Abstract
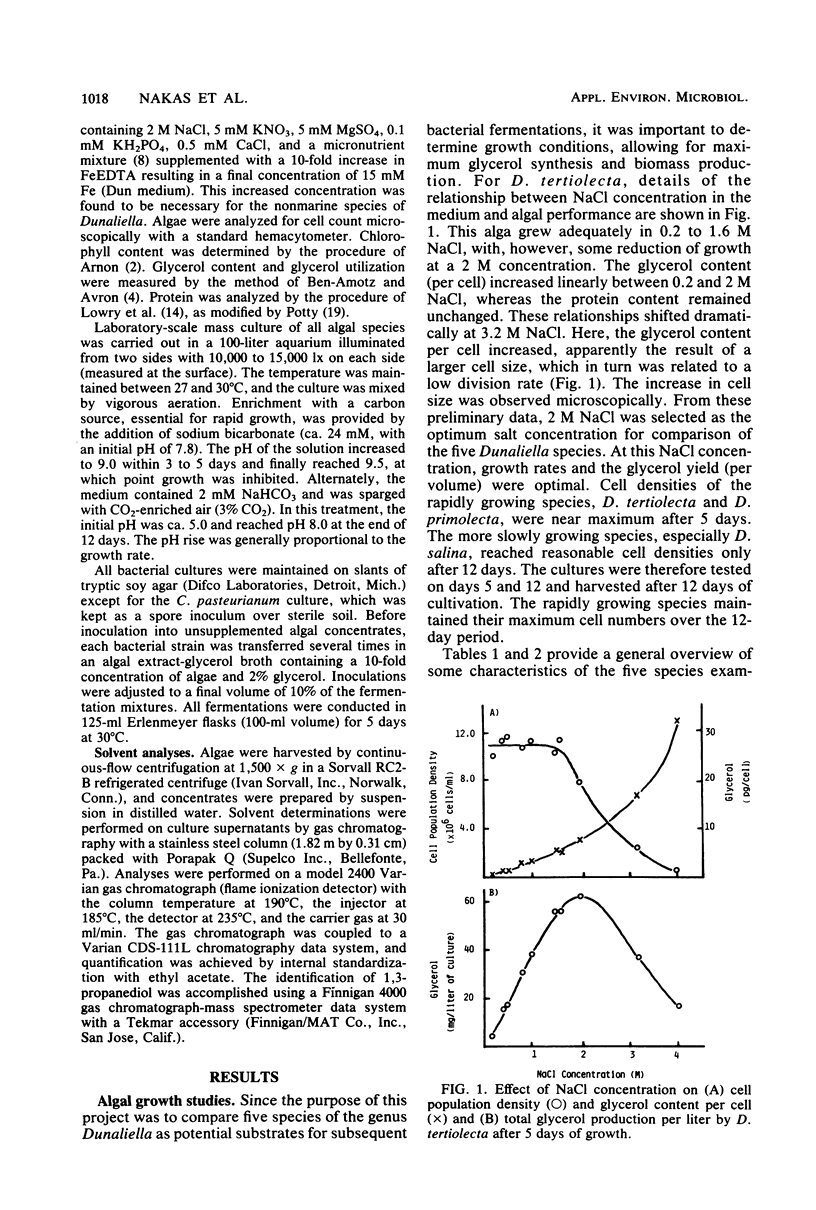
Five species of the genus Dunaliella (D. tertiolecta, D. primolecta, D. parva, D. bardawil, and D. salina) were examined for glycerol accumulation, growth rate, cell density, and protein and chlorophyll content. The suitability of each algal species for use as a fermentation substrate was judged according to glycerol accumulation and quantities of neutral solvents produced after sequential bacterial fermentations. When grown in 2 M NaCl, with 24 mM NaHCO3 or 3% CO2 at 28°C and with 10,000 to 15,000 lx of incident light on two sides of a glass aquarium, four of the five species tested produced ca. 10 to 20 mg of glycerol per liter of culture. Clostridium pasteurianum was found to convert an algal biomass mixture supplemented with 4% glycerol to ca. 16 g of mixed solvents (n-butanol, 1,3-propanediol, and ethanol) per liter. Acetone was not detected. Additionally, it has been demonstrated that Dunaliella concentrates of up to 300-fold can be directly fermented to an identical pattern of mixed solvents. Overall solvent yields were reduced by >50% when fermentations were performed in the presence of 2% NaCl. These results are discussed in terms of practical application in tropical coastal zones.
