Abstract
Helicobacter pylori causes peptic ulceration and gastric adenocarcinoma; the latter is common in Iran but not in Iraq. We hypothesized that more virulent H. pylori strains may be found in Iran than in Iraq and so compared established and newly described virulence factors in strains from these countries. We studied 59 unselected dyspeptic patients from Iran and 49 from Iraq. cagA was found in similar proportions of strains from both countries (76% in Iran versus 71% in Iraq) and was significantly associated with peptic ulcer disease in Iraq (P ≤ 0.01) but not in Iran. cagA alleles encoding four or more tyrosine phosphorylation motifs were found in 12% of the Iranian strains but none of the Iraqi strains (P = 0.02). There were no significant differences in the vacA signal-, middle-, or intermediate-region types between Iranian and Iraqi strains. Among the strains from Iran, vacA genotypes showed no specific peptic ulcer associations, but among the strains from Iraq, vacA i1 strains were associated with gastric ulcer (P ≤ 0.02), mimicking their previously demonstrated association with gastric cancer in Iran. dupA was found in similar proportions of Iranian and Iraqi strains (38% and 32%, respectively) and was associated with peptic ulceration in Iraqi patients (P ≤ 0.01) but not Iranian patients. H. pylori strains from Iraq and Iran possess virulence factors similar to those in Western countries. The presence of cagA with more phosphorylation motifs in Iranian strains may contribute to the higher incidence of gastric cancer. However, the association between strain virulence markers and disease in Iraq but not Iran suggests that other host and environmental factors may be more important in the disease-prone Iranian population.
Helicobacter pylori is a spiral-shaped, gram-negative bacillus which causes gastritis and peptic ulceration (18, 19, 36). Its treatment has become pivotal in the management of peptic ulcer disease (PUD). H. pylori infection is also an important risk factor for gastric adenocarcinoma, the second most important cause of cancer deaths worldwide. Gastric cancer is thought to have a multifactorial etiology; and bacterial strain type, host genotype, and environmental conditions are all thought to be factors contributing to gastric cancer (22). Despite the geographical proximity of Iraq and Iran, the incidence of gastric cancer differs hugely between these countries; in Iran it ranges from 38 to 69 cases/105 population (10, 21, 26, 27, 38), whereas in Iraq the incidence is 5 cases/105 population (10). We hypothesized that this difference may be due to differences in the virulence of the circulating H. pylori strains, and so we set out to type strains from these countries for their virulence. We considered both well-established and more recently described virulence determinants.
Many strains of H. pylori produce the CagA protein, encoded by the cagA gene within the cag pathogenicity island (PAI). H. pylori strains possessing cagA are associated with a significantly increased risk for the development of atrophic gastritis, PUD, and gastric cancer (24, 32). The cag PAI encodes a type IV secretion system that facilitates the translocation of CagA into the host epithelial cytosol, where it becomes phosphorylated with tyrosine at specific phosphorylation motifs by the Src family of kinases (29, 30). Phosphorylated CagA forms a physical complex with SHP-2 phosphatase and stimulates cell signaling pathways, cytoskeletal changes, and abnormal cell proliferation (33). On the basis of the amino acid sequence of the SHP-2 binding site, CagA proteins can be subcategorized into Western and East Asian types. Both have type A and B phosphorylation motifs (usually one of each), but the Western types have additional C motifs (1-3) and the East Asian type has no C motifs but a D motif. The East Asian type CagA possesses stronger SHP-2 binding and transforming activities than the Western type CagA (11). The Western type CagA has a variable number of type C phosphorylation motifs, and the extent of cytoskeletal changes induced by CagA is dependent on this. Strains possessing CagA with greater numbers of type C phosphorylation motifs are more closely associated with gastric carcinogenesis (9). Thus, determination of the degree of CagA phosphorylation or the number of phosphorylation motifs appears to be more important than detection of cagA alone (4, 5).
The vacuolating cytotoxin (VacA) is a well-established H. pylori virulence factor which has multiple effects, including vacuolization of cultured epithelial cells, the induction of apoptosis, increases in the permeability of epithelial monolayers, the formation of pores in cells, and suppression of immune cell function (6, 13). The vacA gene is polymorphic within its signal, intermediate, and middle regions. For the signal region, two distinct allelic sequences, s1 and s2, have been recognized. For the middle region, alleles can be categorized into two classes, m1 or m2. The vacA genotype is associated with in vitro cytotoxin activity (with s1 having greater cytotoxin activity than s2 and m1 having greater cytotoxin activity than m2) (8, 20, 34, 35). Rhead et al. have recently described a novel determinant of VacA toxicity, the intermediate or i region (23). They showed that two allelic variants of this region, i1 and i2, exist. Furthermore, they showed that only s1/m2 strains varied in their i types; s1/m1 and s2/m2 strains were exclusively i1 and i2, respectively. This novel region determines the vacuolating activity among these s1/m2 strains. Most importantly, a significant correlation was found between the i1 type of vacA and gastric cancer in Iran (23).
