Abstract
Although the “gold standard” for diagnosis of tuberculous meningitis (TBM) is bacterial isolation of Mycobacterium tuberculosis, there are still several complex issues. Recently, we developed an internally controlled novel wide-range quantitative nested real-time PCR (WR-QNRT-PCR) assay for M. tuberculosis DNA in order to rapidly diagnose TBM. For use as an internal control calibrator to measure the copy number of M. tuberculosis DNA, an original new-mutation plasmid (NM-plasmid) was developed. Due to the development of the NM-plasmid, the WR-QNRT-PCR assay demonstrated statistically significant accuracy over a wide detection range (1 to 105 copies). In clinical applications, the WR-QNRT-PCR assay revealed sufficiently high sensitivity (95.8%) and specificity (100%) for 24 clinically suspected TBM patients. In conditional logistic regression analysis, a copy number of M. tuberculosis DNA (per 1 ml of cerebrospinal fluid) of >8,000 was an independent risk factor for poor prognosis for TBM (i.e., death) (odds ratio, 16.142; 95% confidence interval, 1.191 to 218.79; P value, 0.0365). In addition, the copy numbers demonstrated by analysis of variance statistically significant alterations (P < 0.01) during the clinical treatment course for 10 suspected TBM patients. In simple regression analysis, the significant correlation (R2 = 0.597; P < 0.0001) was demonstrated between copy number and clinical stage of TBM. We consider the WR-QNRT-PCR assay to be a useful and advanced assay technique for assessing the clinical treatment course of TBM.
Tuberculous meningitis (TBM) is the severest form of infection of Mycobacterium tuberculosis, causing death or severe neurological defects in more than half of those affected in spite of antituberculosis treatment (ATT) (1, 2, 8, 18). The diagnosis of TBM remains a complex issue, because the most widely used conventional bacteriological detection methods, such as direct smear for acid-fast bacilli (AFB) and culture for M. tuberculosis, are unable to rapidly detect M. tuberculosis with sufficient sensitivity in the acute phase of TBM (3-13, 18, 19). In 2006, we designed a novel internally controlled quantitative nested real-time PCR (QNRT-PCR) assay based on TaqMan PCR (Applied Biosystems) (15). Moreover, based on this original QNRT-PCR (OR-QNRT-PCR) assay, an improved wide-range QNRT-PCR (WR-QNRT-PCR) assay was developed (17). For use as a “calibrator” in WR-QNRT-PCR assay, a new internal control was constructed (17). In the preliminary experiments, the WR-QNRT-PCR assay demonstrated significantly improved quantitative accuracy and had a wide detection range (1 to 105 copies) compared to what was seen for the OR-QNRT-PCR assay (17).
In this study, we tried to quantitatively detect M. tuberculosis DNA in actual cerebrospinal fluid (CSF) samples by using the WR-QNRT-PCR assay. In addition, the clinical usefulness of this novel assay technique for the rapid and accurate diagnosis of TBM and for assessing the clinical course of TBM was examined.
MATERIALS AND METHODS
This study was approved by the Nihon University Institutional Review Board.
Clinical specimens and control.
Clinical specimens from 24 patients with clinically suspected TBM and 29 non-TBM control patients were collected between 1998 and 2005. A total of 67 CSF samples were collected from these 24 patients. Of 67 CSF samples, 43 were available serially from the 10 patients (cases 3 and 8 to 16) who had follow-ups of more than at least 2 weeks. In addition, the extracted DNA specimen from M. tuberculosis standard strain H37Rv (ATCC 25618) was used as the positive control in this study.
The 29 non-TBM control patients consisted of 4 cases of bacterial meningitis, 3 of cryptococcal meningitis, 12 of viral meningitis, 6 of multiple sclerosis, and 1 each of central nervous system (CNS) lupus, CNS malignant lymphoma, hepatic insufficiency, and neuro-Behçet's disease. The diagnoses for the non-TBM control cases were based on their specific clinical and laboratory findings. Moreover, to determine the analytical specificity and cross-reactivity of our assays, extracted DNA specimens from six additional reference strains of non-M. tuberculosis species—M. bovis BCG (ATCC 19274), M. avium (ATCC 15769), M. intracellulare (ATCC 15985), and clinically isolated Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas aeruginosa strains—were tested.
Smear and culture.
All CSF samples from patients with suspected TBM were examined microscopically for AFB and by culture for M. tuberculosis. First, CSF aliquots of 1 to 2 ml were concentrated by centrifugation (3,000 × g for 10 min) for conventional bacteriology. The sediments were used to prepare smears for direct examination of AFB by auramine-rhodamine and Ziehl-Neelsen stain and cultured by inoculation on a Bactec MGIT 960 system (Becton Dickinson Diagnostic Instrument Systems, Sparks, MD). The cultures were incubated under an atmosphere containing 5% CO2 at 37°C and observed for 12 weeks before they were discarded.
Conventional single- and nested-PCR assays.
The DNA specimens including M. tuberculosis DNA were extracted and purified from the 250 μl of CSF samples by previously reported conventional phenol-chloroform method and ethanol precipitation (15, 17).
Two sets of primer pairs, outer primers WF1 and WR1 and inner primers WF2 and WR2, which were specific for the MPB64 protein of M. tuberculosis (MPT64; GenBank accession no. NC_000962) were prepared (Table 1). In the single (i.e., first-step) PCR assay, 2 μl of the extracted DNA specimen including M. tuberculosis DNA as a template was added to 18 μl of the previously reported PCR solution mixture (15, 17) containing 20 pM each of outer primers WF1 and WR1 and then subjected to the following protocol using GeneAmp PCR system 9700 (Perkin Elmer, Norwalk, CT): an initial denaturation at 96°C for 3 min followed by 35 cycles of amplification with denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 1 min and a final extension at 72°C for 10 min.
TABLE 1.
