMPV-specific FITC-antibody (Diagnostic Hybrid Inc. [DHI], Athens, OH) was added to the second well. Beginning on 1 February 2007, additional slides were prepared with all specimens as described above and stained with 20 μl of hMPV-specific FITC-antibody (Imagen; Oxoid Ltd., Basingstoke, Hampshire, United Kingdom). The slides were incubated for 30 min at 35°C in a moist chamber. After the incubation, excess antibody was washed away with PBS and the smears were allowed to air dry at room temperature. One drop of mounting fluid was added to the center of each well, and a coverslip was placed over the mounting fluid. The entire well area of the slide was scanned with a fluorescent microscope (9). Both RSV and hMPV were detected by their characteristic granular, bright apple green fluorescence within the cells, which contrasted with the red background staining of uninfected cells.
RNA extraction.
Total nucleic acids were extracted by using a NucliSens easyMAG system (bioMerieux Inc., Durham, NC). Briefly, 0.9 ml of lysis buffer was added to 0.2 ml of thawed nasopharyngeal samples. After a thorough vortex mixing, 200 μl of the mixture was placed in the instrument by using the default extraction protocol (22). Total nucleic acids were eluted in 55 μl of elution buffer (bioMerieux Inc.), and 5 μl of the extracts was used for nucleic acid amplification.
Real-time TaqMan RT-PCR assays.
Two real-time RT-PCR assays that detect RSV and hMPV were performed with an ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA), as described previously (11). In brief, a 25-μl reaction mixture containing 5 μl extracted RNA, 0.5 μM each primer, and 0.2 μM TaqMan probe was mixed with 25 μl TaqMan One-Step RT-PCR 2× master mixture (Applied Biosystems). The reaction conditions were designed as follows: RT at 48°C for 30 min, initial denaturation at 95°C for 10 min, and 40 cycles of denaturation (95°C for 15 s) and annealing/extension (60°C for 1 min). The probes were dual labeled with the reporter dye 6-carboxyfluorescein at the 5′ end and the 6-carboxytetramethyrhodamine quencher at the 3′ end (11).
RT-PCR-EIA.
A microtiter RT-PCR-enzyme immunoassay (EIA) was used to detect RSV, as described previously (21). The PCR mixture (50 μl) contained the following: 1× EN buffer; 18% glycerol; 300 μM dATP, dCTP, and dGTP; 285 μM dUTP; 15 μM digoxigenin-11-dUTP (Roche Diagnostics, Indianapolis, IN); 0.5 μM each primer; 0.01 U/μl uracil N-glycosylase (UNG; Epicenter Technologies, Madison, WI); 0.15 U/μl Tth polymerase (Applied Biosystems); and 10 μl of specimen extract. The reaction mixture was placed in an ABI 9700 thermal cycler programmed for a one-step RT-PCR procedure. The procedure included (i) an initial UNG activation, RT, and UNG inactivation/denaturation of 5 min at 50°C, 30 min at 65°C, and 3 min at 94°C, respectively; (ii) 5 cycles of 15 s at 94°C and 30 s at 60°C; (iii) 45 cycles of 15 s at 90°C and 30 s at 60°C; and (iv) a 10-min extension at 72°C. The output signal was measured at an optical density of 450 (OD450). A positive result was defined as an OD450 − OD490 value greater than or equal to 0.1.
RT-PCR for hMPV detection with commercial reagents.
The RT-PCR was performed with the Pro hMPV real-time assay kit (Prodesse, Inc., Waukesha, WI). Reagents not included in the kit were Platinum Taq DNA polymerase (Invitrogen Corp., Carlsbad, CA) and murine leukemia virus reverse transcriptase (Applied Biosystems). The manufacturer-recommended procedure was followed. The assay was performed on a SmartCycler instrument (Cepheid, Sunnyvale, CA).
Evaluation references.
Samples with inconsistent results between the rapid DFA and the TaqMan assay were retested by the RT-PCR-EIA for RSV and by the Prodesse assay for hMPV. Samples for which the results of the majority of the assays matched (i.e., samples for which the results of two of the three assays or better matched) were considered references.
