Abstract
Transmission of arenaviruses from rodent hosts to humans is generally thought to occur through inhalation or ingestion of dust or droplets containing viral particles. Here we demonstrate that two identified arenavirus receptors, α-dystroglycan (α-DG) and transferrin receptor 1 (TfR1), are expressed in polarized human airway epithelia. Lymphocytic choriomeningitis virus strains with high or low α-DG affinity and Junin virus, which binds TfR1, efficiently infected polarized epithelia only when applied to the basolateral surface or when injury compromised tight junction integrity. Viral egress from infected epithelia exhibited basolateral polarity. This study demonstrates that respiratory entry of arenaviruses occurs via basolateral receptors.
Lymphocytic choriomeningitis virus (LCMV) is generally noncytopathic, and the most common human disease associated with infection is aseptic meningitis. Laboratory arenavirus strains infect several epithelial tissues, including gastric and lung epithelium (3, 7, 15, 18). LCMV is endemic in rodents, which serve as a reservoir. Transmission of arenavirus to humans is believed to occur by more than one route. Evidence suggests that inhalation of infected particulates plays an important role (7, 15), as does direct inoculation from animal bites or abrasions. Rhesus macaques exposed to the Junin arenavirus by aerosol developed acute illness and died within a month (15). Additionally, rhesus and cynomolgus macaques developed morbidity following aerosol infection with LCMV (7). While the respiratory tract is a proposed route of entry, the interactions between LCMV and polarized human respiratory epithelia have not been studied.
Alpha-dystroglycan (α-DG) has been identified as a receptor for some arenaviruses, including the Old World arenaviruses Lassa fever virus and certain strains of LCMV, as well as clade C New World arenaviruses, which include Oliveros and Latino viruses as its sole members (4, 24). Some LCMV strains show little dependence on α-DG (23). Ubiquitously expressed, dystroglycan is transcribed as a precursor peptide and posttranslationally cleaved to yield α-DG and β-DG, noncovalently linked peripheral and integral proteins, respectively (13). Together they form an important transmembrane junction connecting the intracellular cytoskeleton and extracellular matrix. The receptor for the clade B New World arenaviruses, represented by Machupo, Guanarito, Junin, and Sabia viruses, was identified as transferrin receptor 1 (TfR1) (11, 17).
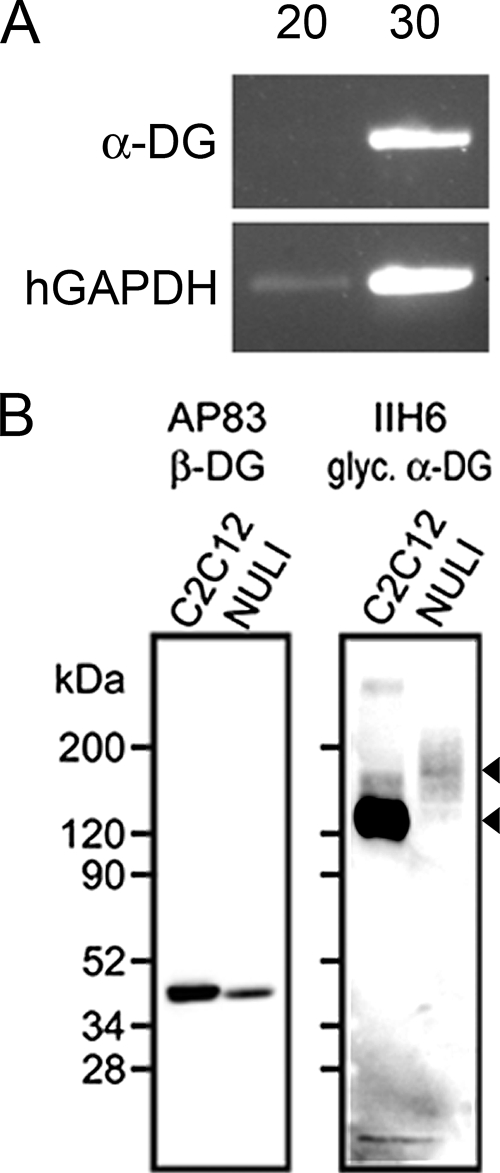
We examined the expression and localization of the identified New World and Old World arenavirus receptors in polarized primary cultures of human airway epithelia. We first asked whether α-DG is an available receptor for LCMV in human airway epithelia. Well-differentiated primary human airway epithelia were prepared as previously described (14). RNA was isolated from polarized airway epithelia using TRIzol (15596-026; Invitrogen). cDNA was generated using SuperScript II reverse transcriptase (18064-022; Invitrogen). Reverse transcription-PCR was performed with primer sets designed for α-DG (α-DG-F [5′ GGTGAAGATCCCGTCAGACACTTT 3′] and α-DG-R [5′ ACCACAGGGATAAACTGTAGGTGC 3′]) or human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (HGAPDH-F [5′ GTCAGTGGTGGACCTGACCT 3′] and HGAPDH-R ′5′ AGGGGTCTACATGGCAACTG 3′]). While α-DG mRNA levels were undetectable after 20 PCR cycles, the mRNA was readily detected after 30 cycles (Fig. 1A).