Sequences of primers and TaqMan probes for PCR assays
| Objective | PCR product size (bp) | Target | Type | Sequencea |
|---|---|---|---|---|
| WR-QNRT-PCR assay | ||||
| First-step PCR | 239 | Wild M. tuberculosis DNA (MPT64) or W-plasmid | WF1: outer wild forward primer | 5′-ATCCGCTGCCAGTCGTCTTCC-3′; total of 21 nucleotides, A:2, T:6, G:4, C:9 (G+C, 62%) |
| WR1: outer wild reverse primer | 5′-CTCGCGAGTCTAGGCCAGCAT-3′; total of 21 nucleotides, A:4, T:4, G:6, C:7 (G+C, 62%) | |||
| New internal control (NM-plasmid) | MF1: outer mutation forward primer | 5′-TCGATTCTGTCCCACCGCCGT-3′; total of 21 nucleotides, A:2, T:6, G:4, C:9 (G+C, 62%) | ||
| MR1: outer mutation reverse primer | 5′-AGACTCGACGCGTAGTCCTCG-3′; total of 21 nucleotides, A:4, T:4, G:6, C:7 (G+C, 62%) | |||
| Second-step PCR | 77 | Wild M. tuberculosis DNA (MPT64) or W-plasmid | TqMn-WF2: TaqMan inner wild forward primer | 5′-GTGAACTGAGCAAGCAGACCG-3′; total of 21 nucleotides, A:7, T:2, G:7, C:5 (G+C, 57%) |
| TqMn-WR2: TaqMan inner wild reverse primer | 5′-GTTCTGATAATTCACCGGGTCC-3′; total of 22 nucleotides, A:4, T:7, G:5, C:6 (G+C, 50%) | |||
| New internal control (NM-plasmid) | TqMn-MF2: TaqMan inner mutation forward primer | 5′-AGATCGGATAGCCAGCACGGA-3′; total of 21 nucleotides, A:7, T:2, G:7, C:5 (G+C, 57%) | ||
| TqMn-MR2: TaqMan inner mutation reverse primer | 5′-TGCGCTGCGTCGACATATTCTA-3′; total of 22 nucleotides, A:4, T:7, G:5, C:6 (G+C, 50%) | |||
| Wild M. tuberculosis DNA (MPT64) or W-plasmid | TqMn-W-VIC: TaqMan probe-wild-VIC | 5′-VIC-TATCGATAGCGCCGAATGCCGG-TAMRA-3′; total of 22 nucleotides, A:5, T:4, G:7, C:6 (G+C, 59%) | ||
| New internal control (NM-plasmid) | TqMn-M-FAM: TaqMan probe-mutation-FAM | 5′-FAM-ATGGGACGGCTAGCAATCCGTC-TAMRA-3′; total of 22 nucleotides, A:5, T:4, G:7, C:6 (G+C, 59%) | ||
| OR-QNRT-PCR assay | ||||
| First-step PCR | 239 | Wild M. tuberculosis DNA (MPT64) and old internal control (M-plasmid) | WF1 | 5′-ATCCGCTGCCAGTCGTCTTCC-3′ |
| WR1 | 5′-CTCGCGAGTCTAGGCCAGCAT-3′ | |||
| Second-step PCR | 77 | Wild M. Tb DNA (MPT64) and old internal control (M-plasmid) | TqMn-WF2 | 5′-GTGAACTGAGCAAGCAGACCG-3′ |
| TqMn-WR2 | 5′-GTTCTGATAATTCACCGGGTCC-3′ | |||
| Wild M. tuberculosis DNA (MPT64) | TqMn-W-VIC | 5′-VIC-TATCGATAGCGCCGAATGCCGG-TAMRA-3′ | ||
| Old internal control (M-plasmid) | TqMn-M-FAM | 5′-FAM-ATGGGACGGCTAGCAATCCGTC-TAMRA-3′ | ||
| Conventional single and nested PCR assays | ||||
| First-step PCR | 239 | Wild M. tuberculosis DNA (MPT64) | WF1 | 5′-ATCCGCTGCCAGTCGTCTTCC-3′ |
| WR1 | 5′-CTCGCGAGTCTAGGCCAGCAT-3′ | |||
| Second-step PCR | 194 | Wild M. tuberculosis DNA (MPT64) | WF2: inner forward primer | 5′-CATTGTGCAAGGTGAACTGAGC-3′ |
| WR2: inner reverse primer | 5′-AGCATCGAGTCGATCGCGGAA-3′ | |||
| Internal control | 196 | Human β-globin gene | HBB-F: human β-globin forward primer | 5′-GGCAGACTTCTCCTCAGGAGTC-3′ |
| HBB-R: human β-globin reverse primer | 5′-CTTAGACCTCACCCTGTGGAGC-3′ |
Underlining indicates artificial sequence; double underlining indicates restriction site. FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine.
In nested (i.e., second-step) PCR, 2 μ1 of the single PCR product as a template was added to 18 μl of the PCR mixture containing 20 pM each of outer primers WF2 and WR2 and then subjected to an initial denaturation at 96°C for 3 min followed by 25 cycles of amplification with denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min and a final extension at 72°C for 10 min. The PCR products were analyzed by Agilent 2100 bioanalyzer system (Agilent Technologies, Waldbronn, Germany). The presence of a 239-bp band for single PCR and a 194-bp band for nested PCR indicated successful amplification.
In addition, for use as an internal control, a pair of primers that were specific for the human β-globin gene (HBB; GenBank accession no. L48217) was prepared (Table 1). The 196-bp HBB fragment as an internal control was amplified in another tube under the same assay conditions.
OR-QNRT-PCR assay.
For the OR-QNRT-PCR assay, two types of original plasmid, wild plasmid (W-plasmid) and mutation plasmid (M-plasmid), were constructed (15). The original W-plasmid was prepared for use as the standard template to construct the standard curve in the second step of this assay (15). The original M-plasmid was constructed based on the W-plasmid for use as the old internal control (15). The DNA specimens were extracted and purified from 250 μl of CSF samples added by the 1,000 copies of M-plasmid in advance by previously reported conventional phenol-chloroform method and ethanol precipitation (15).
The OR-QNRT-PCR assay consists of two consecutive PCR amplification steps, which were conventional PCR at the first step and RT-PCR (TaqMan) at the second step, using an ABI Prism 7700 sequence detector (Applied Biosystems, Foster City, CA). Two sets of primer pairs, outer primers WF1 and WR1 at the first step and inner primers TqMn-WF2 and TqMn-WR2 at the second step, which were specific for wild MPT64, were prepared (Table 1). In addition, for use in second-step PCR, two types of TaqMan probes, TqMn-W-VIC for detecting wild MPT64 and TqMn-M-FAM for detecting the old internal control (i.e., M-plasmid), were prepared (Table 1). As the template, 2 μl of the extracted DNA specimen at the first step and 2 μl of the first-step PCR product at the second step were used. All procedures of this assay were subjected to the previously reported protocol (15). (The first-step PCR was set at 35 amplification cycles.)