The duodenal ulcer (DU)-promoting gene A (dupA) is a recently described virulence factor which comprises both jhp0917 and jhp0918 (16). Lu et al. found a significant relationship between dupA and DU, and the presence of dupA was related to neutrophil infiltration and a high level of interleukin-8 production by epithelial cells. Surprisingly, possession of this gene appeared to be protective against gastric adenocarcinoma (16).
The object of this study was to type the virulence of unselected strains from dyspeptic patients in Iran and Iraq. We aimed to compare the virulence of strains from these neighboring countries, which have very different incidences of gastric cancer, and to assess the association of virulence markers with PUD in each country.
MATERIALS AND METHODS
Patient-derived samples.
Gastric biopsy specimens were obtained from 49 and 59 unselected H. pylori-positive patients from Iraq and Iran, respectively, undergoing routine upper gastrointestinal endoscopy for investigation for dyspepsia. The mean age ± standard deviation of the Iraqi patients was 35 ± 17 years, and that of the Iranian patients was 40 ± 14 years. All Iraqi patients were from the five districts of city of Dohuk. The majority (48/59 [81%]) of Iranian patients were from the city of Tehran; other patients were referred from different regions in Iran. Endoscopic diagnoses were as follows: for DU, 15 in Iraq and 8 in Iran; for gastric ulcer (GU), 5 in Iraq and 9 in Iran; for no ulcer disease, 29 in Iraq and 42 in Iran. During gastroscopy, biopsy samples were taken and either placed in 1 ml of Iso-Sensitest broth (Oxoid, Basingstoke, United Kingdom) containing 15% (vol/vol) glycerol and stored in liquid nitrogen or cultured immediately for H. pylori. In some cases, following prolonged storage and shipment to the United Kingdom, reculture was not possible. In these cases, DNA was extracted directly from the biopsy specimens and used for PCR-based H. pylori typing.
The study protocol was approved by the ethics and research committees of the individual hospitals, and all patients gave informed consent to participation in the study.
Culture.
Each biopsy specimen was spread onto horse blood or Dent agar plates and then incubated under microaerobic conditions generated with a CampyPak system (Becton Dickinson, Baltimore, MD) in an anaerobic jar at 37°C for 2 to 4 days. The organisms were identified as H. pylori by colony morphology, Gram stain, and urease activity. Cultures were harvested as sweeps rather than single colonies and were stored in Iso-Sensitest broth containing 15% (vol/vol) glycerol at −80°C.
Genotyping of H. pylori.
PCR-based typing of the H. pylori isolates was performed with DNA extracted from bacteria or directly from the biopsy specimens. PCR amplification of cagA used previously described primers cag2 and cag4 (25) to amplify the 3′ variable region. PCR amplification of the cag PAI empty site was performed as described previously (1). In the empty-site PCR, primers anneal to sequences adjacent to the cag PAI insertion site in the genome and allow amplification only of a DNA fragment of the expected size in the absence of a complete or partial cag PAI at this locus. Genotyping of the vacA signal, intermediate, and middle regions was performed as described previously (7, 8, 23). dupA was amplified with primer pairs DupAF113-DupAR1083 and DupAF1202-DupA918R, as described previously (3) (Fig. 1). Determination of the number of CagA phosphorylation motifs and the types of motifs was carried out by using the forward primer cag2 and the reverse primers cagA-P1C, cagA-P2CG and cagA-P2TA (the B motif is polymorphic, and reverse primers cagA-P2CG and cagA-P2TA are designed to recognize all types described to date), and cagA-P3E, as described previously (5) (Fig. 2). Five microliters of the PCR products was electrophoresed in 1.5% (wt/vol) agarose gels for 40 min at 80 V in TAE (Tris-acetate-EDTA) buffer. All gels were stained with ethidium bromide (1 mg/liter) and photographed under UV light. A 1-kb DNA ladder (Gibco, Paisley, United Kingdom) was used as a size marker in all gels. Strains with previously determined genotypes were used as positive controls.