RESULTS
A total of 515 pediatric specimens had adequate volumes or numbers of cells with which all the tests could be performed. A specimen was considered adequate if there were at least 1 or more DFA-positive cells per slide or 20 DFA-negative cells per slide. Specimens from the emergency department, outpatient clinics, inpatient non-intensive care unit floors, and inpatient intensive care unit floors accounted for 71%, 6%, 14%, and 9% of all the samples submitted, respectively. A total of 283 (55%) of the samples were collected from male patients and 232 (45%) were collected from female patients. The median age for all children was 4 months. Overall, 67% of the specimens were from children 6 months or age or younger, and 33% were from children older than 6 months of age.
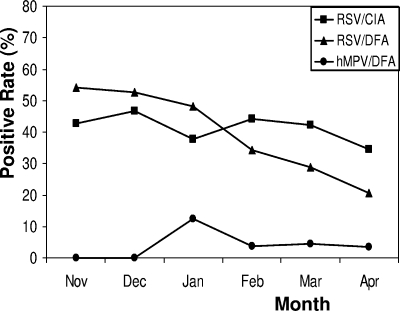
The seasonal distribution of RSV and hMPV detection is presented in Fig. 1. As expected, November, December, January, and February had the highest numbers of RSV-positive samples; of those months, December had the most RSV-positive samples. While the rate of detection of RSV remained relatively high throughout this period, the incidence of RSV peaked in December. In contrast, few samples positive for hMPV were detected except during its peak incidence in January.
FIG. 1.
Seasonal distribution of RSV and hMPV antigens detected in nasopharyngeal specimens.
Of the 515 specimens tested, 272 (53%) were positive for RSV by at least one assay. Overall, 219 samples tested positive by CIA, 233 tested positive by DFA, and 235 tested positive by the TaqMan PCR. A second PCR assay, RT-PCR-EIA, was used to retest specimens with discordant results between the RSV DFA and the TaqMan RT-PCR. Forty specimens tested positive by only one or two assays: 29 by the RSV CIA, 4 by the RSV DFA, and 7 by both RT-PCR and RT-PCR-EIA. Three specimens that had tested positive by the RSV CIA and DFA were found to be negative by RT-PCR but tested positive by RT-PCR-EIA. Similarly, three specimens that had tested positive by CIA and DFA were found to be negative by both PCR tests. These specimens were considered to have false-positive results by CIA and DFA. However, we cannot rule out the possibility that both PCR assays failed to detect the virus in these specimens due to mutations in primer/probe binding regions. When the majority results were used as the evaluation standard, the sensitivity, specificity, positive predicative value (PPV), and negative predictive value (NPV) were 79.8%, 89.5%, 86.8%, and 83.8%, respectively, for the RSV CIA and 94.1%, 96.8%, 96.1%, and 95.0%, respectively, for the RSV DFA (Table 1).
TABLE 1.
Sensitivities, specificities, and predictive values of the four rapid antigen testsa
| Test | No. of specimens with the following result:
|
Sen (%) | Spe (%) | PPV (%) | NPV (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | S+, T+ | S+, T− | S−, T+ | S−, T− | |||||
| RSV CIA | 515 | 190 | 48 | 29 | 248 | 79.8 | 89.5 | 86.8 | 83.8 |
| RSV DFA | 515 | 224 | 14 | 9 | 268 | 94.1 | 96.8 | 96.1 | 95.0 |
| hMPV DFA (DHI) | 515 | 20 | 12 | 1 | 482 | 62.5 | 99.8 | 95.2 | 97.6 |
| hMPV DFA (Imagen)b | 118 | 12 | 7 | 0 | 99 | 63.2 | 100.0 | 100.0 | 93.4 |
Abbreviations and symbols: Sen, sensitivity; Spe, specificity; S, standard; T, test; +, positive result; −, negative result.
Validation of the Imagen DFA kit was initiated in the middle of the study period.
The TaqMan RT-PCR detected hMPV in 32 samples, of which 20 were also positive by the DHI DFA. Tests with the 12 DFA-negative, TaqMan RT-PCR-positive samples were repeated by using the Prodesse real-time PCR, and they all tested positive for hMPV. By using the RT-PCR results as the “gold standard,” the sensitivity, specificity, PPV, and NPV for the DHI hMPV DFA were 62.5%, 99.8%, 95.2%, and 97.6%, respectively. The Imagen hMPV DFA entered the trial and detected hMPV in 12 of 118 specimens, giving a sensitivity, a specificity, a PPV, and an NPV of 63.2%, 100.0%, 100.0%, and 93.4%, respectively (Table 1). One PCR-negative sample tested positive for hMPV by the DHI DFA and tested negative by the Imagen DFA but was shown to be positive for RSV by CIA, DFA, and both PCR assays.