WR-QNRT-PCR assay.
For the WR-QNRT-PCR assay, the original new-mutation plasmid (NM-plasmid) was constructed based on M-plasmid for use as a new internal control (17). The DNA specimens were extracted and purified from the 250 μl of CSF samples added by the 1,000 copies of NM-plasmid in advance by use of the previously reported method (17).
The WR-QNRT-PCR assay consists of two consecutive PCR amplification steps, as does the OR-QNRT-PCR assay (15, 17). In first-step PCR, 2 μl of the extracted DNA specimen including the new internal control as a template was amplified by using the outer primers WF1 and WR1 or MF1 and MR1 (Table 1) at 25 amplification cycles (17). In second-step PCR, 2 μl of the first-step PCR product was used as a template. The wild MPT64 fragment was amplified by the inner primers TqMn-WF2 and TqMn-WR2 and detected by specific TaqMan probe TqMn-W-VIC (Table 1). The new internal control, i.e., NM-plasmid, was amplified by the inner primers TqMn-MF2 and TqMn-MR2 and detected by the specific TaqMan probe TqMn-M-FAM (Table 1). All procedures of this assay were subjected to the previously reported protocol (17).
Quantitative detection for M. tuberculosis DNA.
The initial copy number of M. tuberculosis DNA in CSF samples was calculated based on the amplification ratio against the 1,000 copies of the old internal control (M-plasmid) in the OR-QNRT-PCR assay or the new internal control (NM-plasmid) in the WR-QNRT-PCR assay (15, 17). For M. tuberculosis, it is universally acceptable that a single copy of the MPT64 gene represented one bacterial cell (3, 4). Therefore, we considered that the copy numbers calculated by both the OR- and WR-QNRT-PCR assays corresponded to the M. tuberculosis bacterial cell numbers in CSF samples.
Blinded (randomized) assay.
For comparative evaluation of respective diagnostic assays, conventional single- and nested-PCR assays and OR- and WR-QNRT-PCR assays were performed against the blinded (randomized) 10 CSF samples labeled A to J. These blinded samples were selected in the clinically obtained CSF samples stored at −80°C. The 10 blinded CSF samples, samples A to J, correspond to a cryptococcal meningitis sample, a case 1 sample, a case 2 sample, a viral meningitis sample, a CNS lupus sample, a case 3 sample, a case 8 sample, another viral meningitis sample, a multiple sclerosis sample, and a case 9 sample, respectively.
Statistical analysis.
The statistical analysis was calculated using data analysis software program SPSS 13.0 for Windows. A P value of <0.05 was considered statistically significant.
RESULTS
Clinical features of the patients.
Table 2 summarizes the clinical features of the 24 suspected TBM patients upon admission (before ATT). All 24 patients (i) met the clinical criteria and (ii) demonstrated supporting evidence for TBM (shown in Table 2) (4, 6, 11, 12, 14, 15) and were classified as 8 “confirmed” cases (cases 1 to 8) (CSF culture positive for M. tuberculosis) and 16 “highly probable” cases (cases 9 to 24) (meeting all the clinical criteria and with 3 positive for supporting evidences but having no bacterial isolation). Of these 24 patients, the 2 “confirmed” cases (cases 1 and 2) and 7 “highly probable” cases (cases 9 to 15) correspond to cases 1 to 9 reported in our previous papers (14, 15). In addition, the periods up to initial sample collection available from these 24 patients are shown in Table 2. This period was approximately consistent with the period from the development of symptoms to the beginning of ATT for suspected TBM patients and was therefore considered to be clinically important. The results of respective diagnostic assays, including M. tuberculosis culture, conventional single- and nested-PCR assays, and OR- and WR-QNRT-PCR assays, are summarized and evaluated clinically in Tables 3 and 4.
TABLE 2.
Summary of basal clinical features of 24 patients with suspected TBMa
| Case typeb and patient no. | Age (yr)/sexc | Clinical staged | Basal CSF findings (before treatment)
|
Single-PCR assay | Nested- PCR assay | OR-QNRT-PCR assay (copies/ml CSF) | WR-QNRT-PCR assay (copies/ml CSF) | Cranial MRI findingsf | M. tuberculosis outside CNSg | Period up to initial sample collection | ATT response | Outcome after ATT | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cells (per μl) | Protein (mg/dl) | Glucose (mg/dl) | ADAe (IU/l) | AFB smear | M. tuberculosis culture | ||||||||||||
| Confirmed cases | |||||||||||||||||
| 1 | 73/M | III | 288 | 299 | 13 | 23.4 | − | + | + | + | 1.0 × 105 | 28721 | ME, HC, CVD, IFM | Sp, GA | About 3 wk | Noneffective | Death |
| 2 | 76/M | III | 165 | 569 | 46 | 12.3 | − | + | + | + | 6.4 × 104 | 10028 | ME, CVD | Sp | 2 Days | Effective | Recovery |
| 3 | 28/M | III | 605 | 434 | 25 | 16.3 | − | + | − | + | 8.8 × 104 | 22571 | ME, HC, CVD, IFM | Sp | About 1 mo | Noneffective | Death |
| 4 | 38/M | II | 76 | 637 | 18 | 6.5 | − | + | − | + | 3.0 × 104 | 7161 | HC, IFM | Sp, GA | 1 Day | Effective | Recovery |
| 5 | 53/F | III | 344 | 354 | 38 | 10.3 | − | + | + | + | 6.0 × 104 | 4547 | IFM | − | 1 Day | Effective | Recovery |
| 6 | 72/F | III | 247 | 329 | 57 | 18.4 | − | + | − | + | 3.3 × 103 | 6340 | HC, IFM | − | 7 Days | Noneffective | Death |
| 7 | 34/M | II | 612 | 320 | 18 | 20.2 | − | + | + | + | 3.8 × 103 | 1243 | − | − | Not available | Effective | Recovery |
| 8 | 42/M | II | 418 | 456 | 36 | 22.6 | − | + | + | + | 1.8 × 104 | 10532 | ME, IFM | Sp | About 2 wk | Effective | Recovery |
