Abstract
Human immunodeficiency virus type 1 Vpr is a virion-associated accessory protein that has multiple activities within an infected cell. One of the most dramatic effects of Vpr is the induction of cell cycle arrest at the G2/M boundary, followed by apoptosis. This effect has implications for CD4+ cell loss in AIDS. In normal cell cycle regulation, Wee1, a key regulator for G2-M progression, phosphorylates Tyr15 on Cdc2 and thereby blocks the progression of cells into M phase. We demonstrate that Vpr physically interacts with Wee1 at the N lobe of the kinase domain analogous to that present in other kinases. This interaction with Vpr enhances Wee1 kinase activity for Cdc2. Overexpression of Wee1 kinase-deficient mutants competes for Vpr-mediated cell cycle arrest, and deletion of the region of Wee1 that binds Vpr abrogates that competition. However, the Vpr mutants I74P and I81P, which fail to induce G2 arrest, can bind to and increase the kinase activity of Wee1 to the same extent as wild-type Vpr. Therefore, we conclude that the binding of Vpr to Wee1 is not sufficient for Vpr to activate the G2 checkpoint, and it may reflect an independent function of Vpr.
Human immunodeficiency virus type 1 (HIV-1) is a member of the lentivirus family. In addition to the gag, pol, and env genes, which are found in all simple retroviruses, the lentiviruses also contain a number of accessory genes. Some of these genes are required for HIV-1 replication, whereas others have been implicated in pathogenesis. One such gene, vpr, encodes a 14-kDa, 96-amino-acid (aa) nuclear protein that is highly conserved between HIV-1, HIV-2, and simian immunodeficiency virus (SIV) isolates (15, 57). In addition to vpr, HIV-2 and SIV also contain vpx, which is closely related to vpr and probably arose through a gene duplication event (46). The deletion of both vpr and vpx from SIV resulted in an acute infection, but no disease was observed in rhesus monkeys, indicating that both genes are required for pathogenesis (11). HIV-1 carries vpr alone, which is thought to encompass the functions of both vpr and vpx genes found within HIV-2 and SIV.
A number of functions have been defined in vitro for HIV-1 Vpr that are necessary for viral replication and may be important for pathogenesis (reviewed in reference 25). Vpr was shown to possess a weak transcriptional transactivation activity (6, 37) and was required for productive infection of nondividing cells such as macrophages (3, 8, 9, 12, 52). Analysis of the mature virions has shown that, through an interaction with Gag p6, Vpr is incorporated into virions (15, 26, 29). Vpr has a nuclear subcellular localization (19, 27, 28). After infection, virion-associated Vpr may contribute, in conjunction with the matrix protein (5), integrase (10), and CPPT (60), to localize the preintegration complex to the nucleus (14, 40).
A particularly noteworthy effect of Vpr is the arrest of the division of infected T cells at the G2-to-M phase transition of the cell cycle. The cells subsequently undergo death via apoptosis (1, 44, 45, 51, 55). We hypothesize that this phenotypic effect of the vpr gene could have a profound effect on the T-cell immune function and contribute to the progression of HIV-1 disease. We propose that one role of the arrest induced by Vpr may be to prevent the efficient activation and subsequent clonal expansion of CD4+ T cells that would be engaged in an immune response. Rapid death of infected cells would also limit the recognition of virus-infected cells by immune cells. The death of the cells will contribute to the characteristic CD4+ cell decline in AIDS. In support of this model, Vpr transgenic mice showed effects on T-cell depletion and suppression of cellular immune function (56).
The normal cell cycle transition of cells from G2 to M is regulated by the Cdc2-cyclin B complex. The two primary regulators of Cdc2 activity are Wee1 families and Cdc25 families. Wee1 phosphorylates Tyr15 of Cdc2 to inhibit its activity and prevent the transition from G2 to M, whereas, Cdc25 dephosphorylates Cdc2 to activate Cdc2 kinase activity and promote the G2/M transition. Wee1 is necessary for Vpr-induced G2 arrest, as shown in genetic studies of Schizosaccharomyces pombe (30). Our preliminary results with synchronized HeLa cells indicate that Wee1 is initially stabilized during Vpr-mediated G2 cell cycle arrest, a finding consistent with the inactive state of Cdc2 during G2 cell cycle arrest (13, 18, 41). In contrast, Wee1 levels are diminished after prolonged cell cycle arrest, Cdc2 is activated, and apoptosis occurs. Overexpression of Wee1 inhibits Vpr-mediated apoptosis (59). Therefore, we hypothesize that modulation of Wee1 levels and/or activity may be a critical means by which Vpr induces cell cycle arrest and subsequent apoptosis.
Wee1 is a nuclear protein that is subject to multiple levels of regulation, including reversible phosphorylation, proteolysis, and protein-protein interactions (2, 24, 31, 47, 50). Wee1 kinase activity appears to be controlled by three mechanisms. First, as demonstrated in S. pombe, Wee1 kinase activity is downregulated during the M phase as a result of phosphorylation in its catalytic domain by nim1/cdr1 (7, 39, 54). In addition, phosphorylation at its N-terminal noncatalytic domain is also responsible for its downregulation at the M phase (22, 38). Second, Wee1 levels decrease in M phase as a result of both decreased synthesis and increased degradation (50), the latter of which is mediated by SCFβ-TrCP (48, 49). Finally, Wee1 can be activated and stabilized through association with 14-3-3 proteins (24, 42), although another group reports that the binding of 14-3-3 inactivates Wee1A (21).
We previously demonstrated that Vpr-induced cell cycle arrest results in increased levels of Wee1 kinase in synchronized HeLa cells (58). We observed that Vpr-induced G2 arrest correlated with delayed degradation of Wee1 and that Cdc2 activity is reduced at G2/M in the presence of Vpr. Here, we examine the effects of Vpr upon the kinase activity of Wee1.
MATERIALS AND METHODS
Antibodies and reagents.
Anti-Flag antibody (M2; F1804), anti-Flag antibody-conjugated agarose beads (A1205), anti-β-tubulin antibody (T5201), normal mouse immunoglobulin G (IgG; I5381), normal rabbit IgG (I5006), doxycycline (Dox; D9891), a cocktail of protease inhibitors (P8465), aprotinin (A1153), proteasome inhibitor (C2211), Flag peptide (F3290), nocodazole (M1404), and human transthyretin (P1742) were purchased from Sigma-Aldrich (St. Louis, MO). Anti-hemagglutinin (HA) antibody (HA.11, MMS-101P) and anti-HA antibody-conjugated Sepharose beads (AFC-101P) were obtained from Covance (Princeton, NJ). Anti-actin (SC-1616), anti-Cdc2 (SC-747), anti-Wee1 (SC-325 [polyclonal antibody] and SC-5285 [monoclonal antibody]) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody specific for phosphorylated Tyr15 on Cdc2 was purchased from Cell Signaling Technology (Danvers, MA). [γ-32P]ATP (PB10218), glutathione-Sepharose 4FF, and protein A and protein G-Sepharose 4FF, glutathione S-transferase (GST)-PreScission protease were obtained from GE Healthcare (Piscataway, NJ).
Construction of plasmids.
Flag-tagged wild-type Vpr and Vpr stop mutant were PCR amplified as described previously (23, 32) and cloned into the BS vector (18). Vpr S94/96A mutant was constructed by PCR based mutagenesis according to the protocol of Zhou and Ratner (61). Vpr I74A, Vpr I74P, and Vpr I81P mutants were constructed by PCR-based mutagenesis as described previously (34).