FIG. 1.
α-DG expression in human airway epithelia. (A) Reverse transcription-PCR was performed using cDNA derived from primary epithelia using primers specific for α-DG or human GAPDH (20 or 30 cycles). The human-GAPDH control confirmed mRNA was isolated properly. Results are representative of findings for three different human specimens. (B) Antibodies specific for α-DG or β-DG were used to detect protein expression in a positive-control mouse myoblast cell line (C2C12) and an immortalized human airway epithelial cell line (NuLi).
Immunoblotting confirmed that dystroglycan protein was present in samples of immortalized airway epithelia. An immortalized human respiratory airway cell line (NuLi) (28) and positive-control C2C12 mouse myoblast (ATCC CRL-1772) cell lysates were probed using antibodies specific for β-DG (AP83) or α-DG (IIH6) (10). Both cell types produced abundant β-DG, detected as a band of approximately 43 kDa (Fig. 1B). The airway cell α-DG protein appeared as a broad smear, with a more-prominent band detected at approximately 150 kDa. A likely reason for the increased size and variation in α-DG molecular weights in airway epithelia compared to those with C2C12 cells is differential glycosylation (8). The less-abundant signal in airway epithelia may also represent incomplete recognition of glycosylated isoforms by the antibody or shedding of the noncovalently linked peripheral protein (22, 27).
To localize α-DG and TfR1 expression in polarized airway epithelia, immunohistochemistry was performed. Epithelia were pretreated apically with 100 μl of 1,000-U/ml collagenase (Sigma C-9407) diluted in 50:50 Dulbecco's modified Eagle medium-Ham's F-12 medium (11320-033; Gibco) supplemented with 2% Ultroser G (15950-017; Biosepra) for 2 h at 37°C to remove the extracellular matrix components exposing apical sialic acid residues as previously described (26). α-DG immunolocalization studies utilized a Cy3-labeled β-tubulin antibody (1:100; no. C-4585; Sigma) to label the apical surface cilia and a previously described α-DG antibody, IIH6 (1:20) (10). ZO-1 antibody (1:100; no. 61-7300; Zymed) and an anti-TfR1 antibody (1:100 CD71; no. 555534; BD Pharmingen) were used for TfR1 immulocalization. All immunohistochemistry was performed under permeablizing conditions by blocking in a 0.5% Triton X solution. Control samples were incubated with 1:200 isotype antibody. Following primary antibody incubation, the epithelia were washed and incubated with appropriate secondary antibodies. Primary and secondary antibodies were applied to both the apical and basolateral cell surfaces. The epithelia were then mounted on slides and imaged using laser scanning confocal microscopy.
α-DG displayed no distinct polarity, localizing to both apical and basolateral membranes, with some specimen-to-specimen variation noted (Fig. 2B to D). This is consistent with previous observations made with mouse trachea (9). Diffuse intracellular expression was also observed. No colocalization with cilia was detected. In contrast, TfR1 exclusively localized to basolateral membranes (Fig. 3F and 3G) below the tight junction boundary designated by ZO-1.