A substantial number of false-positive and false-negative results were given by the RSV CIA when the combination of results from DFA and the two RT-PCR assays were used as the evaluation standard (Table 1). The total rates of false results ranged from 12.5 to 24.4%, and these were distributed relatively evenly throughout the season. The false-negative results by the RSV CIA happened mainly in the early part of the season. In contrast, the false-positive results by the RSV CIA happened mainly in the late part of the season (Table 2).
TABLE 2.
Seasonal distribution of false-negative and -positive RSV CIA results
| RSV CIA false results | No. (%) of specimens with the indicated result in:
|
|||||
|---|---|---|---|---|---|---|
| Nov. (n = 96) | Dec. (n = 152) | Jan. (n = 114) | Feb. (n = 79) | March (n = 45) | April (n = 29) | |
| False positive (n = 29) | 0 (0.0) | 3 (2.0) | 2 (1.8) | 10 (12.7) | 9 (20.0) | 5 (17.2) |
| False negative (n = 48) | 12 (12.5) | 16 (10.5) | 14 (12.3) | 4 (5.1) | 2 (4.4) | 0 (0.0) |
| Total false results (n = 77) | 12 (12.5) | 19 (12.5) | 16 (14.0) | 14 (17.7) | 11 (24.4) | 5 (17.2) |
DISCUSSION
RSV and hMPV are the two most common causes of bronchiolitis and pneumonia among infants and children under 1 year of age. Babies (especially those born prematurely), people with immune system problems, people with heart or lung problems, and older adults have an increased risk of developing complications from RSV infection. The clinical symptoms and laboratory findings associated with hMPV infection exhibit a spectrum virtually indistinguishable from those associated with RSV disease. With one possible exception, RSV peaks from December to February, while hMPV is increasingly detected from January to April (14, 24). However, it is generally accepted that during the respiratory illness season, laboratories must take into account the existence of both viruses.
We received 515 clinical samples with adequate volumes with which all of the tests could be performed. While the overall sensitivity and specificity of the CIA for RSV were 79.8% and 89.5%, respectively, CIA was more specific during the peak months of November, December, and January and was far less specific during the off-peak months of February, March, and April. Our data indicated that the false-negative results by RSV CIA happened mainly in the early stage of the season and that the false-positive results happened in the late stage of the season. Because RSV is seen sporadically throughout the year, we concur with other investigators that CIA alone should not be used to detect RSV during off-peak months (20). However, considering the ease of performance and hands-on and turnaround times of 10 and 30 to 60 min, respectively, CIA is very useful for the rapid detection of most positive samples during the peak months of RSV infection. In contrast to CIA, the RSV DFA had a sensitivity and a specificity of 94.1% and 96.8%, respectively, and these remained consistent throughout the season. This is consistent with the manufacturer's performance claim of a sensitivity of 88 to 100%, but we did not attain 100% specificity, as claimed by the manufacturer (20). The relatively high sensitivity of this assay in our study may have been obtained because only specimens with adequate numbers of cells were included in this study.
In recent years, the possibility of hMPV and RSV coinfection has received considerable attention. Semple et al. reported in 2005 that children dually infected with hMPV and RSV present with severe bronchiolitis and an increased risk of admission to a pediatric intensive care unit for mechanical ventilation (19). Others have shown no change in disease severity in patients coinfected with both viruses and a significant variation in the frequency of coinfection on the basis of geographic location and patient population. For example, Cuevas et al., who studied 111 children with acute respiratory infections attending clinics and hospitals in Aracaju, Brazil, reported that 7% of all of the patients were coinfected with RSV and hMPV (2). In contrast, Lazar et al., who studied 46 subjects in 2004, did not see any coinfection at the time that both viruses were circulating in their community in southern Connecticut (10). In this study, we detected eight hMPV-positive cases who tested positive for RSV by CIA, but all cases tested negative for RSV by DFA and RT-PCR. Specimens were collected from these cases in the late study stage of the season and were considered false positive for RSV. Overall, there was evidence of coinfection in specimens from four patients (0.78%), of which only a single specimen was confirmed to contain both viruses by RT-PCR. Unlike the study from Brazil, with its reported coinfection rate of 7%, the low rate of coinfection (0.78%) among our patient population may explain the lack of coinfection observed in the limited number of samples (46 subjects) tested by Lazar et al. during the 2004 respiratory season in Connecticut (10). We conclude that, on rare occasions, RSV and hMPV coinfection does occur among our patient population in northern Connecticut.