Full-length and deletion mutants of Wee1 (human somatic type Wee1, Wee1A) were PCR amplified by using HA-tagged Wee1 (59) as a template with the following primer sets and cloned into the pVAX1 vector (Invitrogen, Carlsbad, CA): 1-646, CGATATCGCCATGACCAGCTACCCATACGATGTTCCAGATTACGCCAGCTTCCTGAGCCGACAGC (HA Wee1 sense) and GACTAGTTCACAGATCCTCTTCAGAGATGAGTTTCTGTTCGTATATAGTAAGGCTGAC; 1-422, HA Wee1 sense and GACTAGTTCACAGATCCTCTTCAGAGATGAGTTTCTGTTCCAAAGACATTGAATGAAT; 1-392, HA Wee1 sense and GACTAGTTCACAGATCCTCTTCAGAGATGAGTTTCTGTTCGTAGTTTTCACTTATAGC; 1-369; HA Wee1 sense and CACTAGTTCACAGATCCTCTTCAGAGATGAGTTTCTGTTCATCTTCTGCCCACGCAGAG; and 1-322, HA Wee1 sense and CACTAGTTCACAGATCCTCTTCAGAGATGAGTTTCTGTTCGCATCCATCCAGCCTCTTC.
For GST fusion constructs of Wee1, full-length and deletion mutants of Wee1 containing a GST tag at the N terminus and an HA tag at the C terminus were PCR amplified by using HA-tagged Wee1 (59) as a template with the following primer sets and cloned into pGEX-6P vector (GE Healthcare): 1-646, GTGCGGATCCATGAGCTTCCTGAGCCGACA and GCTATGCGGCCGCTAAGCATAGTGTGGGAC (HA reverse); 291-646, GCGCCGGATCCATGAAGTCCCGGTATACAAC (291 sense) and HA reverse; 291-422, 291 sense and CGCGACTAGTCTAAGCGTAGTCTGGGACGTCGTATGGGTAAGCCAAAGACATTGAATGAATATAC; 291-392, 291 sense and CGCGACTAGTCTAAGCGTAGTCTGGGACGTCGTATGGGTAAGCGTAGTTTTCACTTATAGC; 291-380, 291 sense and CGCGACTAGTCTAAGCGTAGTCTGGGACGTCGTATGGGTAAGCATTACAATATTCATTCTG; 291-369, 291 sense and AGCTAGCTAAGCGTAGTCTGGGACGTCGTATGGGTAAGCATCTTCTGCCCACGCAGA (369 reverse); 291-323, 291 sense and GCGCGACTAGTCTAAGCGTAGTCTGGGACGTCGTATGGGTAAGCGCATCCATCCAGCCTC; and 324-369, CCGAGATCTATTTATGCCATTAAGCG and 369 reverse.
GST-tagged Wee1 Δ291-369 was constructed by PCR-based mutagenesis with GST-tagged 1-422 as a template and the following primer set: CAATGATCATATGCTTATACAGAATGAATATTG and CTTTCAGTAATTGTAATTCTCTTAGCAGG.
Lentiviral vector pLV-TH encoding HA-tagged Wee1 was constructed by subcloning of HA-tagged Wee1 from HR′Wee1 (59). All plasmid constructs were confirmed by DNA sequencing.
Cell culture and viruses.
Maintenance of HeLa and 293T cells, synchronization at G1/S phase by double thymidine block, gamma irradiation, and cell cycle analysis were performed as described previously (58). HR′EGFP and HR′ wild-type Vpr or Vpr mutants viruses were produced in 293T cells by cotransfection and titrated as described previously (58). The Dox-inducible HeLa cell line stably expressing HA-tagged Wee1 was established by coinfection of HeLa cells with lentiviral vectors pLV-TH encoding HA-tagged Wee1 and pLV-tTR-KRAB (53).
Preparation of recombinant proteins.
All GST fusion proteins were expressed in Rosetta-gami2 (Novagen, Madison, WI) and purified with glutathione-Sepharose 4FF as described previously (20). Plasmids encoding Flag-tagged wild and mutant forms of Vpr or Flag-tagged hrGFP (Stratagene, La Jolla, CA) were transfected into 293T cells by calcium phosphate transfection. At 48 h, the cells were lysed in cold CHAPS buffer (0.5 M NaCl, 0.05 M Tris-HCl [pH 8.5], 0.15 M KCl, 5 mM CHAPS [USB, Cleveland, OH]) containing a cocktail of protease inhibitors, aprotinin, and proteasome inhibitor. Lysates were centrifuged at 4°C for 30 min at 100,000 × g, and supernatants were subjected to immunoprecipitation with anti-Flag antibody-conjugated agarose beads. After five washes with CHAPS buffer, Flag-tagged proteins were eluted with Flag peptide diluted in Wee1 kinase buffer (see below). The purity of each protein was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining (PlusOne silver staining kit; GE Healthcare). The levels of contamination of endogenous Wee1 protein in the purified Flag-tagged protein fractions were checked by Western blotting with anti-Wee1 polyclonal antibody.
Protein-protein interaction.
Interaction between Vpr and Wee1 was analyzed by two methods: immunoprecipitation and GST-pull down assay. For immunoprecipitation of endogenous Wee1, HeLa cells were transfected with plasmid encoding either Flag-tagged Vpr or Vpr stop mutant. For immunoprecipitation of exogenously expressed Wee1, HeLa cells were cotransfected with plasmids encoding HA-tagged Wee1 and either Flag-tagged Vpr or Vpr stop mutant by electroporation (33). At 48 h, cell lysates were prepared in CHAPS buffer containing a cocktail of protease inhibitors, aprotinin, and proteasome inhibitor and then centrifuged at 4°C for 30 min at 100,000 × g. Supernatants were subjected to immunoprecipitation with anti-Flag antibody for Vpr or anti-HA antibody for HA-Wee1. The immunoprecipitates were analyzed by Western blotting with anti-Wee1 monoclonal antibody or anti-Flag antibody, respectively.
For analysis of direct binding between Wee1 and Vpr, GST-tagged Vpr (GST-Vpr) and full-length Wee1 containing a GST tag at the N terminus and an HA tag at the C terminus were expressed and purified as described above. The GST tag on Wee1 was released by on-column cleavage with GST-PreScission protease. The purity and concentration of released Wee1 was confirmed by SDS-PAGE and silver staining. Various amounts of Wee1 were incubated with either GST or GST-Vpr preadsorbed on glutathione-Sepharose 4FF beads in CHAPS buffer for 2 h at 4°C. The beads were washed, and the amount of Wee1 bound to GST-Vpr was analyzed by SDS-PAGE and silver staining.
For analysis of the Vpr-binding region in Wee1, full-length and deletion mutants of Wee1 containing a GST tag at the N terminus and an HA tag at the C terminus were expressed and purified as described above. A total of 50 pmol each of GST-tagged Wee1 was preadsorbed on a glutathione-Sepharose 4FF column and incubated in CHAPS buffer for 2 h at 4°C with 0.5 mg of HeLa cell lysate transfected with Flag-tagged Vpr encoding expression vector. The beads were washed, and the amount of Flag-tagged Vpr bound to Wee1 was analyzed by Western blotting with anti-Flag antibody.
In vitro Wee1 kinase assay.
In vitro Wee1 kinase assay was performed using two different sources of Wee1: (i) affinity-purified HA-tagged Wee1 from HeLa cells coexpressing HA-tagged Wee1 and either Flag-tagged Vpr or Vpr stop mutant using anti-HA antibody-conjugated Sepharose beads and (ii) affinity-purified GST-tagged Wee1 (GST-Wee1) from SF9 cells (Novagen) using glutathione-Sepharose 4FF beads. Affinity-purified HA-tagged Wee1 were incubated with 4 U of Cdc2-cyclinB complex (P6020S; NEB, Ipswich, MA) in 60 μl of Wee1 kinase buffer (50 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 10 mM MnC12, 1 mM dithiothreitol, 0.4 mM ATP) for 15 min at 30°C. Reactions were stopped by adding 5× SDS-PAGE sample buffer, followed by heating to 100°C for 5 min, and then analyzed by SDS-PAGE, followed by Western blotting with antibody specific for phosphorylated Tyr15 on Cdc2. In vitro kinase assay with GST-Wee1 and GST-cyclin B/Cdc2 KR complex as a substrate was performed as described previously (50) in the presence or absence of Flag-tagged Vpr, Vpr mutants, or hrGFP.
Competition for G2 arrest induced by Vpr.