Full text
PDF
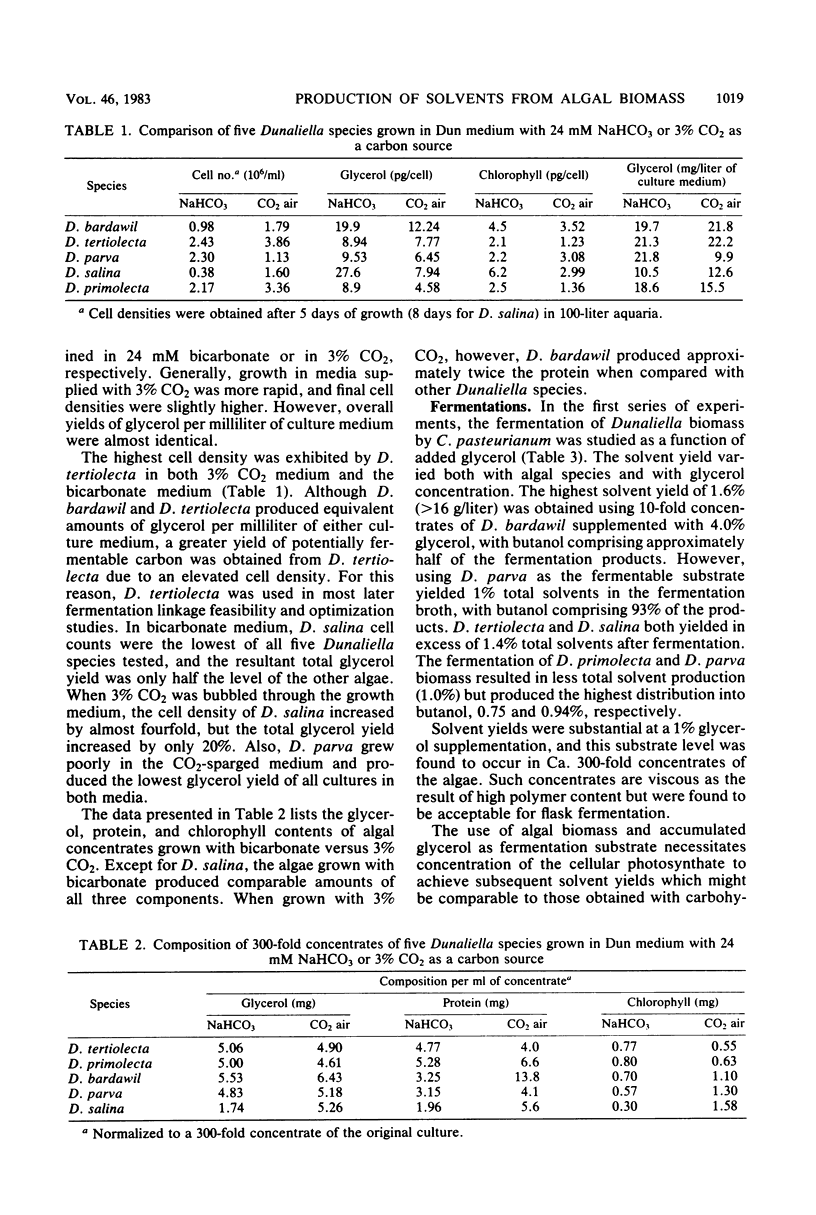



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentrout R. W., Brown R. D. Molecular cloning of genes for cellobiose utilization and their expression in Escherichia coli. Appl Environ Microbiol. 1981 Jun;41(6):1355–1362. doi: 10.1128/aem.41.6.1355-1362.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A., Avron M. The Role of Glycerol in the Osmotic Regulation of the Halophilic Alga Dunaliella parva. Plant Physiol. 1973 May;51(5):875–878. doi: 10.1104/pp.51.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedén C. G. The potential of systems integration in microbial photosynthesis. Biochem Soc Trans. 1979 Feb;7(1):94–98. doi: 10.1042/bst0070094. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Sakajoh M., Halliwell G., Madia A., Demain A. L. Saccharification of Complex Cellulosic Substrates by the Cellulase System from Clostridium thermocellum. Appl Environ Microbiol. 1982 May;43(5):1125–1132. doi: 10.1128/aem.43.5.1125-1132.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Monot F., Martin J. R., Petitdemange H., Gay R. Acetone and Butanol Production by Clostridium acetobutylicum in a Synthetic Medium. Appl Environ Microbiol. 