Thirty-two specimens were positive for hMPV by two RT-PCR assays performed in two separate laboratories. In addition, one specimen that tested strongly positive for RSV by RT-PCR (threshold cycle value, 19), DFA, and CIA was shown to be positive for hMPV by the DHI DFA. The RT-PCR results for this specimen were negative, and the result of the DHI test was considered to be false positive.
Among the 12 RT-PCR-positive samples tested by both the Imagen and the DHI assays, the DHI assay detected 6 positive samples, whereas the Imagen assay detected 7 positive samples. Similarly, the retrospective retesting of all of the specimens that were RT-PCR positive but DHI DFA negative by the Imagen DFA resulted in two additional DFA-positive samples. The overall sensitivity and specificity were 62.5% and 99.8%, respectively, for the DHI assay and 63.2% and 100%, respectively, for the Imagen assay.
We conclude that the RSV CIA lacks sensitivity and specificity, especially during off-peak months, and this conclusion concurs with the conclusions made in previous studies. In contrast, the RSV DFA is more sensitive and specific throughout the year. Similarly, both hMPV DFAs are highly specific compared with the results of RT-PCR, but their sensitivities await further improvement.
Acknowledgments
We thank Carol Latter for editorial assistance during preparation of the manuscript.
Footnotes
Published ahead of print on 12 March 2008.
REFERENCES
- 1.Barenfanger, J., C. Drake, N. Leon, T. Mueller, and T. Troutt. 2000. Clinical and financial benefits of rapid detection of respiratory viruses: an outcomes study. J. Clin. Microbiol. 382824-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuevas, L. E., A. M. Nasser, W. Dove, R. Q. Gurgel, J. Greensill, and C. A. Hart. 2003. Human metapneumovirus and respiratory syncytial virus, Brazil. Emerg. Infect. Dis. 91626-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebihara, T., R. Endo, X. Ma, N. Ishiguro, and H. Kikuta. 2005. Detection of human metapneumovirus antigens in nasopharyngeal secretions by an immunofluorescent-antibody test. J. Clin. Microbiol. 431138-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falsey, A. R., D. Erdman, L. J. Anderson, and E. E. Walsh. 2003. Human metapneumovirus infections in young and elderly adults. J. Infect. Dis. 187785-790. [DOI] [PubMed] [Google Scholar]
- 5.Fenwick, F., B. Young, R. McGuckin, M. J. Robinson, Y. Taha, C. E. Taylor, and G. L. Toms. 2007. Diagnosis of human metapneumovirus by immunofluorescence staining with monoclonal antibodies in the North-East of England. J. Clin. Virol. 40193-196. [DOI] [PubMed] [Google Scholar]
- 6.Freymouth, F., A. Vabret, L. Legrand, N. Eterradossi, F. Lafay-Delaire, J. Brouard, and B. Guillois. 2003. Presence of the new human metapneumovirus in French children with bronchiolitis. Pediatr. Infect. Dis. J. 2292-94. [DOI] [PubMed] [Google Scholar]
- 7.Hamelin, M. E., and G. Boivin. 2005. Development and validation of an enzyme-linked immunosorbent assay for human metapneumovirus serology based on a recombinant viral protein. Clin. Diagn. Lab. Immunol. 12249-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karanfil, L. V., M. Conlon, K. Lykens, C. F. Masters, M. Forman, M. E. Griffith, T. R. Townsend, and T. M. Perl. 1999. Reducing the rate of nosocomially transmitted respiratory syncytial virus. Am. J. Infect. Control 2791-96. [DOI] [PubMed] [Google Scholar]
- 9.Landry, M. L., D. Ferguson, S. Cohen, T. C. Peret, and D. D. Erdman. 2005. Detection of human metapneumovirus in clinical samples by immunofluorescence staining of shell vial centrifugation cultures prepared from three different cell lines. J. Clin. Microbiol. 431950-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazar, I., C. Weibel, J. Dziura, D. Ferguson, M. L. Landry, and J. S. Kahn. 2004. Human metapneumovirus and severity of respiratory syncytial virus disease. Emerg. Infect. Dis. 101318-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, H., M. A. McCormac, R. W. Estes, S. E. Sefers, R. K. Dare, J. D. Chappell, D. D. Erdman, P. F. Wright, and Y. W. Tang. 2007. Simultaneous detection and high-throughput identification of a panel of RNA viruses causing respiratory tract infections. J. Clin. Microbiol. 