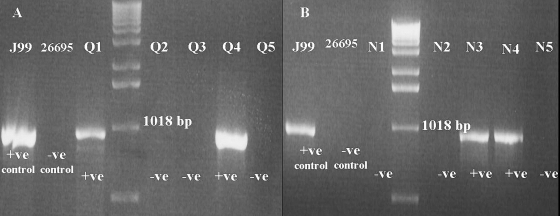
FIG. 1.
Characterization of Iraqi (A) and Iranian (B) strains for dupA by PCR. Image shows the results of PCR typing of dupA with primers DupAF113 and DupAR1083. +ve, positive; −ve, negative.
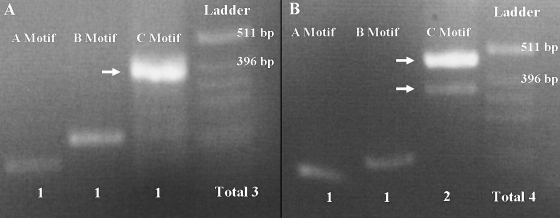
FIG. 2.
Characterization of one Iraqi strain (A) and one Iranian strain (B) for cagA variable-region tyrosine phosphorylation motifs. Genomic DNA samples from H. pylori strains were used to PCR amplify the cagA variable-region EPIYA motifs by using forward primer cag2 and reverse primer cagA-P1C (A motif), reverse primers cagA-P2CG and cagA-P2TA (B motif), or reverse primer cagA-P3E (C motif). No Iraqi strain was found to have more than three phosphorylation motifs.
Data analysis.
Statistical analysis of the data was performed by using logistic regression, the chi-square test, and Fisher's exact test, with significance set at a P value of <0.05. Genotypes with mixed status for vacA were excluded from the calculations of association.
RESULTS
Prevalence of cagA+ strains among dyspeptic Iranian and Iraqi populations.
First, we assessed whether the prevalence of cagA-positive (cagA+) strains was similar or different between the Iraqi and the Iranian populations. cagA+ strains were present in 76% (45/59) and 71% (35/49) of the H. pylori strains from unselected Iranian and Iraqi patients with dyspepsia, respectively (Table 1). To exclude bias from disease association, we also compared subgroups of patients without peptic ulceration: cagA was found in a higher proportion of Iranian strains than Iraqi strains (76% and 55%, respectively), although this did not quite achieve formal statistical significance (P = 0.06). In both countries, all cagA+ strains also typed positive for cagE. Among 14 cagA-negative Iraqi strains, 7 were cag PAI empty-site positive (implying that the whole cag PAI was absent) and 7 were empty-site negative (implying that there was still a partial cag PAI at this locus). Among 14 cagA-negative Iranian strains, 9 were cag PAI empty-site negative. Thus, overall, the cag PAI appeared to be incompletely deleted in 16 strains.
TABLE 1.
cagA status, cagA phosphorylation motif number, and dupA status among H. pylori strains from unselected Iranian and Iraqi patients with dyspepsia
| Country | % of strains positive for:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
cagA
|
More than three cagA phosphorylation motifs
|
dupA
|
|||||||
| PUD | NPUDa | Total | PUD | NPUD | Total | PUD | NPUD | Total | |
| Iraq | 95 (19/20)b,c | 55 (16/29) | 71 (35/49) | 0 (0/20) | 0 (0/29) | 0 (0/49) | 55 (11/20)b | 17 (5/29) | 32 (6/49) |
| Iran | 76 (13/17) | 76 (32/42) | 76 (45/59) | 0 (0/17) | 17 (7/42) | 12 (7/59)d | 35 (6/17) | 40 (17/42) | 39 (23/59) |
NPUD, no PUD.
P < 0.05 for comparison of patients with PUD and patients without PUD.
Values in parentheses indicate the number of strains positive/total number of strains tested.
The presence of cagA alleles with more than three phosphorylation motifs was significantly greater among Iranian strains than among Iraqi strains (P = 0.02).
No significant association was found between cagA status and clinical outcome for the Iranian patients, but a significant correlation was found between cagA and PUDs (P ≤ 0.01; odds ratio, 16.4) in Iraqi patients (Table 1). When DU and GU were considered separately for the Iraqi population, 14/15 (93%) patients with DU had cagA+ strains, whereas 16/29 (55%) patients with no ulcer (P < 0.02) had cagA+ strains. All Iraqi GU patients had cagA+ strains (P was not significant compared with the results for patients with no ulcer, perhaps due to the low number of GU patients).
CagA phosphorylation motif numbers.