1982 Dec;44(6):1318–1324. doi: 10.1128/aem.44.6.1318-1324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. K., Ben-Bassat A., Zeikus J. G. Ethanol Production by Thermophilic Bacteria: Fermentation of Cellulosic Substrates by Cocultures of Clostridium thermocellum and Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1981 Jun;41(6):1337–1343. doi: 10.1128/aem.41.6.1337-1343.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. K., Busche R. M., McDonald C. C., Hardy R. W. Production of feedstock chemicals. Science. 1983 Feb 11;219(4585):733–740. doi: 10.1126/science.219.4585.733. [DOI] [PubMed] [Google Scholar]
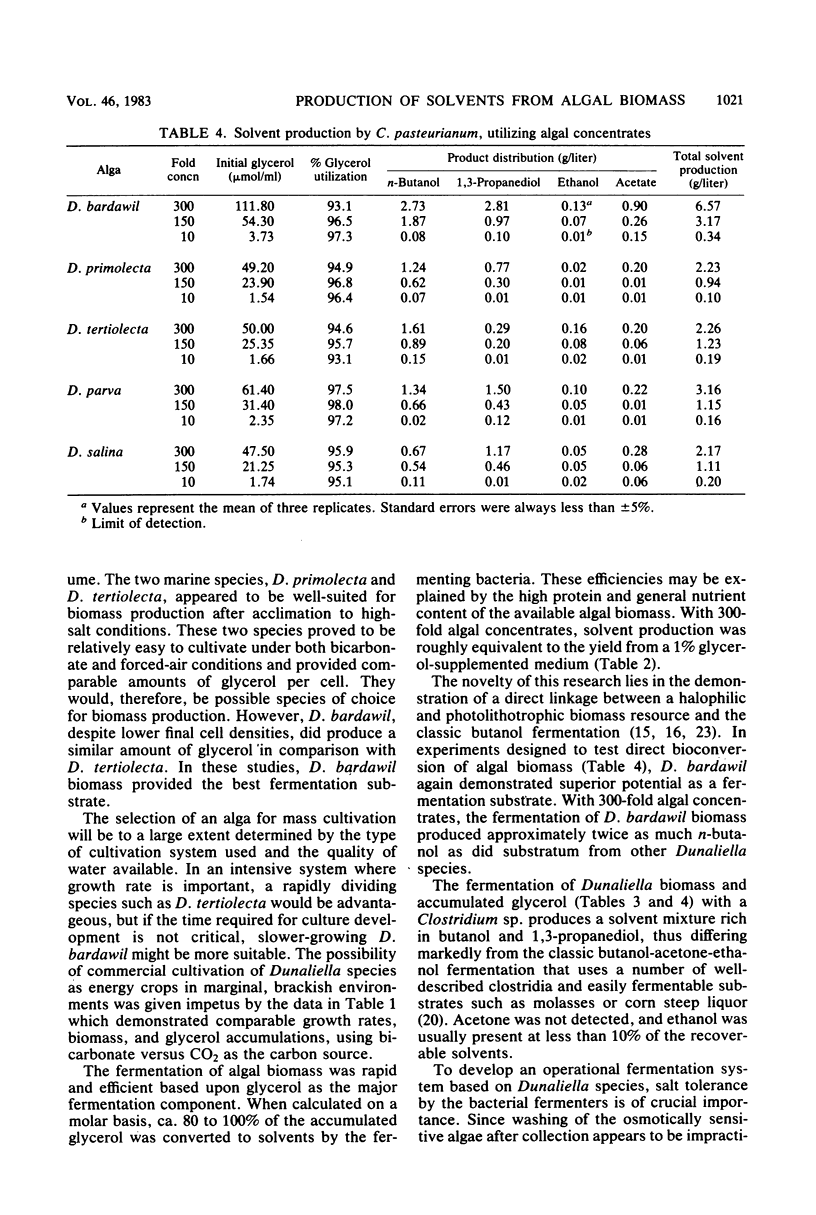
- Potty V. H. Determination of proteins in the presence of phenols and pectins. Anal Biochem. 1969 Jun;29(3):535–539. doi: 10.1016/0003-2697(69)90339-x. [DOI] [PubMed] [Google Scholar]
- Roberts R. E. Reliability of the CES-D Scale in different ethnic contexts. Psychiatry Res. 1980 May;2(2):125–134. doi: 10.1016/0165-1781(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Tang J. C., St Martin E. J., Lin E. C. Derepression of an NAD-linked dehydrogenase that serves an Escherichia coli mutant for growth on glycerol. J Bacteriol. 1982 Dec;152(3):1001–1007. doi: 10.1128/jb.152.3.1001-1007.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Westhuizen A., Jones D. T., Woods D. R. Autolytic Activity and Butanol Tolerance of Clostridium acetobutylicum. Appl Environ Microbiol. 1982 Dec;44(6):1277–1281. doi: 10.1128/aem.44.6.1277-1281.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