The expression plasmids for Wee1 deletion mutants containing an HA tag at the N terminus and a Myc tag at the C terminus were introduced into HeLa cells by nucleofection (Amaxa, Inc., Gaithersburg, MD) using program I-13. We routinely obtained >95% transfection efficiency as monitored by EGFP expression. The cells were then synchronized at G1/S phase by a double thymidine block as described above and infected with HR′EGFP or HR′ wild-type Vpr or gamma irradiated at 4,000 rads. Cell cycle profiles were analyzed at 14 h postinfection.
RESULTS
Vpr enhances the specific kinase activity of Wee1.
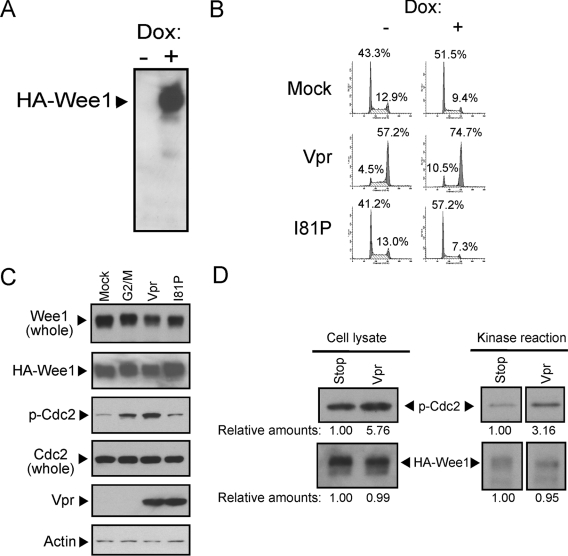
We previously demonstrated that the half-life of Wee1 is enhanced in the presence of Vpr. In the present study we determined whether there were qualitative changes in the kinase activity of Wee1 in the presence of Vpr. In order to control for the various levels of Wee1 in cells, we created an inducible cell line for Wee1 expression (Fig. 1A). Because these cells overexpress an HA-tagged Wee1 in the presence of Dox, the absolute levels of Wee1 are unaffected by the presence of Vpr. These cells are arrested in G2 in the presence of Vpr independent of the induction of Wee1 (Fig. 1B). Despite similar levels of induced and overexpressed Wee1, we observed significant enhancement in phosphorylated Cdc2 in the presence of Vpr but not in the presence of a loss-of-function mutant of Vpr for G2 arrest, the I81P mutant (see Fig. 6C) (34, 35), a finding comparable to levels observed in cells synchronized at the G2/M phase of the cell cycle. The levels of Cdc2 were comparable under all conditions (Fig. 1C).
FIG. 1.
Vpr enhances the specific kinase activity of Wee1. (A) Expression of HA-tagged Wee1 (HA-Wee1) was induced by 10 μg of Dox/ml and analyzed by Western blotting with anti-HA antibody after 72 h of culture. (B) HA-Wee1-expressing HeLa cells were synchronized at the G1/S phase by a double-thymidine block and infected without (mock) or with lentiviral vector encoding either Flag-tagged Vpr (Vpr) or I81P mutant (I81P). Cell cycle profiles with (“+”) or without (“−”) Dox induction were analyzed by flow cytometry at 14 h postinfection. The number in each panel indicates the percentage of cells in each peak. (C) HA-Wee1-expressing HeLa cells were synchronized at the G1/S phase by a double-thymidine block and infected without (mock) or with lentiviral vector encoding Flag-tagged Vpr or Vpr I81P mutant (I81P). Cell lysates were prepared at 8 h after release from the block for cells synchronized in G2/M phase by culture in the presence of 20 μM nocodazole for 4 h before harvesting or at 14 h for mock, Vpr, and I81P mutant. The levels of Wee1, including endogenous and HA-Wee1 (Wee1 whole), HA-Wee1 (HA-Wee1), phosphorylated Cdc2 at Tyr 15 (p-Cdc2), whole Cdc2 (Cdc2 whole), Vpr, and actin (as a loading control), were analyzed by Western blotting. (D) HA-Wee1-expressing HeLa cells were infected with either Flag-tagged wild-type Vpr or Vpr stop mutant encoding lentiviral vector. HA-Wee1 was affinity purified at 24 h postinfection from 0.05 mg of lysate with anti-HA antibody-conjugated Sepharose beads and incubated with 4 U of cyclin B/Cdc2 complex in the presence of 0.4 mM ATP. The levels of phosphate incorporation at Tyr15 of Cdc2 kinase were analyzed by Western blotting with antibody specific for phosphorylated Tyr15 of Cdc2, and the relative amounts were calculated by densitometry.
FIG. 6.
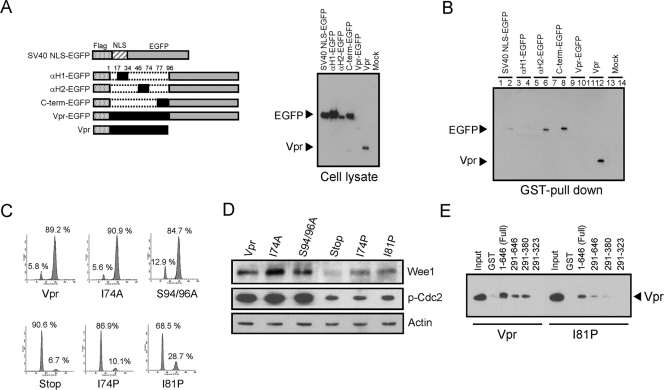
Vpr binds Wee1 through two independent domains, αH2 and C terminus domains. (A) Constructs of Flag-tagged Vpr (Vpr), αH1 (aa 17 to 34), αH2 (aa 46 to 74), C terminus (aa 77 to 96), or the NLS of the large T antigen of SV40 fused to the N terminus of EGFP. Each Vpr domain is represented in black (left panel). Above EGFP fusion proteins (EGFP) and Flag-tagged Vpr (Vpr) were expressed in HeLa cells and analyzed by Western blotting with anti-Flag antibody (right panel). (B) A total of 50 pmol each of GST (lanes 1, 3, 5, 7, 9, 11, and 13) or GST-tagged Wee1 (lanes 2, 4, 6, 8, 10, 12, and 14) preadsorbed on glutathione-Sepharose 4FF beads was incubated with 0.5 mg of HeLa cell lysates either mock transfected (mock) or transfected with expression vectors shown in panel A. The amounts of EGFP fusion proteins (EGFP) and Flag-tagged Vpr (Vpr) bound to Wee1 were analyzed by Western blotting with anti-Flag antibody. (C and D) HeLa cells were synchronized at the G1/S phase by a double-thymidine block and then infected with equivalent amounts of lentiviral vector encoding Flag-tagged wild-type Vpr or Vpr mutants as indicated. Cells were stained with propidium iodide and analyzed for cell cycle by flow cytometry. A total of 10,000 events were collected and analyzed in each sample. The number in each panel indicates the percentage of cells in each peak (C). Whole-cell lysates were separated by SDS-PAGE and probed with antibodies to Wee1, phosphorylated Cdc2 (p-Cdc2), and actin (as a loading control). There were equivalent levels of Vpr expressed from each sample (data not shown) (D). (E) A total of 50 pmol each of GST or GST-tagged full-length and deletion mutants of Wee1 preadsorbed on glutathione-Sepharose 4FF beads was incubated with 0.5 mg of HeLa cell lysate transfected with either Flag-tagged Vpr or Vpr I81P mutant (I81P) encoding expression vector. The amounts of Vpr or I81P mutant bound to Wee1 were analyzed by Western blotting with anti-Flag antibody. Input, 0.5% of cell lysates were loaded as a control for Vpr expression.
Since Wee1 is the primary kinase responsible for negatively regulating the activity of Cdc2, we examined whether the kinase activity of Wee1 in the cells was altered in the presence of Vpr (Fig. 1D). HA-Wee1 was affinity-purified from cells expressing or not expressing Vpr. The purified HA-Wee1 was assayed for kinase activity on purified Cdc2 complexed with cyclin B. Phosphorylation at Tyr15 of Cdc2 kinase was also analyzed by Western blotting with an antibody specific for phosphorylated Tyr15 of Cdc2. Our results demonstrate that in the presence of Vpr the activity of HA-Wee1 to phosphorylate Cdc2 is enhanced by 3.2-fold. The enhanced level of phosphorylated Cdc2 was similar to that seen in the cells expressing Vpr (compare Fig. 1D, upper left and upper right lanes).