FIG. 2.
Immunolocalization of arenavirus receptors in human airway epithelia. En face (A to G) and corresponding confocal vertical sections (inset) of primary human airway epithelia following immunohistochemistry are shown. The localization of α-DG was detected by isotype control antibody (A) or α-DG IIH6 monoclonal antibody (B and C). α-DG expression is represented by a green (FITC) signal, while a red signal indicates cilium-specific β-tubulin Cy3 labeling of the apical surface. (D) TO-PRO 3 (blue) was used to label nuclei. The vertical focus in panel D lies beneath the tight junctions to display basolateral membrane labeling. Scale bar in panel A = 20 μm; n = 12 epithelia from 6 different donors. (E to G) The expression localization of TfR1 detected by secondary antibody only (E) or CD71 monoclonal antibody (F and G). TfR1 expression is represented by a green (Alexa Fluor 488) signal, while a red signal (Alexa Fluor 568) indicates tight junction boundaries detected by ZO-1 antibody. The vertical planes of focus in panels F and G differ to demonstrate unique localization; n = 10 epithelia from 5 different donors.
FIG. 3.
Polarity of infection by infectious arenaviruses. Well-differentiated cultures of primary human airway epithelia were analyzed to determine the polarity of virus entry following either apical or basolateral virus application. Top to bottom, the panels represent apical infection (A, D, G, J, and M), basolateral infection (B, E, H, K, and N), or apical infection following surface injury by scratching the epithelia with a pipette tip (C, F, I, L, and O). LCMV Armstrong 53b (A to C), LCMV Armstrong clone 13 (D to F), and LCMV WE54 (G to I) proteins were detected with a polyclonal antibody followed by FITC-labeled secondary antibody; n = 12 epithelia from 4 different donors. Junin protein (J to L) was detected with monoclonal antibody J3.6.2, followed by Alexa Fluor 488 secondary antibody; n = 6 epithelia from 3 different donors. As a control, Ad-LacZ (M to O) was applied and cells were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside. Scale bar (C) = 100 μm.
Having localized arenavirus receptors in airway epithelia, we examined the polarity of entry and exit of LCMV strains with differing affinities for α-DG as a receptor and the entry polarity of Junin virus. Three different infectious LCMV strains, Armstrong 53b (a gift from John Harty, University of Iowa), Armstrong clone 13, and WE54 (a gift from Michael Oldstone, Scripps Research Institute), were studied. Armstrong 53b has a low affinity for α-DG, and the latter two display high α-DG affinities (4, 23, 24). Cultures of primary human airway cells were infected either apically or basolaterally at a multiplicity of infection (MOI) of 1 (in 100 μl of culture medium) for 2 h, and then the surfaces were rinsed and culture medium replaced. Forty-eight hours postinfection, basolateral culture media were collected and the apical surface of each culture was also rinsed with medium and collected for viral exit titer determination. Cultures were then fixed with 2% paraformaldehyde for 10 min and subsequently blocked with 5% bovine serum albumin plus 0.3% Triton X-100 for 1 h at room temperature. Following two phosphate-buffered-saline washes, a 1:500 dilution of guinea pig LCMV polyclonal antibody (1, 2) (gift of Dan Bonthius, University of Iowa) was incubated on both surfaces of the culture for 1 h at 37°C. After two washes, a 1:1,000 dilution of anti-guinea pig fluorescein isothiocyanate (FITC)-conjugated secondary antibody (FI-7000; Vector Laboratories) was applied under the same conditions as for the primary antibody. Epithelia were mounted on slides and imaged using fluorescence microscopy.
Attenuated Candid 1 Junin virus (supplied by Michael Buchmeier, Scripps Research Institute) (12) was similarly applied to either the apical or basolateral surfaces of primary airway epithelia cultures at an MOI of ∼0.04 (in 100 μl) for 2 h. Afterward, cultures were rinsed and culture media replaced. Three days postinfection, epithelia were processed as described above. Junin monoclonal antibody specific for the nucleoprotein, (J3.6.2) (12) at a 1:500 dilution was incubated on both surfaces of the culture overnight at 4°C. After rinses, a goat antimouse Alexa Fluor 488 (1:500; no. A-21121; Invitrogen) antibody was applied for detection.