| Highly probable cases | |||||||||||||||||
| 9 | 35/F | II | 208 | 300 | 13 | 16.3 | − | − | − | + | 3.6 × 103 | 7892 | ME, HC, CVD, IFM | − | 7 Days | Effective | Recovery |
| 10 | 65/F | I | 107 | 70 | 48 | 7.8 | − | − | − | + | 5.6 × 103 | 1904 | − | − | 3 Days | Effective | Recovery |
| 11 | 52/M | II | 18 | 135 | 54 | 8.6 | − | − | − | + | 2.7 × 103 | 5858 | ME, HC, CVD, IFM | Sp, GA | 1 Day | Effective | Recovery |
| 12 | 24/F | I | 30 | 25 | 30 | 4.4 | − | − | − | + | 1.9 × 104 | 5436 | IFM | − | 1 Day | Effective | Recovery |
| 13 | 44/F | III | 60 | 70 | 52 | N.D. | − | − | − | + | 1.5 × 104 | 9600 | CVD | − | 1 Day | Effective | Recovery |
| 14 | 59/F | II | 40 | 359 | 78 | 3.7 | − | − | − | + | 5.6 × 103 | 5112 | HC | − | About 1 mo | Effective | Recovery |
| 15 | 44/M | III | 117 | 87 | 48 | 3.9 | − | − | − | + | 2.2 × 104 | 8400 | ME | Sp, GA | About 3 wk | Noneffective | Death |
| 16 | 40/M | III | 800 | 188 | 66 | 12 | − | − | − | + | 1.1 × 104 | 7050 | CVD | − | 1 Day | Effective | Recovery |
| 17 | 30/F | III | 720 | 211 | 50 | 9.7 | − | − | − | + | 3.1 × 103 | 5596 | IFM | − | 5 Days | Effective | Recovery |
| 18 | 20/F | II | 442 | 164 | 46 | 17.6 | − | − | − | − | Not detected | Not detected | IFM | GA | Not available | Effective | Recovery |
| 19 | 63/M | III | 75 | 84 | 47 | 15.9 | − | − | − | − | 4.8 × 10 | 76 | ME | Sp | Not available | Effective | Recovery |
| 20 | 63/F | II | 34 | 294 | 30 | 12.7 | − | − | − | + | 3.2 × 102 | 188 | HC | − | Not available | Effective | Recovery |
| 21 | 53/M | III | 76 | 81 | 82 | 16.9 | − | − | − | + | 8.4 × 103 | 2592 | ME, IFM | Sp | 1 Day | Effective | Recovery |
| 22 | 51/M | III | 227 | 155 | 34 | 12.7 | − | − | − | + | 5.2 × 103 | 636 | ME, CVD, IFM | − | 1 Day | Effective | Recovery |
| 23 | 66/M | III | 129 | 120 | 58 | 4.7 | − | − | − | + | 2.2 × 103 | 1600 | ME | − | 4 Days | Effective | Recovery |
| 24 | 2/F | II | 193 | 119 | 30 | 8.3 | − | − | − | + | 1.1 × 103 | 1444 | ME, HC | − | Not available | Effective | Recovery |
The clinical criteria suggestive for TBM are fever, headache, and neck stiffness of more than 1 week in duration. Supporting evidence for TBM includes (i) compatible abnormal CSF findings that included increased white cell counts with lymphocytes predominating, hypoglycorrhachia, a protein concentration of >100 mg/dl, adenosine deaminase at ≥10 IU/liter, and negative results for routine bacterial and fungal cultures; (ii) magnetic resonance imaging findings suggesting tuberculous involvement of the CNS (basal exudates, hydrocephalus, and intracranial focal mass, etc.); (iii) presence of tuberculosis in the body outside of the CNS or a history of tuberculosis; and (iv) clinical response to ATT.
The suspected TBM cases were classified as “confirmed” cases (having bacterial isolation for M. tuberculosis, e.g., being CSF culture positive) or “highly probable” cases (meeting all the above clinical criteria and with all three supporting evidences positive). The 2 “confirmed” cases (cases 1 and 2) and 7 “highly probable” cases (cases 9 to 15) correspond to cases 1 to 9 in our previous papers (see references 14 and 15.
M, male; F, female.
Clinical stages are defined according to the British Medical Research Council as follows: stage 0, no definite neurologic symptoms; stage I, slight signs of meningeal irritation with slight clouding of consciousness; stage II, moderate signs of meningeal irritation with moderate disturbance of consciousness and neurologic defects; stage III, severe disturbance of consciousness and neurologic defects.
ADA, adenosine deaminase.
MRI, magnetic resonance imaging; ME, meningeal enhancement; HC, hydrocephalus; CVD, cerebrovascular disorder; IFM, intracranial focal mass; −, no finding.
Sp, sputum; GA, gastric aspirate; −, no finding.
TABLE 3.
Assay results and clinical parameters
| Assay | Result | No. with indicated result among:
|
Sensitivity (%) | Specificity (%) | Predictive values (%)
|
||
|---|---|---|---|---|---|---|---|
| Clinically suspected TBM cases (24 cases) | Negative control group (35 samples) | Positive | Negative | ||||
| M. tuberculosis culture | + | 8 | 0 | 33.3 | 100 | 100 | 68.6 |
| − | 16 | 35 | |||||
| Single-PCR assay | + | 5 | 0 | 20.8 | 100 | 100 | 64.8 |
| − | 19 | 35 | |||||
| Nested-PCR assay | + | 22 | 0 | 91.7 | 100 | 100 | 94.6 |
| − | 2 | 35 | |||||
| OR-QNRT-PCR assay | + | 23 | 0 | 95.8 | 100 | 100 | 97.2 |
| − | 1 | 35 | |||||
| WR-QNRT-PCR assay | + | 23 | 0 | 95.8 | 100 | 100 | 97.2 |
| − | 1 | 35 | |||||
TABLE 4.
Blind (randomized) assay results
| Blinded (randomized) sample | Result for:
|
Sample source | |||
|---|---|---|---|---|---|
| Single-PCR assaya | Nested-PCR assayb | OR-QNRT-PCR assay (copies/ml CSF)c | WR-QNRT-PCR assay (copies/ml CSF)d | ||
| A | − | − | − | − | Cryptococcal meningitis (negative control) |
| B | + | + | + (2.5 × 105) | + (25,647) | Case 1 |
| C | − | + | + (8.6 × 104) | + (12,214) | Case 2 |
| D | − | − | − | − | Viral meningitis (negative control) |
| E | − | − | − | − | CNS lupus (negative control) |
| F | − | + | + (1.2 × 105) | + (18,236) | Case 3 |
| G | + | + | + (9.2 × 103) | + (9,542) | Case 8 |
| H | − | − | − | − | Viral meningitis (negative control) |
| I | − | − | − | − | Multiple sclerosis (negative control) |
| J | − | + | + (2.7 × 103) | + (8,705) | Case 9 |
Rate of consistency to previous assay results, 90%.