452105-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macfarlane, P., J. Denham, J. Assous, and C. Hughes. 2005. RSV testing in bronchiolitis: which nasal sampling method is best? Arch. Dis. Child. 90634-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohm-Smith, M. J., P. S. Nassos, and B. L. Haller. 2004. Evaluation of the Binax NOW, BD Directigen, and BD Directigen EZ assays for detection of respiratory syncytial virus. J. Clin. Microbiol. 422996-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ordas, J., J. A. Boga, M. Alvarez-Arguelles, L. Villa, C. Rodriguez-Dehli, M. de Ona, J. Rodriguez, and S. Melon. 2006. Role of metapneumovirus in viral respiratory infections in young children. J. Clin. Microbiol. 442739-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Percivalle, E., A. Sarasini, L. Visai, M. G. Revello, and G. Gerna. 2005. Rapid detection of human metapneumovirus strains in nasopharyngeal aspirates and shell vial cultures by monoclonal antibodies. J. Clin. Microbiol. 433443-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peret, T. C., G. Boivin, Y. Li, M. Couillard, C. Humphrey, A. D. Osterhaus, D. D. Erdman, and L. J. Anderson. 2002. Characterization of human metapneumoviruses isolated from patients in North America. J. Infect. Dis. 1851660-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabalais, G. P., G. G. Stout, K. L. Ladd, and K. M. Cost. 1992. Rapid diagnosis of respiratory viral infections by using a shell vial assay and monoclonal antibody pool. J. Clin. Microbiol. 301505-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rovida, F., E. Percivalle, M. Zavattoni, M. Torsellini, A. Sarasini, G. Campanini, S. Paolucci, F. Baldanti, M. G. Revello, and G. Gerna. 2005. Monoclonal antibodies versus reverse transcription-PCR for detection of respiratory viruses in a patient population with respiratory tract infections admitted to hospital. J. Med. Virol. 75336-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semple, M. G., A. Cowell, W. Dove, J. Greensill, P. S. McNamara, C. Halfhide, P. Shears, R. L. Smyth, and C. A. Hart. 2005. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J. Infect. Dis. 191382-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang, Y. W., and J. W. Crowe, Jr. 2007. Respiratory syncytial virus and human metapneumovirus, p. 1361-1377. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, vol. 2, 9th ed. American Society for Microbiology Press, Washington, DC. [Google Scholar]
- 21.Tang, Y. W., P. J. Heimgartner, S. J. Tollefson, T. J. Berg, P. N. Rys, H. Li, T. F. Smith, D. H. Persing, and P. F. Wright. 1999. A colorimetric microtiter plate PCR system detects respiratory syncytial virus in nasal aspirates and discriminates subtypes A and B. Diagn. Microbiol. Infect. Dis. 34333-337. [DOI] [PubMed] [Google Scholar]
- 22.Tang, Y. W., S. E. Sefers, H. Li, D. J. Kohn, and G. W. Procop. 2005. Comparative evaluation of three commercial systems for nucleic acid extraction from urine specimens. J. Clin. Microbiol. 434830-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Hoogen, B. G., J. C. de Jong, J. Groen, T. Kuiken, R. de Groot, R. A. Fouchier, and A. D. Osterhaus. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams, J. V., P. A. Harris, S. J. Tollefson, L. L. Halburnt-Rush, J. M. Pingsterhaus, K. M. Edwards, P. F. Wright, and J. E. Crowe, Jr. 2004. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N. Engl. J. Med. 350443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo, P. C., S. S. Chiu, W. H. Seto, and M. Peiris. 1997. Cost-effectiveness of rapid diagnosis of viral respiratory tract infections in pediatric patients. J. Clin. Microbiol. 351579-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng, X., S. Quianzon, Y. Mu, and B. Z. Katz. 2004. Comparison of two new rapid antigen detection assays for respiratory syncytial virus with another assay and shell vial culture. J. Clin. Virol. 31130-133. [DOI] [PubMed] [Google Scholar]