Regardless of the α-DG affinity of the applied LCMV virus, only epithelia with virus applied to the basolateral surface yielded infected cells detected by abundant LCMV immunoreactivity (Fig. 3B, E, and H). A high percentage of cells expressed LCMV immunoreactivity, with obvious clusters of positive cells visualized. These clusters likely represent a local spread of infection following virus replication rather than cell division, since well-differentiated epithelia have a low mitotic index. Very rarely, a positive cell was observed following apical infection (Fig. 3G). These results imply that only basolaterally localized α-DG is efficiently used or that a different receptor is used. It is possible that correct α-DG glycosylation for arenavirus recognition is limited to the basolateral surfaces. However, this is unlikely, because the IIH6 antibody recognizes glycosylated α-DG necessary for virus binding (16, 21). Alternatively, differences in viral trafficking at the apical and basolateral surfaces due to unique endocytic pathways (20) may account for the observed differences. Adenovirus serotype 5 expressing β-galactosidase was used as a control and demonstrated basolateral entry polarity detected by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside staining, as expected (Fig. 3N).
The polarity of entry for Junin virus was also basolateral (Fig. 3K), consistent with TfR1 immunolocalization. Despite a titer-limited MOI ∼2 logs lower than that of LCMV strains, basolateral infection with Junin yielded an average of 212 (±86) nucleoprotein-positive colonies/epithelial sheet studied (n = 6 from 3 donors). Apical Junin infection yielded no positive colonies (n = 6). Our observed basolateral polarity of Junin entry contradicts a previous report of apical entry in a polarized respiratory epithelial cell line (6). We stress that polarized primary epithelia more accurately model the in vivo pseudostratified epithelium.
To further confirm that only basolaterally expressed receptors are used by arenaviruses, we physically disrupted tight junction integrity. Epithelial sheets were gently scratched once on the apical side with a pipette tip, followed immediately by application of each LCMV strain apically for 1 h. Two days postinfection, immunohistochemistry was performed to label infected cells. LCMV applied to the apical surface of injured epithelia displayed distinct tracts of labeled cells along scratch sites (Fig. 3C, F, and I). Similar results were observed with serotype 5 adenovirus, known to use a basolateral receptor (CAR) (29). Junin virus did not exhibit similar results, likely due to a greatly reduced effective MOI. In summary, these findings further suggest that only basolateral α-DG serves as a functional receptor for Old World arenavirus strains.
The polarity of viral egress was examined by collecting apical washes and basolateral media following infections. For all three LCMV strains, we observed LCMV release from infected human airway epithelia exclusively from the basolateral surface (data not shown), consistent with observations that the entry and exit of many viruses occur from the same surface in polarized cell types (5, 6, 25, 29). Low starting titers of the Junin vaccine strain did not allow conclusive evaluation of viral exit.
This study is the first demonstration of polarized entry and exit of arenaviruses in well-differentiated human airway epithelia. Previously, aerosol delivery of LCMV (7) or Junin virus (15) to macaques resulted in virus spread to several visceral organs. Despite the common notion that the respiratory epithelium is a primary site of arenavirus infection in humans, we were surprised to discover that entry through the apical surface of polarized airway epithelia does not occur. We speculate that arenavirus entry in the respiratory tract likely occurs when epithelial tight junction permeability and integrity are compromised by injury or disease. The virus might also spread lymphatically or via primarily infected mononuclear or dendritic cells. Basolateral release of virus produced in respiratory epithelia may also facilitate secondary infection of the spleen and liver, sites associated with arenavirus intragastric infection (19).
Acknowledgments
We acknowledge the support of the University of Iowa Cell and Tissue Core and the Cell Morphology Core, partially supported by the Cystic Fibrosis Foundation, NHLBI (PPG HL-51670), and the Center for Gene Therapy for Cystic Fibrosis (NIH P30 DK-54759). This work was supported by NIH Research Service Award Institutional Training Grant 2 T32 GM008629, NIH grant RO1 HL-61460 (to P.B.M.), and grant PPG HL-51670 (to P.B.M.).