Functional interaction between Vpr and Wee1.
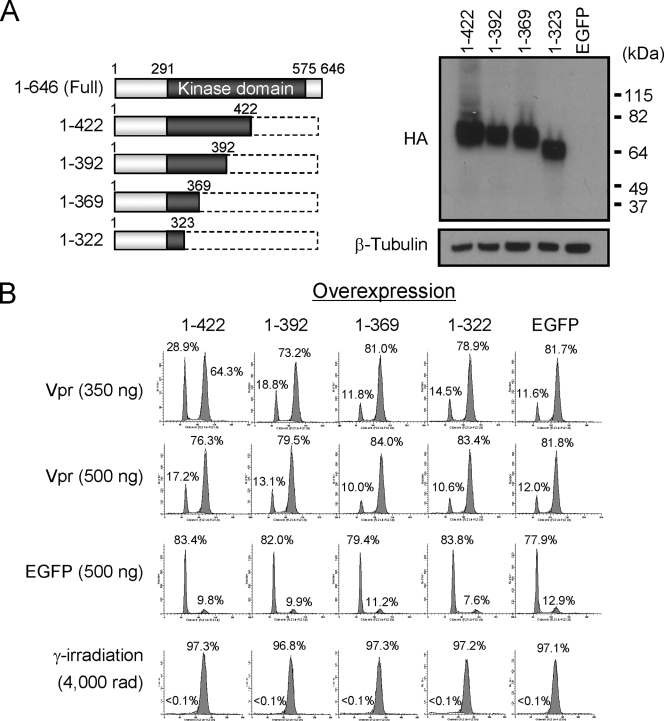
We further analyzed by genetic means whether Vpr interacts in the same pathway leading to G2 arrest as does Wee1. We tested whether mutants of Wee1 lacking the core kinase domain, located between aa 291 and 575 (43) could compete for Vpr-mediated cell cycle arrest (Fig. 2). Two Wee1 mutants, 1-422 and 1-392, alleviated the cell cycle arrest when transfected into cells prior to the introduction of virion-associated Vpr. In the absence of Wee1 mutants, the percentage of cells in G2/M was 82%. This was reduced to 64 and 73%, respectively, for 1-422 and 1-392. Larger truncations of Wee1 did not alleviate cell cycle arrest (1-369 and 1-322). The alleviation of G2 arrest was specific to G2 arrest induced by Vpr; no alleviation was observed when cells were arrested in G2 by gamma irradiation. Furthermore, the alleviation of cell cycle arrest could be partially abrogated by increasing the dosage of Vpr through increasing the multiplicity of infection (MOI) of virion-containing Vpr. At a virion input of 500 ng of virion p24 antigen, the level of G2 arrest in the presence of the competing Wee1 1-422 increased from 64 to 76%. Therefore, these results suggest that Vpr interacts with Wee1 at a common point in the pathway leading to cell cycle arrest, which is consistent with the enhanced kinase activity of Wee1 in the presence of Vpr.
FIG. 2.
Alleviation of Vpr-induced G2 arrest by overexpression of Wee1 deletion mutants. (A) Constructs of Wee1 deletion mutants (left panel). Dark boxes represent the Wee1 kinase domain (43). All mutants contain an HA tag at the N terminus and a Myc tag at the C terminus. Wee1 deletion mutants and EGFP-encoding plasmids were introduced into HeLa cells by nucleofection according to the manufacturer's instructions (program I-13; Amaxa). The expression of Wee1 deletion mutants was analyzed by Western blotting with anti-HA antibody. β-Tubulin was the loading control (right panel). (B) Cells were synchronized at the G1/S phase by a double-thymidine block and then infected with lentiviral vector encoding either Flag-tagged Vpr (Vpr) or EGFP (as a control) at two different MOIs (350 and 500 ng of viral p24 antigen per 105 cells), or gamma irradiated at 4,000 rads. Cells were stained with propidium iodide at 12 h postinfection, and cell cycle profiles were analyzed by flow cytometry. A total of 10,000 events were collected and analyzed per sample. The number in each panel indicates the percentage of cells in each peak.
Vpr directly interacts with Wee1.
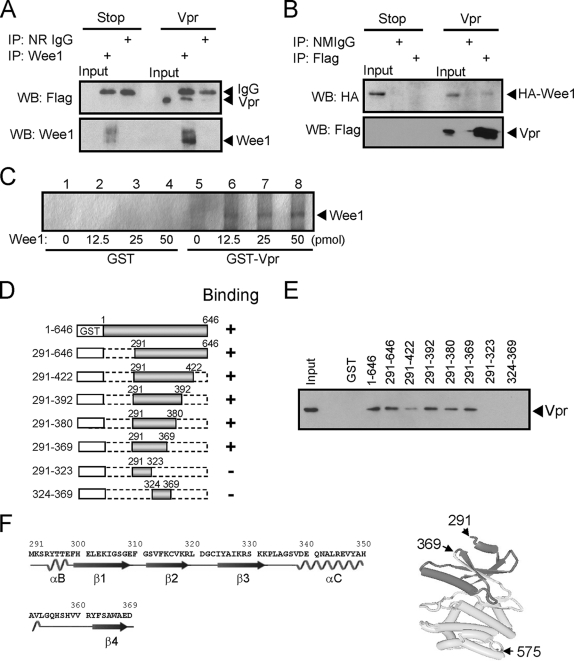
Given the results here demonstrating enhancements in Wee1 kinase activity and alleviation of cell cycle arrest by kinase-defective mutants of Wee1, as well as our previous studies demonstrating an increase in the half-life of Wee1 by Vpr, we tested whether the alterations in Wee1 kinase activity in the presence of Vpr might be the result of direct physical interactions between Wee1 and Vpr. HeLa cells were transfected with Flag-tagged Vpr. Coimmunoprecipitation of Vpr with antibody for endogenous Wee1 was analyzed by Western blotting (Fig. 3A). The results demonstrate that Vpr is coimmunoprecipitated with antibodies to endogenous Wee1. HA-tagged Wee1, which is overexpressed in HeLa cells, is also coimmunoprecipitated with Flag antibodies to Flag-tagged Vpr (Fig. 3B). We further tested the direct interaction between Vpr and Wee1 using GST-tagged Vpr and bacterially expressed and affinity-purified Wee1 visualized by silver staining (Fig. 3C). Thus, Vpr can directly physically interact with Wee1.
FIG. 3.
Vpr directly interacts with Wee1. (A) Flag-tagged Vpr (Vpr) or Vpr stop mutant (Stop) encoding plasmid was transfected into HeLa cells. Cells were lysed in CHAPS buffer and used for immunoprecipitation. The lysates (2 mg) were incubated with either control normal rabbit IgG (NR IgG) or anti-Wee1 rabbit polyclonal antibody. The immunocomplexes were collected with protein A-Sepharose 4FF beads and analyzed by Western blotting with anti-Flag antibody for Vpr. Endogenous Wee1 in immunocomplexes was detected with anti-Wee1 mouse monoclonal antibody. (B) HA-tagged Wee1 (HA-Wee1) and either Flag-tagged Vpr (Vpr) or Vpr stop mutant (Stop) encoding plasmids were cotransfected into HeLa cells. Cells were lysed in CHAPS buffer and Flag-tagged Vpr was immunoprecipitated with either control normal mouse IgG (NMIgG) or anti-Flag antibody. The immunocomplexes were collected with protein G-Sepharose 4FF and analyzed by Western blotting with anti-HA antibody for HA-Wee1. Flag-tagged Vpr in immunocomplexes was detected with anti-Flag antibody. IgG, light chain of antibody used for immunoprecipitation. Input, 1% of each lysate was loaded as controls for the expression of Vpr, Wee1, and HA-Wee1. (C) GST or GST-tagged Vpr was incubated with various amounts of recombinant Wee1 (0, 12.5, 25, or 50 pmol) and pulled down by using glutathione-Sepharose 4FF beads. The interaction was monitored by SDS-PAGE, followed by silver staining. (D, E, and F) Identification of the Vpr-binding domain in Wee1 using GST pulldown assays. (D) Constructs of GST-tagged full-length and deletion mutants of Wee1. (E) A total of 50 pmol each of GST-tagged full-length or deletion mutants of Wee1 preadsorbed on glutathione-Sepharose 4FF beads was incubated with 0.5 mg of HeLa cell lysate transfected with Flag-tagged Vpr (Vpr) encoding expression vector. The amounts of Vpr bound to Wee1 were analyzed by Western blotting with anti-Flag antibody. Input, 0.5% of cell lysate was loaded as a control for Vpr expression. (F) Primary and secondary structures for Wee1 between aa 291 and 369 (left panel) and the three-dimensional structures for Wee1 between aa 291 and 575 (PDB 1x8B, right panel) (43). The Vpr-binding domain located from aa 291 to 369 is indicated by dark gray area on the three-dimensional structure. α, α-helix structure; β, β-sheet structure.
