Abstract
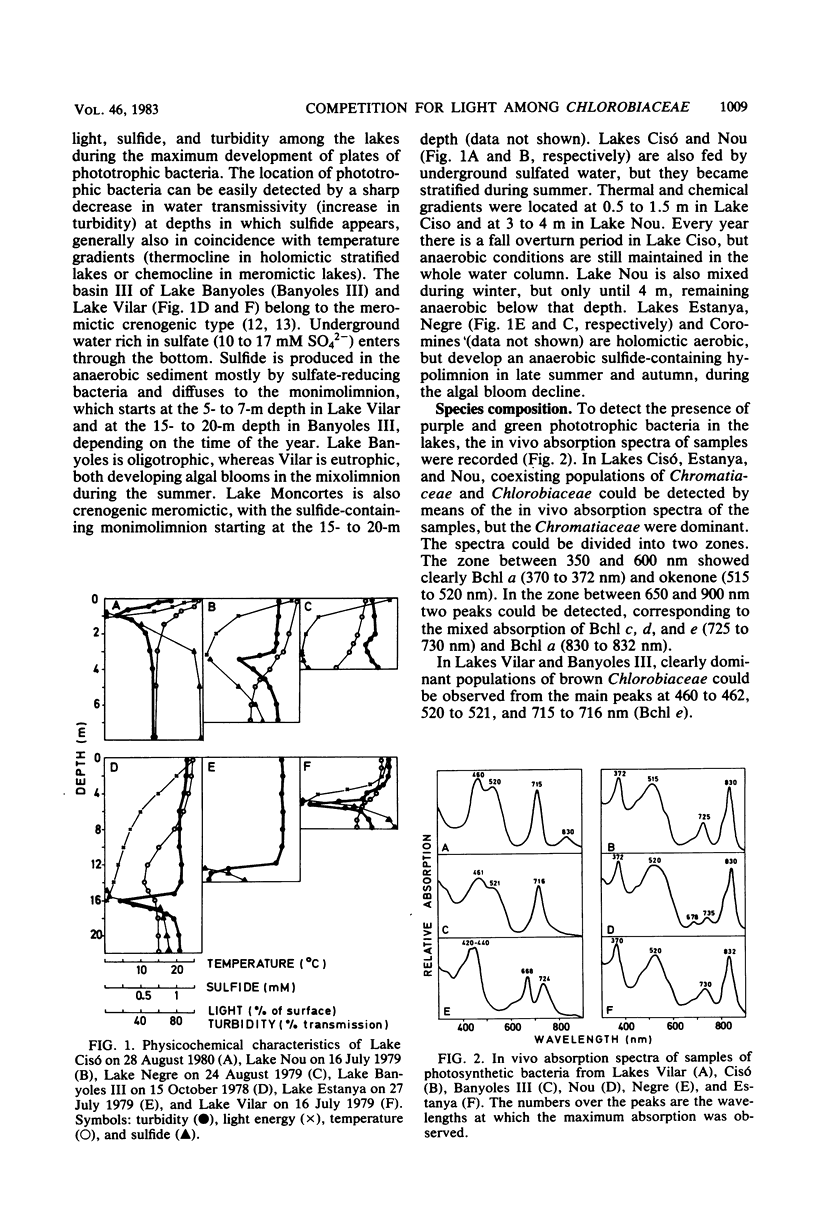
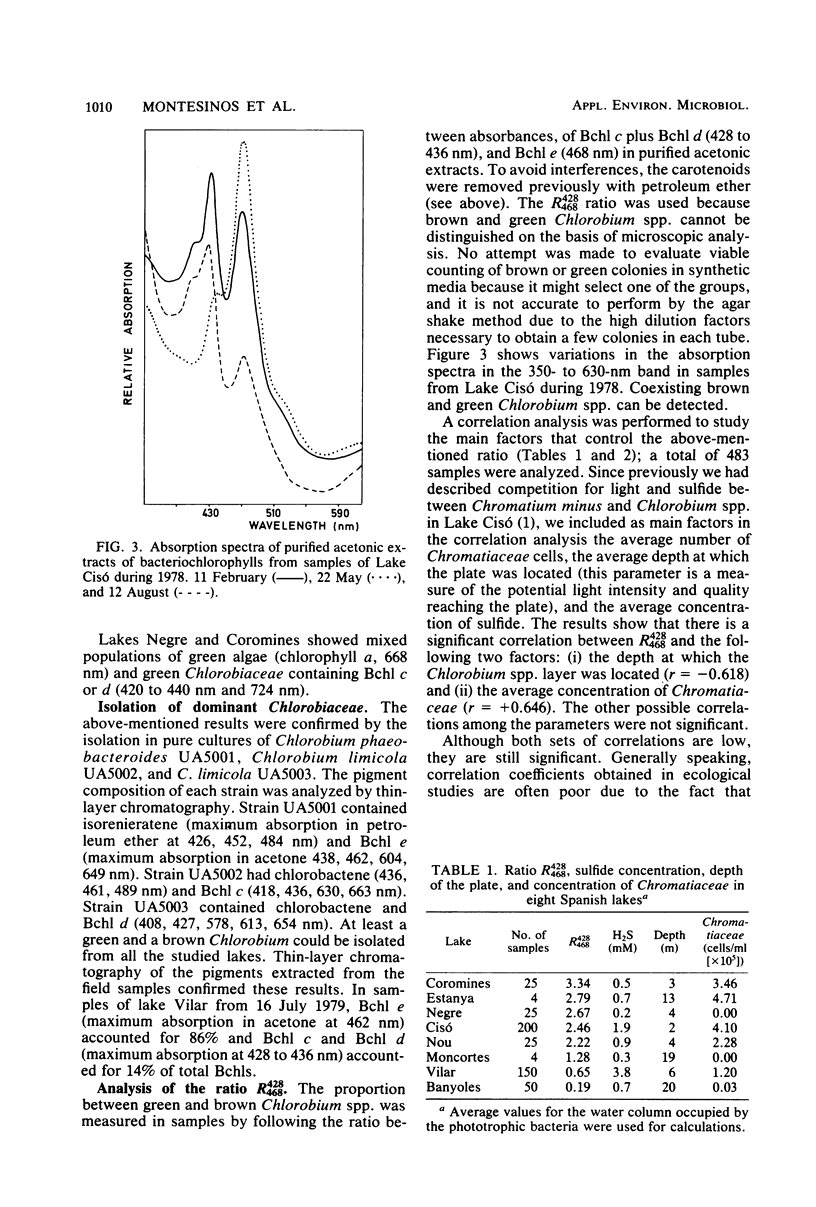
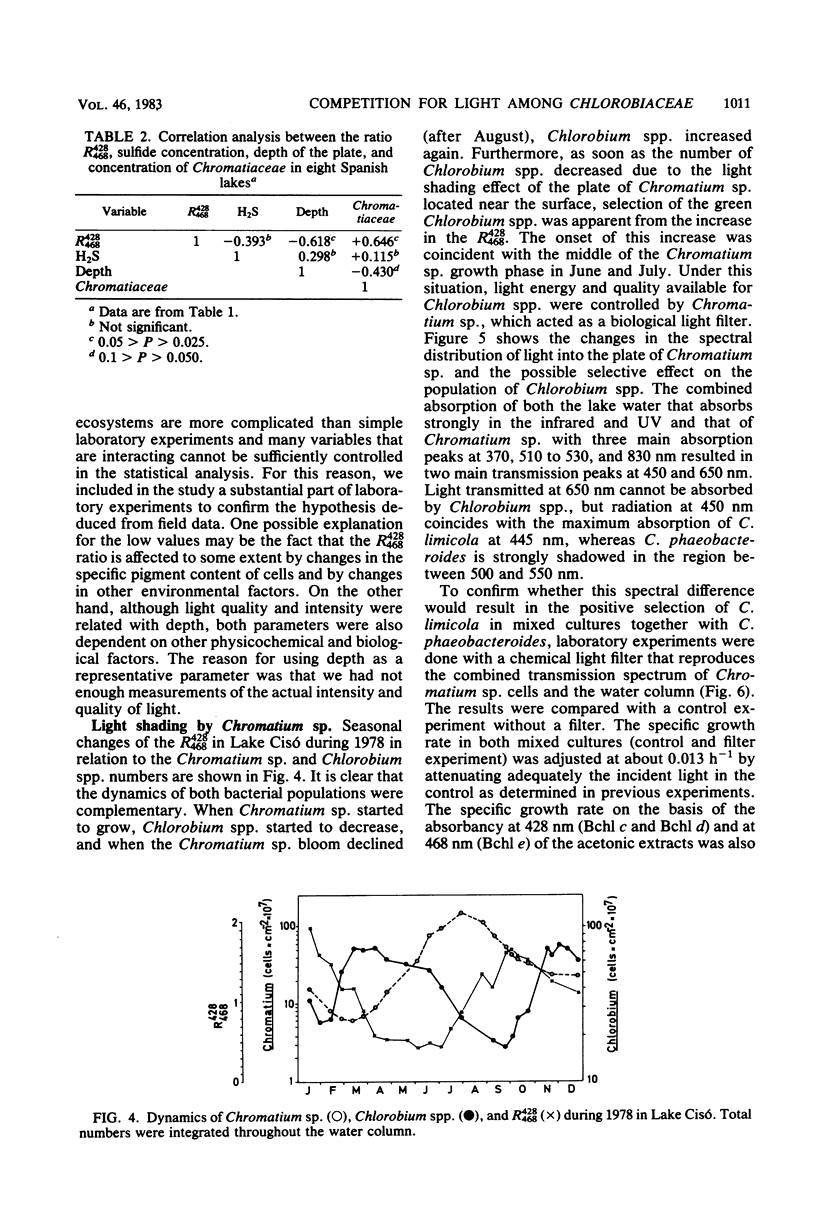
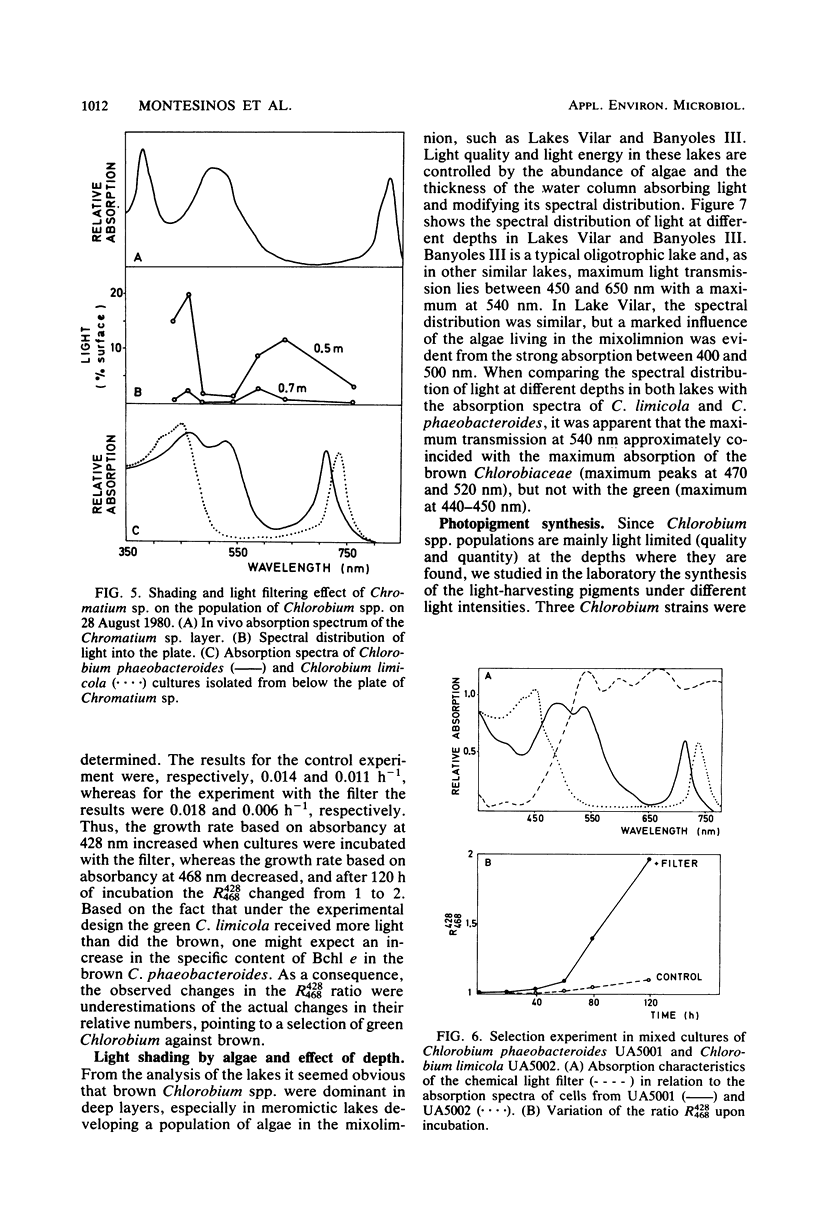
Accurate studies of the pigment composition and isolation in pure cultures of Chlorobiaceae from samples of eight Spanish lakes show that there are two main coexisting groups of green and brown Chlorobium spp. represented respectively by Chlorobium limicola and Chlorobium phaeobacteroides. Laboratory experiments with pure and mixed cultures of the isolated strains show that light quality plays a selective role on the species composition among Chlorobiaceae. This selection depends on the pigment composition which determines the in vivo absorption spectrum of the cells as well as on their ability to adjust the intracellular concentration of light-harvesting pigments to the spectral distribution and energy of light. Correlation analysis performed with field