Rate of consistency to previous assay results, 100%.
Rate of consistency to previous assay results, 100%. Wilcoxon signed-rank test P value, 0.0052.
Rate of consistency to previous assay results, 100%. Wilcoxon signed-rank test P value, 0.147 (no significant difference).
WR-QNRT-PCR assay results for CSF samples. (i) Upon admission.
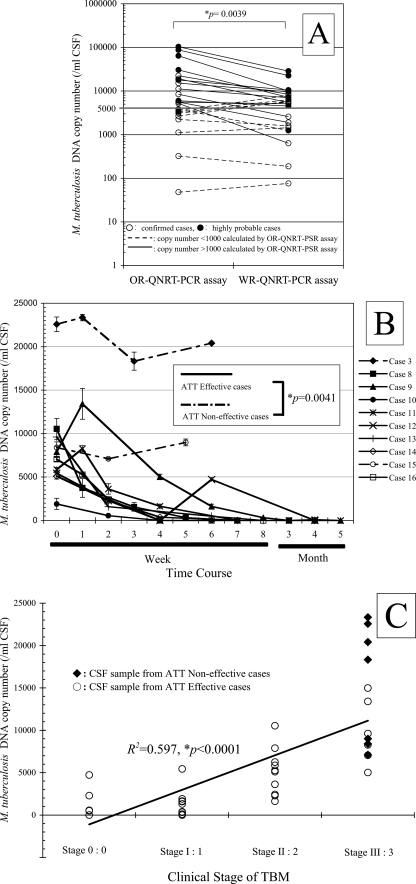
For WR-QNRT-PCR assay, the quantitative detection of M. tuberculosis DNA was possible in 23 cases out of the 24 suspected TBM patients (95.8%) upon admission (Tables 2 and 3). The measured copy numbers of M. tuberculosis DNA (per 1 ml of CSF) are shown in Table 2. However, the WR-QNRT-PCR assay revealed no amplification for all 35 CSF samples collected from the 29 patients in the non-TBM control group and the six reference strains of non-M. tuberculosis species (Table 3). OR-QNRT-PCR assay results are also shown in Table 2. The sensitivities and specificities of both the OR- and WR-QNRT-PCR assays were equivalent (Table 3). However, there was the significant difference (P = 0.0039) between the OR- and WR-QNRT-PCR results by Wilcoxon signed-rank testing (Fig. 1A). In particular, copy numbers of more than 1,000 calculated by the OR-QNRT-PCR assay tend to reveal more values than do WR-QNRT-PCR assay results (Fig. 1A). This trend indicated the unfavorable influence of overamplification and the instability of the M-plasmid as the old internal control in the OR-QNRT-PCR assay.
FIG. 1.
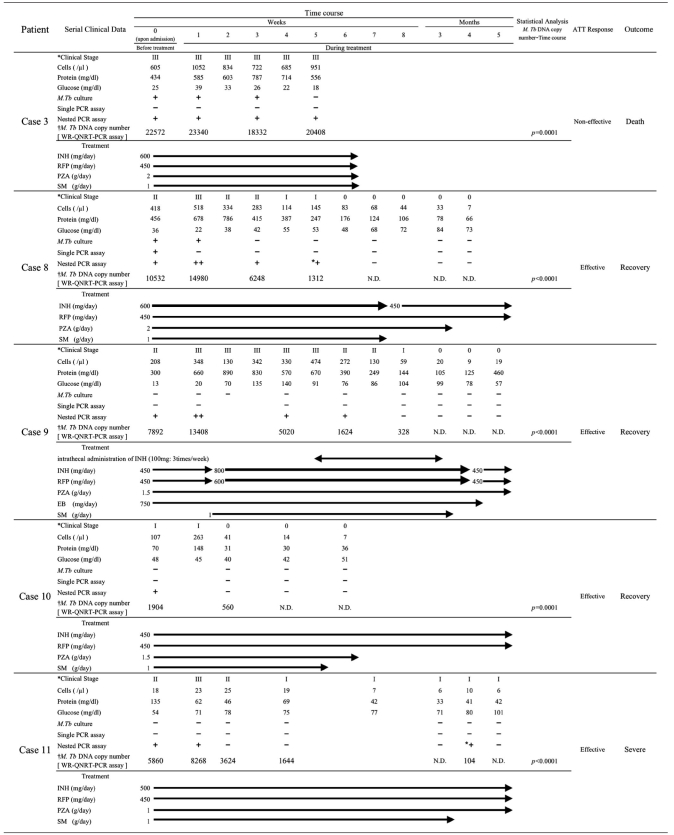
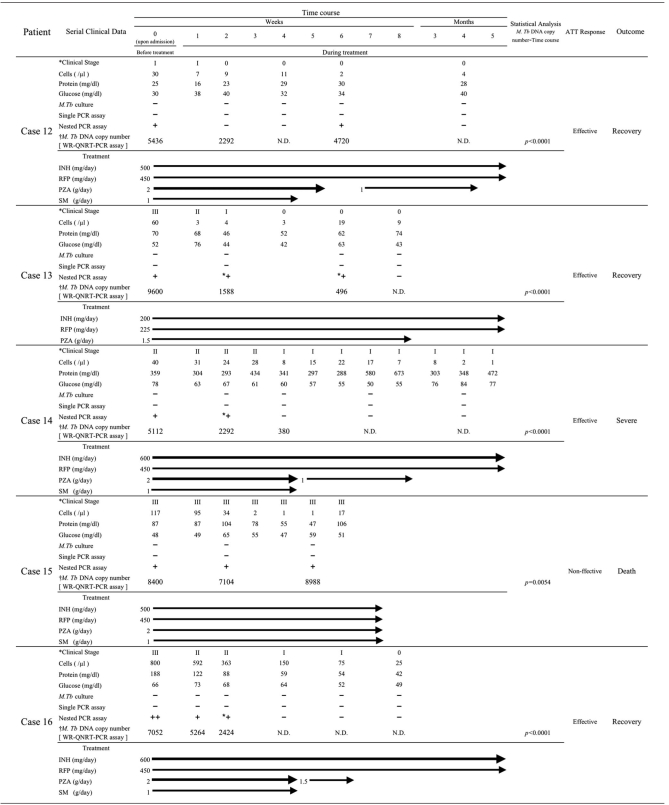
Statistical analysis for WR-QNRT-PCR assay in clinical application. (A) Statistical comparison between OR- and WR-QNRT-PCR results by Wilcoxon signed-rank test. (B) The progress of M. tuberculosis DNA copy numbers calculated by the WR-QNRT-PCR assay during a clinical time course for 10 suspected TBM patients (cases 3 and 8 to 16). A statistical comparison between the ATT-effective cases (cases 8 to 14 and 16) and the ATT-noneffective cases (cases 3 and 8) was calculated by repeated-measures ANOVA. (C) Result of simple regression analysis between M. tuberculosis DNA copy number (y axis) and clinical stage of TBM (x axis).