Vpr interacts with Wee1 aa 291 to 369.
The region responsible for the interaction between Wee1 and Vpr was confirmed by expressing full-length Wee1 and deletion mutants of Wee1 in Escherichia coli as GST fusion proteins (Fig. 3D and E). Purified GST fusion proteins were tested for interaction with Flag-tagged Vpr expressed in HeLa cells. Interaction was assayed by pulldown of GST fusion proteins and analysis by Western blotting for coprecipitating Vpr. Successive truncations of Wee1 from the amino and carboxy termini of Wee1 delineated a region of Wee1 from aa 291 to 369 that retained binding activity to Vpr. Further deletion abolished the binding activity to Vpr.
While these studies were in progress, a crystal structure was attained for the catalytic domain of human Wee1A complexed with an active-site inhibitor (PD0407824) (43). The catalytic domain of Wee1 is comprised of two parts, N and C lobes. The N lobe is composed of two α helical domains (αB and αC) and five β strands (β1 to β5). The αC helix is adjacent to a key functional structure found in many kinases, known as the “activation segment.” In several kinases this activation segment forms a loop that regulates kinase activity through the binding of proteins to αC (16). In the case of cyclin-dependent kinase (CDK), the binding of cyclin to CDK through αC helix (also called the PSTAIRE) reorients αC helix and subsequently causes the conformational change of the “activation segment,” resulting in the activation of CDK (17). Interestingly, the Vpr-binding domain of Wee1 contains the αC helix located between aa 338 and 352; thus, it is possible that interaction of Vpr with the domain of Wee1 located between aa 291 and 369 enhances the kinase activity of Wee1, thereby increasing phosphorylation at the inhibitory Tyr15 and contributing to cell cycle arrest.
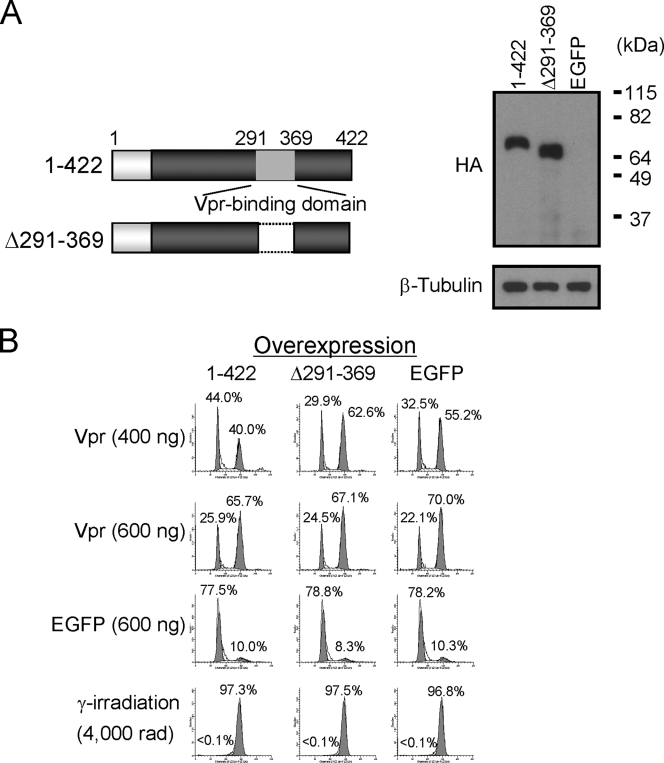
The Vpr-binding domain located between aa 291 and 369 in Wee1 is required for the dominant-negative effect.
In Fig. 2, we showed that two Wee1 deletion mutants, 1-422 and 1-396, partially abrogated G2 arrest induced by Vpr. We then tested whether a mutant of Wee1 lacking the Vpr-binding domain located between aa 291 and 369 competes for Vpr-mediated G2 arrest (Fig. 4). In the absence of Wee1 mutants, the percentage of cells in G2/M was ca. 55%. This was reduced to 40% for 1-422, which is similar to the results shown in Fig. 2. Importantly, the alleviation of G2 arrest could be abrogated by deletion of the Vpr-binding domain (Δ291-369) in 1-422; the percentage of cells in G2/M was increased to 63%. These results provide further support for the model that Wee1 interacts with Vpr through the region located between aa 291 and 369, and this interaction may be involved in G2 arrest induced by Vpr.
FIG. 4.
Vpr-binding domain located between aa 291 and 369 in Wee1 is required for the dominant-negative effect. (A) Constructs of Wee1 deletion mutants (left panel). Dark boxes represent a part of the Wee1-kinase domain located from aa 291 to 575. The light gray box represents the Vpr-binding domain located from aa 291 to 369. All mutants contain an HA tag at the N terminus and a Myc tag at the C terminus. Wee1 deletion mutants and EGFP-encoding plasmids were introduced into HeLa cells by nucleofection according to the manufacturer's instructions. The expression of Wee1 deletion mutants was analyzed by Western blotting with anti-HA specific antibody. β-Tubulin was the loading control (right panel). (B) Cells were synchronized at G1/S by a double-thymidine block and then infected with lentiviral vector encoding either Flag-tagged Vpr (Vpr) or EGFP (as a control) at two different MOIs (400 and 600 ng of viral p24 antigen per 105 cells), or gamma irradiated at 4,000 rads. Cells were then stained with propidium iodide at 12 h postinfection and analyzed by flow cytometry. A total of 10,000 events were collected and analyzed per sample. The number in each panel indicates the percentage of cells in each peak.
Vpr stimulates Wee1 kinase activity.
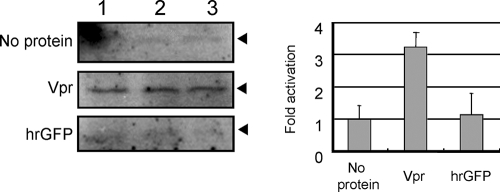
We determined whether Vpr would stimulate Wee1 activity in an in vitro assay using purified proteins (Fig. 5). Full-length Vpr has been notoriously difficult to purify as a recombinant protein in nonmammalian expression systems. Therefore, we chose to express Vpr in the 293T cells and purify the expressed Vpr by affinity purification using a Flag tag specific antibody. GST-tagged Wee1 was incubated with purified complexes of Cdc2KR, a kinase-defective form of Cdc2, and GST-tagged cyclin B (50). Under appropriate reaction conditions, Wee1 will catalyze the incorporation of [γ-32P]ATP into Tyr15 of Cdc2KR. The degree of Tyr15 phosphorylation was analyzed in the presence or absence of Vpr affinity purified from the transfected 293T cells by using anti-Flag antibody-conjugated agarose beads. In the presence of Vpr, we observed an approximately threefold enhancement in 32P incorporation into Cdc2KR relative to a reaction without (no protein) or reaction with an irrelevant protein, Flag-tagged hrGFP (hrGFP), purified in the same manner as Flag-tagged Vpr. The increased levels of 32P incorporation into Cdc2KR by Vpr were not observed in the absence of GST-tagged Wee1 (data not shown), indicating that it was Wee1-dependent phosphorylation. Thus, these results demonstrate that Vpr can stimulate the kinase activity of Wee1 in an in vitro cell-free assay. The major species detected by silver staining after purification is Flag-tagged Vpr (see Fig. 7A), and there is no detectable level of Wee1 derived from 293T cells on Western blotting analysis (see Fig. 7B); however, we cannot completely exclude the presence of other proteins that may contribute to the activities we observed.