data resulted in significant, but low, correlation coefficients. Nevertheless, they were consistent with laboratory data showing that brown Chlorobiaceae were dominant in deep layers in meromictic lakes, whereas green Chlorobiaceae dominated in layers nearer the surface or underneath plates of Chromatiaceae. The combination of laboratory and field observations stress the role of biological light filtering in determining the species composition among Chlorobiaceae in lakes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergstein T., Henis Y., Cavari B. Z. Investigations on the photosynthetic sulfur bacterium Chlorobium phaeobacteroides causing seasonal blooms in Lake Kinneret. Can J Microbiol. 1979 Sep;25(9):999–1007. doi: 10.1139/m79-154. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., PFENNIG N., KUNISAWA R. THE FINE STRUCTURE OF GREEN BACTERIA. J Cell Biol. 1964 Jul;22:207–225. doi: 10.1083/jcb.22.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruden D. L., Stanier R. Y. The characterization of chlorobium vesicles and membranes isolated from green bacteria. Arch Mikrobiol. 1970;72(2):115–134. doi: 10.1007/BF00409518. [DOI] [PubMed] [Google Scholar]
- GOEDHEER J. C. Energy transfer between carotenoids and bacteriochlorophyll in chromatophores of purple bacteria. Biochim Biophys Acta. 1959 Sep;35:1–8. doi: 10.1016/0006-3002(59)90328-2. [DOI] [PubMed] [Google Scholar]
- Jensen S. L. Bacterial carotenoids. 18. Aryl-carotenes from Phaeobium. Acta Chem Scand. 1965;19(5):1025–1030. doi: 10.3891/acta.chem.scand.19-1025. [DOI] [PubMed] [Google Scholar]
- Matheron R., Baulaigue R. Influence de la énétration de la lumière solaire sur le développement des bactéries phototrophes sulfureuses dans les environnements marins. Can J Microbiol. 1977 Mar;23(3):267–270. [PubMed] [Google Scholar]
- Pedrós-Alió C., Montesinos E., Guerrero R. Factors determining annual changes in bacterial photosynthetic pigments in holomictic lake cisó, Spain. Appl Environ Microbiol. 1983 Nov;46(5):999–1006. doi: 10.1128/aem.46.5.999-1006.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig N. Photosynthetic bacteria. Annu Rev Microbiol. 1967;21:285–324. doi: 10.1146/annurev.mi.21.100167.001441. [DOI] [PubMed] [Google Scholar]
- Pfennig N. Phototrophic green and purple bacteria: a comparative, systematic survey. Annu Rev Microbiol. 1977;31:275–290. doi: 10.1146/annurev.mi.31.100177.001423. [DOI] [PubMed] [Google Scholar]
- STANIER R. Y., SMITH J. H. The chlorophylis of green bacteria. Biochim Biophys Acta. 1960 Jul 15;41:478–484. doi: 10.1016/0006-3002(60)90045-7. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Pfennig N., Jensen S. L. Carotenoids of Thiorhodaceae IV. The carotenoid composition of 25 pure isolates. Arch Mikrobiol. 1965 Oct 14;52(2):132–146. [PubMed] [Google Scholar]