In the blinded (randomized) assay (Table 4), both OR- and WR-QNRT-PCR results were completely consistent with the assay results shown in Table 2. However, there was a significant difference (P = 0.0052) in copy numbers (per ml of CSF) between the blinded assay and the previous assay results of OR-QNRT-PCR by Wilcoxon signed-rank test (Table 4). In WR-QNRT-PCR, the copy numbers were not statistically significantly different between blinded assay and previous assay results (Table 4). These results indicated that the WR-QNRT-PCR assay had sufficient reproducibility and was more stable and reliable as a diagnostic method than conventional assay techniques, including OR-QNRT-PCR.
In conditional logistic regression analysis, M. tuberculosis culture positivity, M. tuberculosis DNA copy numbers of >8,000 (per ml of CSF) calculated by WR-QNRT-PCR assay, and more than 2 weeks up to initial sample collection, were independent risk factors for a poor prognosis for TBM (i.e., death) (Table 5) (for these three risk factors, statistics were, respectively, as follows: odds ratio [OR] = 37.368, 95% confidence interval [95% CI] = 1.233 to 1132.781, P = 0.0375; OR = 16.142, 95% CI = 1.191 to 218.79, P = 0.0365; OR = 32.501, 95% CI = 1.709 to 618.21, P = 0.0205). The copy number of >8,000 (per ml of CSF) as the threshold value was set based on the 75th percentile (8,146 copies) of WR-QNRT-PCR assay results in all 24 cases (Table 2). However, the conventional single- and nested-PCR assays, which were qualitative examinations, were not statistically significant risk factors for a poor prognosis for TBM (Table 5).
TABLE 5.
Independent predictors for poor prognosis of TBM (conditional logistic regression model)a
| Independent predictor | OR | 95% CI | P valueb |
|---|---|---|---|
| Age (yr) | 1.040 | 0.953-1.134 | 0.3803 (NSD) |
| Culture for M. tuberculosis | 37.368 | 1.233-1132.781 | 0.0375 |
| Single-PCR assay | 0.069 | 0.001-3.236 | 0.1731 (NSD) |
| Nested-PCR assay | 6.125 | 0.489-76.770 | 0.1600 (NSD) |
| M. tuberculosis DNA copy no. of >8,000 (WR-QNRT-PCR assay) | 16.142 | 1.191-218.79 | 0.0365 |
| More than 2 wk to initial sample collection | 32.501 | 1.709-618.21 | 0.0205 |
The association of the specifically diagnostic parameters with risk for the poor prognosis for TBM (death) was tested using conditional logistic regression analysis. A backwards elimination procedure was used for multivariable analysis. For multivariate risk predictors, the adjusted OR values are given with the 95% CI values.
NSD, no significant difference.
(ii) Diachronic study.
Table 6 summarizes the diachronic study results, which include M. tuberculosis cultures, conventional single- and nested-PCR assays, the WR-QNRT-PCR assay, and other routine CSF findings, for the 10 patients: 2 “confirmed” cases (cases 3 and 8) and 8 “highly probable” cases (cases 8 to 16). Among these 10 patients, cases 8 to 15 correspond to cases 1 to 8 in our previous paper (16). The cultures for M. tuberculosis revealed positive results for only 3 (from cases 3 and 8) out of 43 serial CSF samples collected during the clinical treatment course. In contrast, the quantitative detection of M. tuberculosis DNA was possible for 25 CSF samples (58.1%) in the WR-QNRT-PCR assay. In analysis of variance (ANOVA), the WR-QNRT-PCR assay results revealed significant alterations (P < 0.01) during the clinical treatment course in 10 patients (Table 6 and Fig. 1B). In addition, the copy numbers of M. tuberculosis DNA (per 1 ml of CSF) gradually decreased to below the detection limit of the WR-QNRT-PCR assay for the eight patients (cases 8 to 14 and 16) for whom ATT was effective and who demonstrated improvement in both their clinical stages and routine CSF findings during the clinical treatment course (Table 6 and Fig. 1B). However, for cases 3 and 15, for whom ATT was noneffective and who died due to aggravation of TBM, the copy numbers were continually at a high level throughout the clinical course (Table 6 and Fig. 1B). The pattern (trend) in the alterations of M. tuberculosis DNA copy numbers during the clinical treatment course demonstrated significant difference (P = 0.0041) between the ATT-effective cases (cases 8 to 14 and 16) and the ATT-noneffective cases (cases 3 and 8) by repeated-measures ANOVA (Fig. 1B). Moreover, in simple regression analysis, significant correlation (R2 = 0.597, P < 0.0001) was demonstrated between the M. tuberculosis DNA copy number and the clinical stage of TBM (Fig. 1C).
TABLE 6.
Diachronic study results during the clinical course in the 10 patients with suspected TBMa
M. Tb, M. tuberculosis; +, positive; ++, strongly positive; *+, slightly positive; −, negative; †, per 1 ml CSF; INH, isoniazid; RFP, rifampin; PZA, pyrazinamide; SM, streptomycin sulfate; EB, ethambutol; N.D., not determined. Clinical stages are defined according to the British Medical Research Council as follows: stage 0, no definite neurologic symptoms; stage I, slight signs of meningeal irritation with slight clouding of consciousness; stage II, moderate signs of meningeal irritation with moderate disturbance of consciousness and neurologic defects; stage III, severe disturbance of consciousness and neurologic defects.
DISCUSSION
We have developed an improved WR-QNRT-PCR assay technique for the accurate quantitative detection of M. tuberculosis DNA in CSF samples collected from patients with clinically suspected TBM.