FIG. 5.
Vpr stimulates Wee1 kinase activity. Flag-tagged Vpr (Vpr) and Flag-tagged hrGFP (hrGFP) were expressed in 293T cells and affinity purified using anti-Flag antibody-conjugated agarose beads. GST-tagged cyclin B and kinase-inactive form of Cdc2 complex (cyclin B/Cdc2KR) and GST-tagged Wee1 (GST-Wee1) were expressed and purified from SF9 by using glutathione-Sepharose 4FF beads. Cyclin B/Cdc2KR, GST-Wee1, and either Vpr or hrGFP (1 pmol each) or buffer only (No protein) was mixed and incubated for 15 min at 30°C in the presence of [γ-32P]ATP (10 μCi). The levels of 32P incorporation into Cdc2KR were analyzed by SDS-PAGE and autoradiography (arrowheads, left panel). The data shown are the results obtained in three independent reactions (lanes 1 to 3). The fold activation is calculated relative to the value for “no protein” by densitometry (right panel). The bars represent the standard deviations of three independent reactions.
FIG. 7.
A single point mutation in Vpr does not affect on activation for Wee1 kinase activity. (A) Silver staining for affinity-purified Flag-tagged hrGFP (hrGFP), wild-type Vpr (Vpr), Vpr I74P (I74P) and Vpr I81P (I81P) mutants. Various amounts of human transthyretin were used as standards. (B) Affinity-purified Flag-tagged proteins, as well as total cell lysates (5 μg), were analyzed by Western blotting with anti-Wee1 polyclonal and anti-Flag antibodies. (C) Cyclin B/Cdc2KR, GST-Wee1, and either Flag-tagged hrGFP, Vpr, or Vpr mutants (1.3 pmol each) were mixed and incubated for 15 min at 30°C in the presence of [γ-32P]ATP (10 μCi). The levels of 32P incorporation into Cdc2KR were analyzed by SDS-PAGE and autoradiography (arrowhead, left panel). The fold activation was calculated relative to the value for “no protein” by densitometry (right panel).
Domains of Vpr that interact with Wee1.
We expressed three domains of Vpr—α-helical region 1 (αH1), α-helical region 2 (αH2), and the C terminus (C-term) of Vpr—as EGFP fusion proteins (19) and tested them in a GST pulldown assay for interaction with GST-Wee1 (Fig. 6). Since the enhanced green fluorescent protein (EGFP) fusion proteins with each Vpr domain predominantly localize in the nucleus (19), we used simian virus 40 large T nuclear localization signal-conjugated EGFP as a control. Both αH2 and the C-terminal region of Vpr independently reacted with Wee1 (Fig. 6B). αH 1 did not interact with Wee1. The expression of wild-type Vpr-EGFP fusion protein could not be assayed since it was not stable in cells (Fig. 6A, right panel).
Point mutations in each of the above-determined domains have been shown to abrogate Vpr-mediated cell cycle arrest. Figure 6C shows a typical result with mutation I74P in αH2 and mutation I81P in the C-terminal region of Vpr. These mutations strongly impair the G2 arrest activity of Vpr (34, 35). Wild-type Vpr and other mutants (I74A and S94/96A) that induce cell cycle arrest increase the levels of Wee1 and the levels of phosphorylated Cdc2 (Fig. 6D). These mutants show diminished levels of Wee1 and decreased phosphorylation of Cdc2. However, GST pulldown studies utilizing full-length or truncated mutants of Wee1 still demonstrate interaction of these functionally defective Vpr mutants with Wee1 (Fig. 6E). Similar to wild-type Vpr, the I81P mutant interacts with full-length Wee1 and truncation variants of Wee1 that include the kinase and Vpr-binding domain of Wee1, but not further truncations; Wee1 291-380 binds Vpr and I81P mutant, whereas Wee1 291-323 does not. Similar binding was observed when the I74P mutant was used instead of the I81P mutant (data not shown). These mutants of Vpr defective for cell cycle arrest still bind to Wee1. Therefore, solely the interaction between Vpr and Wee1 appears to be insufficient for the cell cycle-arresting properties of Vpr and suggests that additional properties or activities of the complex that includes Vpr and Wee1 is necessary for cell cycle arrest by Vpr.
Since the mutants of Vpr still bind Wee1, we tested whether the mutations had an effect upon the kinase activity of Wee1 in an in vitro kinase assay (Fig. 7). Flag-tagged hrGFP, wild-type Vpr, and I74P and I81P mutants were affinity purified from 293T cells (Fig. 7A and B). Additions of the Vpr protein to the in vitro Wee1 kinase assay demonstrated increased phosphorylation of Cdc2KR by both mutants at levels comparable to those seen with wild-type Vpr. The increased levels of 32P incorporation into Cdc2KR by wild-type Vpr or Vpr mutants were not observed in the absence of GST-tagged Wee1 (data not shown). These results are consistent with the interaction studies described above wherein mutants that abrogate cell cycle arrest are still able to bind Wee1. Therefore, interaction between Vpr and Wee1 correlates with increased kinase activity of Wee1; however, this increased activity is not sufficient for the induction of cell cycle arrest by Vpr, implicating other factors in the Vpr-mediated cell cycle arrest.
DISCUSSION
These results provide biochemical and genetic evidence for the interaction between Vpr and Wee1. Ectopic expression of a C-terminal truncated Wee1 partially alleviates Vpr-mediated cell cycle arrest. Physical interaction between Wee1 and Vpr is evidenced in GST pulldown assays and in coimmunoprecipitation assays. Importantly, we defined the domain of Wee1 with which Vpr interacts as a region that corresponds to the conserved αC helix regulatory domain found in many kinases (16). Finally, we demonstrate that the interaction of Vpr with Wee1 leads to an enhancement of Wee1 kinase activity on its primary physiologic substrate, Cdc2. Despite these interactions between Vpr and Wee1, our results also show that these interactions are not solely responsible for the induction of cell cycle arrest by Vpr. Mutants of Vpr that do not induce cell cycle arrest can still bind to Wee1 and enhance in vitro kinase activity.
Our structural mapping studies of the Vpr-binding domain delineate a key domain, the αC helix domain of Wee1. This domain is found in the β-sheet-rich N lobe of other kinases and is the only conserved helix structure; it is generically termed the αC helix domain. The αC helix domain induces conformational change within the catalytic domain of kinases through the interaction with N-terminal region of the activation loop (see the reviews in references 16 and 36). As a typical example, the regulation of CDK by cyclin has been well studied. CDKs are in a catalytically inactive state in the absence of cyclin. Cyclin binds the αC helix domain of CDKs and induces conformational changes in the αC helix domain and activation loop, resulting in the activation of CDKs (17). The crystal structure of the Wee1 kinase domain suggested that the αC helix domain of Wee1 is present in an active conformation; however, our results indicate that it may still serve a regulatory function. It should be noted that the crystal structure of Wee1 was resolved only for its catalytic domain that lacks N-terminal regulatory domain located between aa 1 and 290 (43). Thus, it is possible that the conformation of the αC helix of Wee1 may be inactive for mature protein, or other cellular proteins may also recognize this domain and participate in the regulation of Wee1 kinase activity. Like other kinases, Wee1 activity may also be modulated via this αC helix domain, and Vpr might act as an activator of Wee1.