Second, we turned our attention to cagA polymorphisms and, in particular, the number of CagA phosphorylation motifs, which we assessed using our recently described PCR-based typing system (5). Among the cagA+ strains, 12% (7/49) of the strains from Iran carried a cagA variable region of >550 bp (when the region was amplified with primers cag2 and cag4), indicating the presence of more than three CagA phosphorylation motifs. This was a significantly higher proportion than that found in the strains from Iraq, where all strains possessed cagA with a variable-region size of 550 bp, indicating the presence of CagA with three phosphorylation motifs (P = 0.02) (Table 1). In the analysis confined to patients without ulcers, 22% (7/32) of the cagA+ Iranian strains had more than three phosphorylation sites, whereas none of the Iraqi strains did. Previous studies with other populations have linked multiple CagA phosphorylation motif numbers with an increased risk of cancer but not with an increased risk of ulcer (9, 15): in agreement with this, no strains with more than three phosphorylation motifs were found in the ulcer group from Iran (Table 1).
vacA polymorphism.
We then turned to vacA polymorphisms in Iranian and Iraqi strains, examining both established s and m genotypes, and also the recently described polymorphic i-region type. Since individual H. pylori isolates possess only a single copy of vacA, the presence of more than one vacA s, i, or m genotype in a DNA sample indicates colonization by two or more strains with different vacA genotypes (8). Among the Iraqi isolates, a single vacA signal region was observed in all samples, but 8/49 (16%) of the specimens examined possessed both middle-region types, and 9/49 (18%) possessed both i-region genotypes. Among the Iranian samples, all isolates possessed a single signal-region type, but 2/59 (3%) carried both m-region types and 7/59 (12%) possessed both i-region types. There was no difference in the prevalence of strains with different vacA genotypes among the unselected dyspeptic populations from Iran and Iraq, whether strains with multiple genotypes were excluded (planned analysis; Table 2) or classified as the more pathogenic or the less pathogenic type (exploratory analyses).
TABLE 2.
Distribution of vacA allelic types among H. pylori strains isolated from unselected dyspeptic patients from Iraq and Iran
| Country | No. of patients positive for the following allelic type/total no. (%):
|
|||||
|---|---|---|---|---|---|---|
| s1/i1/m1 | s1/i1/m2 | s1/i2/m1 | s1/i2/m2 | s2/i2/m2 | Mixed | |
| Iraq | 8/49 (16.3) | 2/49 (4.1) | 1/49 (2.0) | 20/49 (40.8) | 4/49 (8.2) | 14/49 (28.5) |
| Iran | 15/59 (25.4) | 4/59 (6.7) | 1/59 (1.7) | 16/59 (27.1) | 16/59 (27.1) | 7/59 (11.9) |
Next, we examined associations between vacA allelic variation and peptic ulceration within the Iranian and Iraqi populations. For the Iranian strains, no significant associations were found. For the Iraqi strains, no significant association was found for duodenal ulceration, but 80% (4/5) of the strains isolated from GU patients were of the vacA i1 genotype, which was significantly greater than the 13% (4/29) of the strains from patients without ulcers (P < 0.02). Although this subgroup analysis is exploratory, it is interesting, given the described association between vacA i1 genotype and gastric cancer and the similarities in epidemiology and pathogenesis between GU and gastric cancer. Associations were not seen between gastric ulceration and the vacA s and m types, again supporting the recent finding that the vacA i type is a better marker of strain virulence (23).
dupA status.
Third, we examined strains for the recently described putative virulence gene dupA. Similar proportions of Iranian and Iraqi strains possessed dupA (Table 1). Among the Iranian patients, we found no association between dupA and the clinical outcome. However, among the Iraqi patients, 55% (11/20) of the peptic ulcer patients carried dupA+ strains, significantly more than the 17% (5/29) of the patients without ulcers who carried dupA+ strains (P < 0.01; odds ratio, 6.2) (Table 1). When we looked at DU and GU separately, 60% (9/15) of the H. pylori isolates from DU patients were dupA+ (P < 0.01 compared with the results for patients without ulcers) and 40% (2/5) of the H. pylori isolates from GU patients were dupA+ (P was not significant compared with the results for the patients without ulcers, but note the small number of GU patients).
Associations between virulence factors, particularly for cagA phosphorylation motif number.