In clinical application, the WR-QNRT-PCR assay demonstrated equivalence in sensitivity (95.8%) and specificity (100%) to the OR-QNRT-PCR assay (Table 3). However, for the actual clinical CSF samples, the copy numbers calculated by the OR-QNRT-PCR assay revealed the unfavorable influence of overamplification and the instability of the M-plasmid as the old internal control (Fig. 1A), as well as the preliminary experiment results described in the companion methodology paper (17). In addition, this instability of OR-QNRT-PCR assay was also recognized in the blinded (randomized) assay (Table 4). However, the WR-QNRT-PCR assay results in the blinded assay demonstrated sufficient reproducibility and strengthened the clinical significance of this assay (Table 4). Therefore, the WR-QNRT-PCR assay was considered to be not only more accurate but also a more stable and reliable diagnostic method than conventional assay techniques, including OR-QNRT-PCR. Moreover, it was statistically evaluated using conditional logistic regression analysis whether the specifically diagnostic parameters, including M. tuberculosis cultures, conventional single- and nested-PCR assays, and the WR-QNRT-PCR assay for CSF and the period up to initial sample collection associated with risk for a poor prognosis for TBM (i.e., death) (Table 5). The conditional logistic regression analysis results indicated that an M. tuberculosis DNA copy number of >8,000 calculated by the WR-QNRT-PCR assay was an independent risk factor for a poor prognosis for TBM, as was M. tuberculosis culture positivity (Table 5). Interestingly, a period of more than 2 weeks up to initial sample collection was also one of the most important risk factors for a poor prognosis for TBM (Table 5). This result suggests that a delay in making the appropriate clinical decision, including starting ATT, has a serious influence against the prognosis for TBM. In the present diachronic study, the copy numbers demonstrated statistically significant alterations (P < 0.01) during the clinical treatment course for 10 patients (Table 6). In addition, these alterations of M. tuberculosis DNA copy numbers were significantly correlated with the patient's clinical condition (stage) and the ATT response (Fig. 1B and C). In cases 3 and 15, although the appropriate ATT was started immediately after admission to our hospital, ATT was noneffective for these two patients and they died due to aggravation of TBM (Table 6). These unsuccessful ATT outcomes may be due not only to the high copy numbers of M. tuberculosis DNA in these two patients but also to the delay (more than 2 weeks) in appropriate CSF examination or in starting treatment (Table 2).
We consider that accurate quantitative analysis by WR-QNRT-PCR assay may provide a significantly reliable foundation for appropriate clinical decision, such as the start of ATT, the additional use of corticosteroids and other anti-inflammatory or immune-modulatory adjunctive treatments, and prediction of prognosis in patients with suspected TBM. The present diachronic study results indicate that quantitative analysis by the WR-QNRT-PCR assay is very useful for assessing the clinical course of TBM and ATT response. To our knowledge, there has been no previous study which serially assessed the quantity of DNA or bacterial cell numbers of M. tuberculosis in CSF samples throughout the clinical course of TBM patients. Previously (in 2006), to quantitatively detect M. tuberculosis DNA in CSF samples, we designed the OR-QNRT-PCR assay (15). However, the OR-QNRT-PCR assay was incomplete and insufficient for massive clinical application and commercial evolution, since this assay technique held the unfavorable influence of overamplification caused by the instability of the M-plasmid, used as the old internal control (15, 17). In this study, the WR-QNRT-PCR assay was developed as a novel improved assay technique for wider use in the clinical practice (17). In actual clinical application, this novel assay technique demonstrated significant accuracy and reliability for the quantitative detection of M. tuberculosis DNA in CSF samples due to the development of NM-plasmid, which was used as the new internal control.
The clinical usefulness of the WR-QNRT-PCR assay is based on its capacity for the accurate quantitative detection of M. tuberculosis DNA with a wide detection range. However, this assay technique does not have the ability to evaluate the viability of bacteria. Therefore, the copy number of M. tuberculosis DNA calculated by WR-QNRT-PCR assay may not necessarily be consistent with the viable bacterial number of M. tuberculosis in the CSF sample. At present, the only assay method for detecting viable M. tuberculosis in the CSF sample is culture examination. Although the culture for M. tuberculosis in CSF samples is the “gold standard” for TBM diagnosis, it is inadequate for early diagnosis due to its poor sensitivity or the long time required (4 to 8 weeks) (3-14, 18). Rapid and accurate diagnosis in the acute phase of TBM and an early start to ATT are the most important factors with regard to the prognosis and the prevention of long-term neurological sequelae (3-19). Based on the present assay results, we considered that the WR-QNRT-PCR assay is a reliable assay technique for assessing ATT response and the clinical course of TBM. Particularly, in clinical practice, the WR-QNRT-PCR assay would demonstrate its capacity in rapid and accurate diagnosis for the difficult cases in which conventional assay methods cannot detect M. tuberculosis.
At present, despite an overall decrease in the total numbers of tuberculosis cases in the advanced nations, for example, the United States, a gradual and continuous increase in the proportion of extrapulmonary tuberculosis cases has been reported (1, 2). The causes for the increase of extrapulmonary tuberculosis cases are mainly in the recent rise in immune-compromised patients and in the human immunodeficiency virus/AIDS epidemic (1, 2). Although the overall population-based mortality rate from tuberculosis is low and decreasing, several studies have shown that mortality rates are substantially higher for patients with several forms of extrapulmonary tuberculosis, including CNS tuberculosis or TBM and disseminated disease (1, 2, 8, 18). As the proportion of extrapulmonary tuberculosis cases, including those with CNS tuberculosis, continues to increase, particularly in immune-compromised patients, the WR-QNRT-PCR assay technique may become increasingly important for the rapid and accurate diagnosis of TBM.
Certainly, the WR-QNRT-PCR assay may be inadequate for screening examinations dealing with many samples, since this novel assay technique requires additional complicated experimental procedures. However, in actual clinical practice, definitive diagnosis of TBM is required, and this is not available from present screening examination procedures. Therefore, the WR-QNRT-PCR assay will become a prominently useful assay technique if used for well-defined and appropriate clinical specimens collected from “highly probable” TBM patients. We speculate that if the WR-QNRT-PCR assay is widely and appropriately adopted within clinical practice, it will be a powerful tool for the rapid and accurate diagnosis of TBM.
Acknowledgments
We thank Hiroki Nagase and many doctors who collected CSF samples in the following institutions: Department of Neurology and Pediatrics, Kasugai Hospital; Department of Neurology, Nagoya University School of Medicine; Department of Neurology, Kariya Hospital; Department of Neurology, Tokai University School of Medicine; Fourth Department of Internal Medicine, Saitama Medical Center; Department of Neurology, Metropolitan Bokutoh Hospital; Kohnodai Hospital; and National Center of Neurology and Psychiatry, Japan.