We previously reported that the presence of Vpr leads to enhanced levels of Wee1 through protein stability. Here we show that Vpr also enhances the specific kinase activity of Wee1. The dual effect of Vpr of increasing the Wee1 activity through the previously reported protein stabilization and increasing the specific kinase activity are likely to play a role in the mechanism by which Vpr induces cell cycle arrest. Although Wee1 is a key regulator of Cdc2 kinase, the activation of Wee1 activity by Vpr appears to be insufficient for cell cycle arrest by Vpr. This conclusion is evidenced by our observations with loss-of-function mutants of Vpr. Although overexpression of a C-terminal truncated version of Wee1 partially alleviates cell cycle arrest, supporting a common pathway for Vpr and Wee1 in the induction of cell cycle arrest, mutants of Vpr that do not lead to cell cycle arrest and do not affect the phosphorylation levels of Cdc2 kinase in cells are still capable of binding to Wee1 and enhance the phosphorylation of Cdc2 in vitro. Therefore, Vpr-mediated cell cycle arrest is unlikely to solely involve enhancements in Wee1 levels or activity. A number of other factors also play a role in the regulation of Cdc2 kinase, including the related Myt1 kinase related to Wee1 and Cdc25 phosphatases, positive regulators of Cdc2, which in turn are regulated by 14-3-3 proteins. Vpr-mediated cell cycle arrest has also been shown to act through the ataxia-telangiectasia mutated and Rad3-related (ATR) kinase pathway. ATR kinase responses to double-stranded DNA breaks with ataxia-telangiectasia mutated kinase and phosphorylates checkpoint kinase 1 (Chk1), an effector ATR kinase. By this phosphorylation, Chk1 kinase is activated and then phosphorylates Wee1, as well as Cdc25, resulting in the activation of Wee1 kinase activity and the degradation of Cdc25, respectively (4, 24). There are likely to be as-yet-undefined interactions between these factors, Vpr and Wee1, that in combination lead to cell cycle arrest by Vpr. Further work will be required to elucidate the interactions among the multiple pathways potentially acting in concert to ultimately induce cell cycle arrest.
Acknowledgments
We thank Didier Trono for lentiviral vectors, pLV-TH, and pLV-tTR-KRAB. We are grateful to Betty Poon for proofreading of the manuscript.
This study was supported by the NIH grants CA070018-13 (I.S.Y.C) and AI1028697-18 (UCLA CFAR).
Footnotes
Published ahead of print on 2 April 2008.
REFERENCES
- 1.Andersen, J. L., J. L. Dehart, E. S. Zimmerman, O. Ardon, B. Kim, G. Jacquot, S. Benichou, and V. Planelles. 2006. HIV-1 Vpr-induced apoptosis is cell cycle dependent and requires Bax but not ANT. PLoS Pathog. 2e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azad, A. A. 2000. Could Nef and Vpr proteins contribute to disease progression by promoting depletion of bystander cells and prolonged survival of HIV-infected cells? Biochem. Biophys. Res. Commun. 267677-685. [DOI] [PubMed] [Google Scholar]
- 3.Balliet, J. W., D. L. Kolson, G. Eiger, F. M. Kim, K. A. McGann, A. Srinivasan, and R. Collman. 1994. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology 200623-631. [DOI] [PubMed] [Google Scholar]
- 4.Bartek, J., C. Lukas, and J. Lukas. 2004. Checking on DNA damage in S phase. Nat. Rev. Mol. Cell. Biol. 5792-804. [DOI] [PubMed] [Google Scholar]
- 5.Bukrinsky, M. I., S. Haggerty, M. P. Dempsey, N. Sharova, A. Adzhubel, L. Spitz, P. Lewis, D. Goldfarb, M. Emerman, and M. Stevenson. 1993. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature 365666-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, E. A., E. F. Terwilliger, Y. Jalinoos, J. Proulx, J. G. Sodroski, and W. A. Haseltine. 1990. Identification of HIV-1 vpr product and function. J. Acquir. Immune Defic. Syndr. 311-18. [PubMed] [Google Scholar]
- 7.Coleman, T. R., Z. Tang, and W. G. Dunphy. 1993. Negative regulation of the wee1 protein kinase by direct action of the nim1/cdr1 mitotic inducer. Cell 72919-929. [DOI] [PubMed] [Google Scholar]
- 8.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206935-944. [DOI] [PubMed] [Google Scholar]
- 9.Eckstein, D. A., M. P. Sherman, M. L. Penn, P. S. Chin, C. M. De Noronha, W. C. Greene, and M. A. Goldsmith. 2001. HIV-1 Vpr enhances viral burden by facilitating infection of tissue macrophages but not nondividing CD4+ T cells. J. Exp. Med. 1941407-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallay, P., T. Hope, D. Chin, and D. Trono. 1997. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc. Natl. Acad. Sci. USA 949825-9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbs, J. S., A. A. Lackner, S. M. Lang, M. A. Simon, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1995. Progression to AIDS in the absence of a gene for vpr or vpx. J. Virol. 692378-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hattori, N., F. Michaels, K. Fargnoli, L. Marcon, R. C. Gallo, and G. Franchini. 1990. The human immunodeficiency virus type 2 vpr gene is essential for productive infection of human macrophages. Proc. Natl. Acad. Sci. USA 878080-8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 696705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinzinger, N. K., M. I. Bukinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 917311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang, L.-M., and K. T. Jeang. 1995. HIV Vpr: roles in viral replication and cellular metabolism, p. III-3-III-9. In G. Myers, B. Hahn, J. Mellors, and L. Henderson (ed.), Human retroviruses and AIDS. Los Alamos National Laboratory, Los Alamos, NM.
- 16.Huse, M., and J. Kuriyan. 2002. The conformational plasticity of protein kinases. Cell 109275-282. [DOI] [PubMed] [Google Scholar]
- 17.Jeffrey, P. D., A. A. Russo, K. Polyak, E. Gibbs, J. Hurwitz, J. Massague, and N. P. Pavletich. 1995. Mechanism of CDK activation revealed by the structure of a cyclin A-CDK2 complex. Nature 376313-320. [DOI] [PubMed] [Google Scholar]
- 18.Jowett, J. B., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 696304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamata, M., and Y. Aida. 2000. Two putative alpha-helical domains of human immunodeficiency virus type 1 Vpr mediate nuclear localization by at least two mechanisms. J. Virol. 747179-7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamata, M., Y. Nitahara-Kasahara, Y. Miyamoto, Y. Yoneda, and Y. Aida. 2005. Importin-alpha promotes passage through the nuclear pore complex of human immunodeficiency virus type 1 Vpr. J. Virol. 793557-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katayama, K., N. Fujita, and T. Tsuruo. 2005. Akt/protein kinase B-dependent phosphorylation and inactivation of WEE1Hu promote cell cycle progression at G2/M transition. Mol. Cell. Biol. 255725-5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, S. Y., E. J. Song, K. J. Lee, and J. E. Ferrell, Jr. 2005. Multisite M-phase phosphorylation of Xenopus Wee1A. Mol. Cell. Biol. 2510580-10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuramitsu, M., C. Hashizume, N. Yamamoto, A. Azuma, M. Kamata, N. Yamamoto, Y. Tanaka, and Y. Aida. 2005. A novel role for Vpr of human immunodeficiency virus type 1 as a regulator of the splicing of cellular pre-mRNA. Microbes Infect. 71150-1160. [DOI] [PubMed] [Google Scholar]
- 24.Lee, J., A. Kumagai, and W. G. Dunphy. 2001. Positive regulation of Wee1 by Chk1 and 14-3-3 proteins. Mol. Biol. Cell 12551-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Rouzic, E., and S. Benichou. 2005. The Vpr protein from HIV-1: distinct roles along the viral life cycle. Retrovirology 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu, Y. L., R. P. Bennett, J. W. Wills, R. Gorelick, and L. Ratner. 1995. A leucine triplet repeat sequence (LXX)4 in p6gag is important for Vpr incorporation into human immunodeficiency virus type 1 particles. J. Virol. 696873-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu, Y. L., P. Spearman, and L. Ratner. 1993. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J. Virol. 676542-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahalingam, S., R. G. Collman, M. Patel, C. E. Monken, and A. Srinivasan. 1995. Functional analysis of HIV-1 Vpr: identification of determinants essential for subcellular localization. Virology 212331-339. [DOI] [PubMed] [Google Scholar]