Next, we assessed associations between virulence factors in strains from Iran and Iraq. As in virtually all strain populations worldwide, we found that cagA+ strains were more likely to be vacA s1 than s2: in Iran, 37/45 (82%) cagA+ strains were vacA s1, whereas 5/14 (36%) of the cagA-negative strains were vacA s1 (P < 0.005); in Iraq, all cagA+ strains typed s1, whereas 10/14 (71%) of the cagA-negative strains typed s1 (P < 0.005). No significant associations were found between cagA status and other vacA polymorphisms or between cagA status and dupA status. As strains with a larger cagA are thought to be more pathogenic than those with a smaller cagA, we examined the association between the size of cagA and other virulence factors among Iranian strains. This analysis was not possible for Iraqi strains, as they all had the same number (n =3) of CagA phosphorylation motifs. Seven of 45 (15%) cagA+ Iranian strains carried a larger cagA (with more than three phosphorylation motifs). In the association analysis with vacA genotypes, we excluded patients with mixed genotypes. The small numbers of strains studied meant that most associations were not statistically significant, but for the vacA m region, 6/7 (86%) strains with more than three phosphorylation motifs were type m1, significantly more than the 10/37 (27%) strains with only three phosphorylation motifs (P = 0.01). Lastly, we examined the association between the size of cagA and dupA status: 6/7 (86%) strains with more than three phosphorylation motifs were dupA+, significantly more than the 14/38 (36%) strain with only three phosphorylation motifs (P = 0.03).
DISCUSSION
The study of H. pylori virulence factors in populations is important, as they contribute to disease risk. For example, in Japan, where gastric cancer is common, more than 90% of H. pylori strains are cagA positive (17). The gastric cancer rate in Iraq is lower than that in Iran; we hypothesized that differences in the virulence factors of the H. pylori strains between these two countries may partially explain this difference. We found no difference in the prevalence of cagA+ strains between unselected dyspeptic populations from these countries, although among patients without ulcers, cagA+ strains were 21% more prevalent in Iran (P = 0.06). Furthermore, Iranian patients with cagA+ strains were more likely to have the more pathogenic forms of cagA encoding four or more tyrosine phosphorylation sites, and among patients without ulcers, this difference was 22%. Taking these results together, this represents a considerable difference in potential cagA-associated pathogenicity which could contribute to the differences in gastric cancer rates seen between these populations: both cagA status and the number of cagA phosphorylation motifs have been linked with cancer prevalence in a number of populations (4, 11). However, we found no significant differences between Iranian and Iraqi populations in vacA types and, in particular, in the i-region type, which has recently been linked with gastric cancer risk in Iran (23). Also, we found no difference in dupA status, which we studied because dupA has been reported to have a negative association with gastric cancer (16), although recent data from us dispute this (3).
In the present report, we have shown that 71% and 76% of the H. pylori strains isolated from Iraqi and Iranian samples, respectively, were cagA+. This value is closer to the values for Western countries and Turkey than to the values for East or Southeast Asia (2, 14, 28, 31). Our strains had the Western type of CagA and the Western types of vacA. Thus, it appears that the high cancer rate in Iran is not due to the presence of strains of the East Asian type in that country.
We looked within the Iranian and Iraqi populations for associations between virulence factors and PUD. Among the Iraqi strains but not the Iranian strains, we observed an association between cagA+ status and PUD. Reports from a neighboring country, Turkey, have shown results similar to those from Iraq (28). No Iraqi strains had cagA with more than three phosphorylation motifs, so we could not perform an examination for disease associations. The situation in Iran was interesting: no strains with more than three phosphorylation motifs were found in patients with peptic ulcer. This may imply that the presence of more than three phosphorylation motifs is protective against ulcers rather than being a specific predisposition to cancer, as reported previously (4, 11). For vacA polymorphisms, we found no association between the vacA i region and the clinical outcome for the Iranian samples. However, for the Iraqi specimens, a novel association was found between the presence of vacA i1 strains and gastric ulcer. This is not unexpected, as GU and gastric cancer are epidemiologically similar. However, our results need confirmation by the performance of studies with other populations, as only a small number of GU patients were enrolled in this study. For dupA, a significant link with PUD was present in the Iraqi population, but no association was found in Iranians. Thus, overall, we showed that the Iraqi population was similar to Western populations in terms of the association of many virulence factors with ulcer disease. In contrast, these associations were not seen in the Iranian population. This may imply that factors other than bacterial virulence are the most important for ulcer risk in Iran.
Many previous reports have shown a clustering of active virulence factors within H. pylori strains, for example, associations between cagA+ status and the vacA s1 genotype (37). In agreement with the findings presented in those reports, we found a significant correlation between cagA+ status and the presence of the vacA s1 genotype in Iran and Iraq. In addition, we showed a significant association between the presence of a greater number of cagA phosphorylation motifs and the presence of both the vacA m1 genotype and dupA+ status. This further supports the concept of the clustering of virulence factors, such that the majority of H. pylori strains possess either many or a few, and the fact that it is favorable for H. pylori to be either strongly pathogenic or nonpathogenic.