This work was supported by “Academic Frontier” Project for Private Universities: matching fund subsidy from MEXT (Ministry of Education, Culture, Sports, Science and Technology), 2006-2010.
Footnotes
Published ahead of print on 12 March 2008.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2005. Reported tuberculosis in the United States. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA.
- 2.Kourbatova, E. V., M. K. Leonard, Jr., J. Romero, C. Kraft, C. del Rio, and H. M. Blumberg. 2006. Risk factors for mortality among patients with extrapulmonary tuberculosis at an academic inner-city hospital in the US. Eur. J. Epidemiol. 21715-721. [DOI] [PubMed] [Google Scholar]
- 3.Lee, B. W., J. A. Tan, S. C. Wong, C. B. Tan, H. K. Yap, P. S. Low, J. N. Chia, and J. S. Tay. 1994. DNA amplification by the polymerase chain reaction for the rapid diagnosis of tuberculous meningitis. Comparison of protocols involving three mycobacterial DNA sequences, IS6110, 65 kDa antigen, and MPB64. J. Neurol. Sci. 123173-179. [DOI] [PubMed] [Google Scholar]
- 4.Liu, P. Y., Z. Y. Shi, Y. J. Lau, and B. S. Hu. 1994. Rapid diagnosis of tuberculous meningitis by a simplified nested amplification protocol. Neurology 441161-1164. [DOI] [PubMed] [Google Scholar]
- 5.Marin, M., D. Garcia de Viedma, M. J. Ruiz-Serrano, and E. Bouza. 2004. Rapid direct detection of multiple rifampin and isoniazid resistance mutations in Mycobacterium tuberculosis in respiratory samples by real-time PCR. Antimicrob. Agents Chemother. 484293-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medical Research Council. 1948. Streptomycin treatment of tuberculous meningitis. Lancet i582-596. [PubMed] [Google Scholar]
- 7.Nakajima, H., K. Ashida, H. Yamasaki, K. Shinoda, and N. Ohsawa. 1995. Intracranial tuberculoma with spontaneous recovery. Rinsho Shinkeigaku 35521-525. (In Japanese.) [PubMed] [Google Scholar]
- 8.Porkert, M. T., M. Sotir, P. Parrott-Moore, and H. M. Blumberg. 1997. Tuberculous meningitis at a large inner-city medical center. Am. J. Med. Sci. 313325-331. [DOI] [PubMed] [Google Scholar]
- 9.Rindi, L., N. Lari, D. Bonanni, and C. Garzelli. 2004. Detection of Mycobacterium tuberculosis genotypic groups by a duplex real-time PCR targeting the katG and gyrA genes. J. Microbiol. Methods 59283-287. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz, M., M. J. Torres, A. C. Llanos, A. Arroyo, J. C. Palomares, and J. Aznar. 2004. Direct detection of rifampin- and isoniazid-resistant Mycobacterium tuberculosis in auramine-rhodamine-positive sputum specimens by real-time PCR. J. Clin. Microbiol. 421585-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scarpellini, P., S. Racca, P. Cinque, F. Delfanti, N. Gianotti, M. R. Terreni, L. Vago, and A. Lazzarin. 1995. Nested polymerase chain reaction for diagnosis and monitoring treatment response in AIDS patients with tuberculous meningitis. AIDS 9895-900. [DOI] [PubMed] [Google Scholar]
- 12.Shankar, P., N. Manjunath, K. K. Mohan, K. Prasad, M. Behari, Shriniwas, and G. K. Ahuja. 1991. Rapid diagnosis of tuberculous meningitis by polymerase chain reaction. Lancet 3375-7. [DOI] [PubMed] [Google Scholar]
- 13.Stranska, R., R. Schuurman, M. de Vos, and A. M. van Loon. 2004. Routine use of a highly automated and internally controlled real-time PCR assay for the diagnosis of herpes simplex and varicella-zoster virus infections. J. Clin. Virol. 3039-44. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi, T., T. Nakayama, M. Tamura, K. Ogawa, H. Tsuda, A. Morita, M. Hara, M. Togo, H. Shiota, Y. Suzuki, M. Minami, H. Ishikawa, K. Miki, E. Shikata, S. Takahashi, T. Kuragano, K. Matsumoto, S. Sawada, and T. Mizutani. 2005. Nested polymerase chain reaction for assessing the clinical course of tuberculous meningitis. Neurology 641789-1793. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi, T., and T. Nakayama. 2006. Novel technique of quantitative nested real-time PCR assay for Mycobacterium tuberculosis DNA. J. Clin. Microbiol. 441029-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi, T., M. Tamura, S. N. Takahashi, K. Matsumoto, S. Sawada, E. Yokoyama, T. Nakayama, T. Mizutani, T. Takasu, and H. Nagase. 2007. Quantitative nested real-time PCR assay for assessing the clinical course of tuberculous meningitis. J. Neurol. Sci. 25569-76. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi, T., M. Tamura, Y. Asami, E. Kitamura, K. Saito, T. Suzuki, S. N. Takahashi, K. Matsumoto, S. Sawada, E. Yokoyama, and T. Takasu. 2008. Novel wide-range quantitative nested real-time PCR assay for Mycobacterium tuberculosis DNA: development and methodology. J. Clin. Microbiol. 461708-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thwaites, G. E., D. B. Nguyen, H. D. Nguyen, T. Q. Hoang, T. T. Do, T. C. Nguyen, Q. H. Nguyen, T. T. Nguyen, N. H. Nguyen, T. N. Nguyen, N. L. Nguyen, H. D. Nguyen, N. T. Vu, H. H. Cao, T. H. Tran, P. M. Pham, T. D. Nguyen, K. Stepniewska, N. J. White, T. H. Tran, and J. J. Farrar. 2004. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N. Engl. J. Med. 3511741-1751. [DOI] [PubMed] [Google Scholar]
- 19.Wada, T., S. Maeda, A. Tamaru, S. Imai, A. Hase, and K. Kobayashi. 2004. Dual-probe assay for rapid detection of drug-resistant Mycobacterium tuberculosis by real-time PCR. J. Clin. Microbiol. 425277-5285. [DOI] [PMC free article] [PubMed] [Google Scholar]