- 29.Mahalingam, S., B. MacDonald, K. E. Ugen, V. Ayyavoo, M. G. Agadjanyan, W. V. Williams, and D. B. Weiner. 1997. In vitro and in vivo tumor growth suppression by HIV-1 Vpr. DNA Cell Biol. 16137-143. [DOI] [PubMed] [Google Scholar]
- 30.Masuda, M., Y. Nagai, N. Oshima, K. Tanaka, H. Murakami, H. Igarashi, and H. Okayama. 2000. Genetic studies with the fission yeast Schizosaccharomyces pombe suggest involvement of wee1, ppa2, and rad24 in induction of cell cycle arrest by human immunodeficiency virus type 1 Vpr. J. Virol. 742636-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGowan, C. H., and P. Russell. 1995. Cell cycle regulation of human WEE1. EMBO J. 142166-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishino, Y., T. Myojin, M. Kamata, and Y. Aida. 1997. Human immunodeficiency virus type 1 Vpr gene product prevents cell proliferation on mouse NIH 3T3 cells without the G2 arrest of the cell cycle. Biochem. Biophys. Res. Commun. 232550-554. [DOI] [PubMed] [Google Scholar]
- 33.Nishizawa, M., M. Kamata, R. Katsumata, and Y. Aida. 2000. A carboxy-terminally truncated form of the human immunodeficiency virus type 1 Vpr protein induces apoptosis via G1 cell cycle arrest. J. Virol. 746058-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishizawa, M., M. Kamata, T. Mojin, Y. Nakai, and Y. Aida. 2000. Induction of apoptosis by the Vpr protein of human immunodeficiency virus type 1 occurs independently of G2 arrest of the cell cycle. Virology 27616-26. [DOI] [PubMed] [Google Scholar]
- 35.Nishizawa, M., T. Myojin, Y. Nishino, Y. Nakai, M. Kamata, and Y. Aida. 1999. A carboxy-terminally truncated form of the Vpr protein of human immunodeficiency virus type 1 retards cell proliferation independently of G2 arrest of the cell cycle. Virology 263313-322. [DOI] [PubMed] [Google Scholar]
- 36.Nolen, B., S. Taylor, and G. Ghosh. 2004. Regulation of protein kinases; controlling activity through activation segment conformation. Mol. Cell 15661-675. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa, K., R. Shibata, T. Kiyomasu, I. Higuchi, Y. Kishida, A. Ishimoto, and A. Adachi. 1989. Mutational analysis of the human immunodeficiency virus vpr open reading frame. J. Virol. 634110-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamoto, K., and N. Sagata. 2007. Mechanism for inactivation of the mitotic inhibitory kinase Wee1 at M phase. Proc. Natl. Acad. Sci. USA 1043753-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker, L. L., S. A. Walter, P. G. Young, and H. Piwnica-Worms. 1993. Phosphorylation and inactivation of the mitotic inhibitor Wee1 by the nim1/cdr1 kinase. Nature 363736-738. [DOI] [PubMed] [Google Scholar]
- 40.Popov, S., M. Rexach, G. Zybarth, N. Reiling, M. A. Lee, L. Ratner, C. M. Lane, M. S. Moore, G. Blobel, and M. Bukrinsky. 1998. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 17909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Re, F., D. Braaten, E. K. Franke, and J. Luban. 1995. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 696859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothblum-Oviatt, C. J., C. E. Ryan, and H. Piwnica-Worms. 2001. 14-3-3 binding regulates catalytic activity of human Wee1 kinase. Cell Growth Differ. 12581-589. [PubMed] [Google Scholar]
- 43.Squire, C. J., J. M. Dickson, I. Ivanovic, and E. N. Baker. 2005. Structure and inhibition of the human cell cycle checkpoint kinase, Wee1A kinase: an atypical tyrosine kinase with a key role in CDK1 regulation. Structure 13541-550. [DOI] [PubMed] [Google Scholar]
- 44.Stewart, S. A., B. Poon, J. B. Jowett, and I. S. Chen. 1997. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J. Virol. 715579-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart, S. A., B. Poon, J. Y. Song, and I. S. Chen. 2000. Human immunodeficiency virus type 1 vpr induces apoptosis through caspase activation. J. Virol. 743105-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tristem, M., C. Marshall, A. Karpas, and F. Hill. 1992. Evolution of the primate lentiviruses: evidence from vpx and vpr. EMBO J. 113405-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, Y., C. Jacobs, K. E. Hook, H. Duan, R. N. Booher, and Y. Sun. 2000. Binding of 14-3-3beta to the carboxyl terminus of Wee1 increases Wee1 stability, kinase activity, and G2-M cell population. Cell Growth Differ. 11211-219. [PubMed] [Google Scholar]
- 48.Watanabe, N., H. Arai, J. Iwasaki, M. Shiina, K. Ogata, T. Hunter, and H. Osada. 2005. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways. Proc. Natl. Acad. Sci. USA 10211663-11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe, N., H. Arai, Y. Nishihara, M. Taniguchi, N. Watanabe, T. Hunter, and H. Osada. 2004. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFβ-TrCP. Proc. Natl. Acad. Sci. USA 1014419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watanabe, N., M. Broome, and T. Hunter. 1995. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 141878-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watanabe, N., T. Yamaguchi, Y. Akimoto, J. B. Rattner, H. Hirano, and H. Nakauchi. 2000. Induction of M-phase arrest and apoptosis after HIV-1 Vpr expression through uncoupling of nuclear and centrosomal cycle in HeLa cells. Exp. Cell Res. 258261-269. [DOI] [PubMed] [Google Scholar]
- 52.Westervelt, P., T. Henkel, D. B. Trowbridge, J. Orenstein, J. Heuser, H. E. Gendelman, and L. Ratner. 1992. Dual regulation of silent and productive infection in monocytes by distinct human immunodeficiency virus type 1 determinants. J. Virol. 663925-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiznerowicz, M., and D. Trono. 2003. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J. Virol. 778957-8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu, L., and P. Russell. 1993. Nim1 kinase promotes mitosis by inactivating Wee1 tyrosine kinase. Nature 363738-741. [DOI] [PubMed] [Google Scholar]
- 55.Yao, X. J., N. Rougeau, G. Duisit, J. Lemay, and E. A. Cohen. 2004. Analysis of HIV-1 Vpr determinants responsible for cell growth arrest in Saccharomyces cerevisiae. Retrovirology 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yasuda, J., T. Miyao, M. Kamata, Y. Aida, and Y. Iwakura. 2001. T-cell apoptosis causes peripheral T-cell depletion in mice transgenic for the HIV-1 vpr gene. Virology 285181-192. [DOI] [PubMed] [Google Scholar]
- 57.Yu, X. F., M. Matsuda, M. Essex, and T. H. Lee. 1990. Open reading frame vpr of simian immunodeficiency virus encodes a virion-associated protein. J. Virol. 645688-5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan, H., M. Kamata, Y. M. Xie, and I. S. Chen. 2004. Increased levels of Wee-1 kinase in G2 are necessary for Vpr- and gamma irradiation-induced G2 arrest. J. Virol. 788183-8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan, H., Y. M. Xie, and I. S. Chen. 2003. Depletion of Wee-1 kinase is necessary for both human immunodeficiency virus type 1 Vpr- and gamma irradiation-induced apoptosis. J. Virol. 772063-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zennou, V., C. Petit, D. Guetard, U. Nerhbass, L. Montagnier, and P. Charneau. 2000. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 101173-185. [DOI] [PubMed] [Google Scholar]
- 61.Zhou, Y., and L. Ratner. 2000. Phosphorylation of human immunodeficiency virus type 1 Vpr regulates cell cycle arrest. J. Virol. 746520-6527. [DOI] [PMC free article] [PubMed] [Google Scholar]