Our study has several limitations. First, and perhaps most importantly, we studied H. pylori strains from dyspeptic patients rather than a random community sample of H. pylori strains, and this may have introduced bias. The reason that we did this is that strain genotyping requires upper gastrointestinal endoscopy, which is difficult to perform with randomly selected asymptomatic individuals. We argue that the prevalence of virulent strains in the dyspeptic group without ulcer disease is likely to be similar to that in asymptomatic community members: the association between H. pylori infection and nonulcer dyspepsia is controversial, and if such an association is present at all, it is weak; so any association with virulence factors is likely to be weaker still. Second, our study was not large. However, this is more likely to hide true-positive associations rather than to produce false-positive results and is most unlikely to have produced the multiple associations of virulence factors with disease that we have demonstrated. Third, we genotyped strains for virulence rather than perform virulence phenotyping. Many studies have shown good but imperfect associations between the genotype and the phenotype of a strain, but any error is likely to be in favor of designating a strain as virulent when, in fact, it is avirulent. For example, a seemingly virulent cagA+ strain may not be able to translocate CagA into epithelial cells (if it has a mutation elsewhere in the cag PAI which inactivates the type IV secretion system) and so may be avirulent. In contrast, a seemingly avirulent cagA-negative strain will never be able to translocate CagA (as it lacks it), and so such a strain will never be virulent. Thus, any errors from genotyping rather than errors from phenotyping are also likely to be conservative. Taken together these study deficiencies should encourage others to perform better studies to repeat and extend our investigation, but they do not negate our findings.
To summarize, the virulence factors of both Iraqi and Iranian H. pylori strains appear to be more closely related to strains from Western countries than to strains from Asian countries. Iranian strains appear to be more virulent, but the difference appears to be unlikely completely to explain the difference in disease prevalence between these countries. This suggests that unidentified strain, host, and environmental factors may contribute to these differences. In the absence of an East Asian type of cagA and almost universally virulent strains (as are found in Japan and parts of China), the very high gastric cancer rate in Iran remains largely unexplained. Similarly, the cancer rates in Iraq appear to be lower than those that would be expected from the circulating H. pylori strain types, an enigma similar to that reported (controversially) in Africa (12).
Acknowledgments
We thank the Helicobacter pylori Research Group at the Biotechnology Research Center of the Pasteur Institute of Iran and the clinical staff at the Cancer Research Center of the Tehran University of Medical Sciences. We also thank the physicians and nurses in the gastroenterology departments in both Iraq and Iran for assisting with biopsy specimen collection. We are grateful to Marouf Jaro, Karwan Fendi, and Halat Majed for their kind help in Azadi Hospital in Dohuk, Iraq.
Footnotes
Published ahead of print on 19 March 2008.
REFERENCES
- 1.Akopyants, N. S., S. W. Clifton, D. Kersulyte, J. E. Crabtree, B. E. Youree, C. A. Reece, N. O. Bukanov, E. S. Drazek, B. A. Roe, and D. E. Berg. 1998. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 2837-53. [DOI] [PubMed] [Google Scholar]
- 2.Arents, N. L., A. A. van Zwet, J. C. Thijs, A. M. Kooistra-Smid, K. R. van Slochteren, J. E. Degener, J. H. Kleibeuker, and L. J. van Doorn. 2001. The importance of vacA, cagA, and iceA genotypes of Helicobacter pylori infection in peptic ulcer disease and gastroesophageal reflux disease. Am. J. Gastroenterol. 962603-2608. [DOI] [PubMed] [Google Scholar]
- 3.Argent, R. H., A. Burette, V. Y. M. Deyi, and J. C. Atherton. 2007. The presence of dupA in Helicobacter pylori is not significantly associated with duodenal ulceration in Belgium, South Africa, China, or North America. Clin. Infect. Dis. 451204-1206. [DOI] [PubMed] [Google Scholar]
- 4.Argent, R. H., M. Kidd, R. J. Owen, R. J. Thomas, M. C. Limb, and J. C. Atherton. 2004. Determinants and consequences of different levels of CagA phosphorylation for clinical isolates of Helicobacter pylori. Gastroenterology 127514-523. [DOI] [PubMed] [Google Scholar]
- 5.Argent, R. H., Y. Zhang, and J. C. Atherton. 2005. Simple method for determination of the number of Helicobacter pylori CagA variable-region EPIYA tyrosine phosphorylation motifs by PCR. J. Clin. Microbiol. 43791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atherton, J. 2006. The pathogenesis of H. pylori-induced gastro-duodenal diseases. Annu. Rev. Pathol. Mech. Dis. 163-96. [DOI] [PubMed] [Google Scholar]
- 7.Atherton, J. C. 1998. H. pylori virulence factors. Br. Med. Bull. 54105-120. [DOI] [PubMed] [Google Scholar]
- 8.Atherton, J. C., P. Cao, R. M. Peek, Jr., M. K. Tummuru, M. J. Blaser, and T. L. Cover. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 27017771-17777. [DOI] [PubMed] [Google Scholar]
- 9.Azuma, T., A. Yamakawa, S. Yamazaki, K. Fukuta, M. Ohtani, Y. Ito, M. Dojo, Y. Yamazaki, and M. Kuriyama. 2002. Correlation between variation of the 3′ region of the cagA gene in Helicobacter pylori and disease outcome in Japan. J. Infect. Dis. 1861621-1630. [DOI] [PubMed] [Google Scholar]
- 10.Globocan, IARC. 2002. Cancer map: male stomach cancer, age-standardized incidence rate per 100,000. http://www-dep.iarc.fr/2007/05/30.