Ariadna Arias and Jennifer Springsteen provided valuable technical assistance.
Footnotes
Published ahead of print on 16 April 2008.
REFERENCES
- 1.Baldridge, J. R., B. D. Pearce, B. S. Parekh, and M. J. Buchmeier. 1993. Teratogenic effects of neonatal arenavirus infection on the developing rat cerebellum are abrogated by passive immunotherapy. Virology 197669-677. [DOI] [PubMed] [Google Scholar]
- 2.Bonthius, D. J., J. Mahoney, M. J. Buchmeier, B. Karacay, and D. Taggard. 2002. Critical role for glial cells in the propagation and spread of lymphocytic choriomeningitis virus in the developing rat brain. J. Virol. 766618-6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchmeier, M. J., M. D. Bowen, and C. J. Peters. 2001. Arenaviridae: the viruses and their replication, p. 1635-1668. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA.
- 4.Cao, W., M. D. Henry, P. Borrow, H. Yamada, J. H. Elder, E. V. Ravkov, S. T. Nichol, R. W. Compans, K. P. Campbell, and M. B. Oldstone. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 2822079-2081. [DOI] [PubMed] [Google Scholar]
- 5.Chu, J. J., and M. L. Ng. 2002. Infection of polarized epithelial cells with flavivirus West Nile: polarized entry and egress of virus occur through the apical surface. J. Gen. Virol. 832427-2435. [DOI] [PubMed] [Google Scholar]
- 6.Cordo, S. M., M. Cesio y Acuna, and N. A. Candurra. 2005. Polarized entry and release of Junin virus, a New World arenavirus. J. Gen. Virol. 861475-1479. [DOI] [PubMed] [Google Scholar]
- 7.Danes, L., R. Benda, and M. Fuchsova. 1963. Experimental inhalation infection of monkeys of the Macacus cynomolgus and Macacus rhesus species with the virus of lymphocytic choriomeningitis (We). Bratisl. Lek. Listy 271-79. (In Czech.) [PubMed] [Google Scholar]
- 8.Durbeej, M., and K. P. Campbell. 1999. Biochemical characterization of the epithelial dystroglycan complex. J. Biol. Chem. 27426609-26616. [DOI] [PubMed] [Google Scholar]
- 9.Durbeej, M., M. D. Henry, M. Ferletta, K. P. Campbell, and P. Ekblom. 1998. Distribution of dystroglycan in normal adult mouse tissues. J. Histochem. Cytochem. 46449-457. [DOI] [PubMed] [Google Scholar]
- 10.Ervasti, J. M., and K. P. Campbell. 1991. Membrane organization of the dystrophin-glycoprotein complex. Cell 661121-1131. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan, M. L., J. Oldenburg, T. Reignier, N. Katz-Holt, G. A. Hamilton, V. K. Martin, and P. M. Cannon. 2008. New World clade B arenaviruses can use transferrin receptor 1 (TfR1)-dependent and independent entry pathways, and glycoproteins from human pathogenic strains are associated with the use of TfR1. J. Virol. 82938-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard, C. R., H. Lewicki, L. Allison, M. Salter, and M. J. Buchmeier. 1985. Properties and characterization of monoclonal antibodies to Tacaribe virus. J. Gen. Virol. 661383-1395. [DOI] [PubMed] [Google Scholar]
- 13.Ibraghimov-Beskrovnaya, O., J. M. Ervasti, C. J. Leveille, C. A. Slaughter, S. W. Sernett, and K. P. Campbell. 1992. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355696-702. [DOI] [PubMed] [Google Scholar]
- 14.Karp, P. H., T. O. Moninger, S. P. Weber, T. S. Nesselhauf, J. L. Launspach, J. Zabner, and M. J. Welsh. 2002. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol. Biol. 188115-137. [DOI] [PubMed] [Google Scholar]
- 15.Kenyon, R. H., K. T. McKee, Jr., P. M. Zack, M. K. Rippy, A. P. Vogel, C. York, J. Meegan, C. Crabbs, and C. J. Peters. 1992. Aerosol infection of rhesus macaques with Junin virus. Intervirology 3323-31. [DOI] [PubMed] [Google Scholar]