- 11.Higashi, H., R. Tsutsumi, A. Fujita, S. Yamazaki, M. Asaka, T. Azuma, and M. Hatakeyama. 2002. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc. Natl. Acad. Sci. USA 9914428-14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holcombe, C. 1992. Helicobacter pylori: the African enigma. Gut 33429-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwamoto, H., D. M. Czajkowsky, T. L. Cover, G. Szabo, and Z. Shao. 1999. VacA from Helicobacter pylori: a hexameric chloride channel. FEBS Lett. 450101-104. [DOI] [PubMed] [Google Scholar]
- 14.Kersulyte, D., A. K. Mukhopadhyay, B. Velapatino, W. Su, Z. Pan, C. Garcia, V. Hernandez, Y. Valdez, R. S. Mistry, R. H. Gilman, Y. Yuan, H. Gao, T. Alarcon, M. Lopez-Brea, G. Balakrish Nair, A. Chowdhury, S. Datta, M. Shirai, T. Nakazawa, R. Ally, I. Segal, B. C. Wong, S. K. Lam, F. O. Olfat, T. Boren, L. Engstrand, O. Torres, R. Schneider, J. E. Thomas, S. Czinn, and D. E. Berg. 2000. Differences in genotypes of Helicobacter pylori from different human populations. J. Bacteriol. 1823210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidd, M., A. J. Lastovica, J. C. Atherton, and J. A. Louw. 1999. Heterogeneity in the Helicobacter pylori vacA and cagA genes: association with gastroduodenal disease in South Africa? Gut 45499-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu, H., P. I. Hsu, D. Y. Graham, and Y. Yamaoka. 2005. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology 128833-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda, S., K. Ogura, H. Yoshida, F. Kanai, T. Ikenoue, N. Kato, Y. Shiratori, and M. Omata. 1998. Major virulence factors, VacA and CagA, are commonly positive in Helicobacter pylori isolates in Japan. Gut 42338-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall, B. J., and J. R. Warren. 2001. One hundred years of discovery and rediscovery of Helicobacter pylori and its association with peptic ulcer disease, p. 19-24. In H. L. T. Mobley, G. L. Mendz, and S. L. Hazell. (ed.), Helicobacter pylori: physiology and genetics. ASM Press, Washington, DC. [PubMed]
- 19.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i1311-1315. [DOI] [PubMed] [Google Scholar]
- 20.McClain, M. S., P. Cao, H. Iwamoto, A. D. Vinion-Dubiel, G. Szabo, Z. Shao, and T. L. Cover. 2001. A 12-amino-acid segment, present in type s2 but not type s1 Helicobacter pylori VacA proteins, abolishes cytotoxin activity and alters membrane channel formation. J. Bacteriol. 1836499-6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nouraie, M., A. Pourshams, F. Kamangar, M. Sotoudeh, M. H. Derakhshan, M. R. Akbari, H. Fakheri, M. J. Zahedi, K. Caldwell, C. C. Abnet, P. R. Taylor, R. Malekzadeh, and S. M. Dawsey. 2004. Ecologic study of serum selenium and upper gastrointestinal cancers in Iran. World J. Gastroenterol. 102544-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peek, R. M., Jr., and M. J. Blaser. 2002. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 228-37. [DOI] [PubMed] [Google Scholar]
- 23.Rhead, J. L., D. P. Letley, M. Mohammadi, N. Hussein, M. A. Mohagheghi, M. Eshagh Hosseini, and J. C. Atherton. 2007. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology 133926-936. [DOI] [PubMed] [Google Scholar]
- 24.Rokkas, T., S. Ladas, C. Liatsos, E. Petridou, G. Papatheodorou, S. Theocharis, A. Karameris, and S. Raptis. 1999. Relationship of Helicobacter pylori CagA status to gastric cell proliferation and apoptosis. Dig. Dis. Sci. 44487-493. [DOI] [PubMed] [Google Scholar]