- 16.Kunz, S., J. M. Rojek, M. Kanagawa, C. F. Spiropoulou, R. Barresi, K. P. Campbell, and M. B. Oldstone. 2005. Posttranslational modification of alpha-dystroglycan, the cellular receptor for arenaviruses, by the glycosyltransferase LARGE is critical for virus binding. J. Virol. 7914282-14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radoshitzky, S. R., J. Abraham, C. F. Spiropoulou, J. H. Kuhn, D. Nguyen, W. Li, J. Nagel, P. J. Schmidt, J. H. Nunberg, N. C. Andrews, M. Farzan, and H. Choe. 2007. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature 44692-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rai, S. K., D. S. Cheung, M. S. Wu, T. F. Warner, and M. S. Salvato. 1996. Murine infection with lymphocytic choriomeningitis virus following gastric inoculation. J. Virol. 707213-7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rai, S. K., B. K. Micales, M. S. Wu, D. S. Cheung, T. D. Pugh, G. E. Lyons, and M. S. Salvato. 1997. Timed appearance of lymphocytic choriomeningitis virus after gastric inoculation of mice. Am. J. Pathol. 151633-639. [PMC free article] [PubMed] [Google Scholar]
- 20.Rojek, J. M., M. Perez, and S. Kunz. 2008. Cellular entry of lymphocytic choriomeningitis virus. J. Virol. 821505-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rojek, J. M., C. F. Spiropoulou, K. P. Campbell, and S. Kunz. 2007. Old World and clade C New World arenaviruses mimic the molecular mechanism of receptor recognition used by alpha-dystroglycan's host-derived ligands. J. Virol. 815685-5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh, J., Y. Itahana, S. Knight-Krajewski, M. Kanagawa, K. P. Campbell, M. J. Bissell, and J. Muschler. 2004. Proteolytic enzymes and altered glycosylation modulate dystroglycan function in carcinoma cells. Cancer Res. 646152-6159. [DOI] [PubMed] [Google Scholar]
- 23.Smelt, S. C., P. Borrow, S. Kunz, W. Cao, A. Tishon, H. Lewicki, K. P. Campbell, and M. B. Oldstone. 2001. Differences in affinity of binding of lymphocytic choriomeningitis virus strains to the cellular receptor alpha-dystroglycan correlate with viral tropism and disease kinetics. J. Virol. 75448-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiropoulou, C. F., S. Kunz, P. E. Rollin, K. P. Campbell, and M. B. Oldstone. 2002. New World arenavirus clade C, but not clade A and B viruses, utilizes α-dystroglycan as its major receptor. J. Virol. 765140-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng, C. T., J. Tseng, L. Perrone, M. Worthy, V. Popov, and C. J. Peters. 2005. Apical entry and release of severe acute respiratory syndrome-associated coronavirus in polarized Calu-3 lung epithelial cells. J. Virol. 799470-9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, G., G. Williams, H. Xia, M. Hickey, J. Shao, B. L. Davidson, and P. B. McCray. 2002. Apical barriers to airway epithelial cell gene transfer with amphotropic retroviral vectors. Gene Ther. 9922-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White, S. R., K. R. Wojcik, D. Gruenert, S. Sun, and D. R. Dorscheid. 2001. Airway epithelial cell wound repair mediated by alpha-dystroglycan. Am. J. Respir. Cell Mol. Biol. 24179-186. [DOI] [PubMed] [Google Scholar]
- 28.Zabner, J., P. Karp, M. Seiler, S. L. Phillips, C. J. Mitchell, M. Saavedra, M. Welsh, and A. J. Klingelhutz. 2003. Development of cystic fibrosis and noncystic fibrosis airway cell lines. Am. J. Physiol. Lung Cell Mol. Physiol. 284L844-L854. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, L., M. E. Peeples, R. C. Boucher, P. L. Collins, and R. J. Pickles. 2002. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 765654-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]