- 25.Rudi, J., C. Kolb, M. Maiwald, D. Kuck, A. Sieg, P. R. Galle, and W. Stremmel. 1998. Diversity of Helicobacter pylori vacA and cagA genes and relationship to VacA and CagA protein expression, cytotoxin production, and associated diseases. J. Clin. Microbiol. 36944-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadjadi, A., R. Malekzadeh, M. H. Derakhshan, A. Sepehr, M. Nouraie, M. Sotoudeh, A. Yazdanbod, B. Shokoohi, A. Mashayekhi, S. Arshi, A. Majidpour, M. Babaei, A. Mosavi, M. A. Mohagheghi, and M. Alimohammadian. 2003. Cancer occurrence in Ardabil: results of a population-based cancer registry from Iran. Int. J. Cancer 107113-118. [DOI] [PubMed] [Google Scholar]
- 27.Sadjadi, A., M. Nouraie, M. A. Mohagheghi, A. Mousavi-Jarrahi, R. Malekezadeh, and D. M. Parkin. 2005. Cancer occurrence in Iran in 2002, an international perspective. Asian Pac. J. Cancer Prev. 6359-363. [PubMed] [Google Scholar]
- 28.Saribasak, H., B. A. Salih, Y. Yamaoka, and E. Sander. 2004. Analysis of Helicobacter pylori genotypes and correlation with clinical outcome in Turkey. J. Clin. Microbiol. 421648-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selbach, M., S. Moese, C. R. Hauck, T. F. Meyer, and S. Backert. 2002. Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J. Biol. Chem. 2776775-6778. [DOI] [PubMed] [Google Scholar]
- 30.Stein, M., F. Bagnoli, R. Halenbeck, R. Rappuoli, W. J. Fantl, and A. Covacci. 2002. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol. Microbiol. 43971-980. [DOI] [PubMed] [Google Scholar]
- 31.Stephens, J. C., J. A. Stewart, A. M. Folwell, and B. J. Rathbone. 1998. Helicobacter pylori cagA status, vacA genotypes and ulcer disease. Eur. J. Gastroenterol. Hepatol. 10381-384. [DOI] [PubMed] [Google Scholar]
- 32.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388539-547. [DOI] [PubMed] [Google Scholar]
- 33.Tsutsumi, R., H. Higashi, M. Higuchi, M. Okada, and M. Hatakeyama. 2003. Attenuation of Helicobacter pylori CagA × SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J. Biol. Chem. 2783664-3670. [DOI] [PubMed] [Google Scholar]
- 34.van Doorn, L. J., C. Figueiredo, F. Megraud, S. Pena, P. Midolo, D. M. Queiroz, F. Carneiro, B. Vanderborght, M. D. Pegado, R. Sanna, W. De Boer, P. M. Schneeberger, P. Correa, E. K. Ng, J. Atherton, M. J. Blaser, and W. G. Quint. 1999. Geographic distribution of vacA allelic types of Helicobacter pylori. Gastroenterology 116823-830. [DOI] [PubMed] [Google Scholar]
- 35.van Doorn, L. J., C. Figueiredo, R. Sanna, S. Pena, P. Midolo, E. K. Ng, J. C. Atherton, M. J. Blaser, and W. G. Quint. 1998. Expanding allelic diversity of Helicobacter pylori vacA. J. Clin. Microbiol. 362597-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitfield, J. 2003. The ulcer bug: gut reaction. Nature 423583-584. [DOI] [PubMed] [Google Scholar]
- 37.Xiang, Z., S. Censini, P. F. Bayeli, J. L. Telford, N. Figura, R. Rappuoli, and A. Covacci. 1995. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect. Immun. 6394-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yavari, P., T. G. Hislop, C. Bajdik, A. Sadjadi, M. Nouraie, M. Babai, and R. Malekzadeh. 2006. Comparison of cancer incidence in Iran and Iranian immigrants to British Columbia, Canada. Asian Pac. J. Cancer Prev. 786-90. [PubMed] [Google Scholar]