Abstract
A challenge in hepatitis C virus (HCV) vaccine development is defining conserved protective epitopes. A cluster of these epitopes comprises an immunodominant domain on the E2 glycoprotein, designated domain B. CBH-2 is a neutralizing human monoclonal antibody to a domain B epitope that is highly conserved. Alanine scanning demonstrated that the epitope involves residues G523, G530, and D535 that are also contact residues for E2 binding to CD81, a coreceptor required for virus entry into cells. However, another residue, located at position 431 and thus at a considerable distance in the linear sequence of E2, also contributes to the CBH-2 epitope. A single amino acid substitution at this residue results in escape from CBH-2-mediated neutralization in a genotype 1a virus. These results highlight the challenges inherent in developing HCV vaccines and show that an effective vaccine must induce antibodies to both conserved and more invariant epitopes to minimize virus escape.
The ability of passively administered neutralizing polyclonal antibody preparations to delay and suppress hepatitis C virus (HCV) infection in experimental animals (11), coupled with subsequent correlations with antibody titers inhibiting HCV E2 glycoprotein binding to CD81 (16) and in vitro infection with retrovirus-based pseudotyped particles (HCVpp) bearing genotype 1a HCV E1E2 glycoproteins (1), provides a strong impetus to develop a vaccine that is able to elicit these antibodies. A significant challenge is defining protective epitopes that are conserved broadly among different HCV genotypes and subtypes. Antibodies to these epitopes generally recognize conformational determinants on the HCV E2 envelope glycoprotein. Many bind to epitopes involving amino acid residues W420, Y527, W529, G530, and/or D535 that are also contact residues for E2 binding to CD81, an essential coreceptor for HCV entry (5, 7, 8, 12, 15). This cluster of overlapping neutralizing epitopes, which we refer to here as “domain B,” is conserved across most HCV genotypes and thus is an attractive target for vaccine design. However, a concern for vaccine development is whether a strong antibody response to this region could select for virus escape variants. The high error rate inherent in the replication of HCV RNA leads to high levels of genetic diversity, particularly within the E2-coding segment of the genome. Selection of escape variants during chronic infection has been associated with progressive mutations within a hypervariable region at the N terminus of E2 (17). Thus, it remains possible that a similar escape mechanism could occur with antibodies directed against domain B epitopes. Here, we describe the fine mapping of a highly conserved epitope within domain B that is recognized by a human monoclonal antibody (HMAb), CBH-2, and demonstrate how a single amino acid substitution within it can lead to a high level of neutralization escape without apparent loss of replication fitness.
CBH-2 is an HMAb derived from B cells of a donor who was chronically infected with genotype 1b HCV (5). The antibody is directed against a conformational epitope, as suggested by its ability to immunoprecipitate E2, but it is unable to detect denatured E2 by Western blot analysis. Initially, we found that CBH-2 neutralizes the infectivity of retrovirus-based pseudotyped particles (HCVpp) bearing the genotype 1a HCV E1E2 glycoproteins from an H strain variant (Hvar) that is closely related to the prototypical genotype 1a H77c strain (accession number AAB67037) (2, 6). In a subsequent study, we found that this antibody also neutralized HCVpp bearing envelope proteins from multiple other genotypes (1b, 2a, 2b, 4, 5, and 6) but failed to neutralize HCVpp bearing E1E2 from a genotype 3a virus or, surprisingly, E1E2 from the 1a H77c strain itself (13).
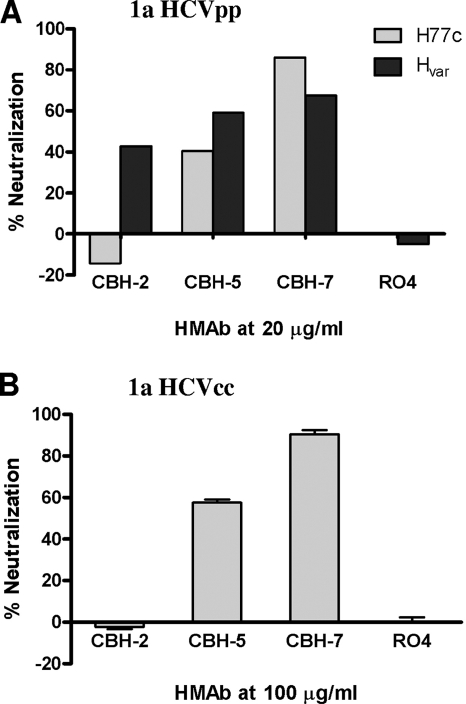
To follow up on these observations, we carried out comparative neutralization studies of HCVpp bearing the Hvar and H77c E1E2 proteins, and a cell culture infectious chimeric HCV containing the H77c structural proteins (with a Y361H substitution in E1) placed in the background of the replication-competent JFH1 strain: H-NS2/NS3-J/Y361H/Q1251L (HJ3-5) virus (18) (referred to here simply as HCVcc). We studied neutralization mediated by CBH-2, as well as by two other neutralizing anti-HCV HMAbs, CBH-5 and CBH-7, which are representatives of antibodies recognizing two distinct immunogenic domains in E2: domain B and domain C (10). R04, an isotype-matched HMAb directed against an unrelated antigen, was used as a negative control. Neutralization of HCVpp and the chimeric HCVcc was done as described previously (8, 18). As shown in Fig. 1A, CBH-2, CBH-5, and CBH-7 (at 20 μg/ml) each neutralized the Hvar HCVpp (40, 60, and 70% neutralization, respectively). However, CBH-2 failed to neutralize H77c HCVpp, whereas CBH-5 and CBH-7 were capable of doing so (0, 40, and 90%, respectively). Similar results were obtained with the infectious chimeric HCVcc, in which E1E2 are derived from H77c (18). It was resistant to neutralization by CBH-2 but effectively neutralized by CBH-5 and CBH-7 (60 and 85% reduction, respectively, in infected cell foci) (Fig. 1B). Importantly, the finding that the H77c envelope was resistant to CBH-2 neutralization but not neutralization by CBH-5 and CBH-7 suggests that the relevant differences in the E2 proteins of Hvar and H77c are not global but rather localized specifically to the CBH-2 epitope.
FIG. 1.
Neutralization of HCVpp and HCVcc by CBH-2. (A) HCVpp containing either H77c or Hvar envelope proteins were preincubated with 20 μg of HMAb CBH-2, CBH-5, or CBH-7/ml before infection of Huh7 cells. The negative control R04 is an isotype-matched HMAb to cytomegalovirus. The results are expressed as the percentage of neutralization as measured by luciferase activity. (B) Neutralization of 1a HCVcc by HMAbs CBH-2, CBH-5, and CBH-7. Infectious genotype 1a HJ3-5 chimeric virus inoculum was incubated with each antibody at different concentrations, as indicated for 1 h at 37°C prior to inoculation onto Huh-7.5 cells preseeded into eight-well tissue culture chamber slides. Cells were fixed and stained by an indirect immunofluorescence procedure using MAb to core antigen at day 4 postinoculation, followed by enumeration of the foci of infected cells. Neutralization is reported as the percent reduction in focus-forming units of virus.
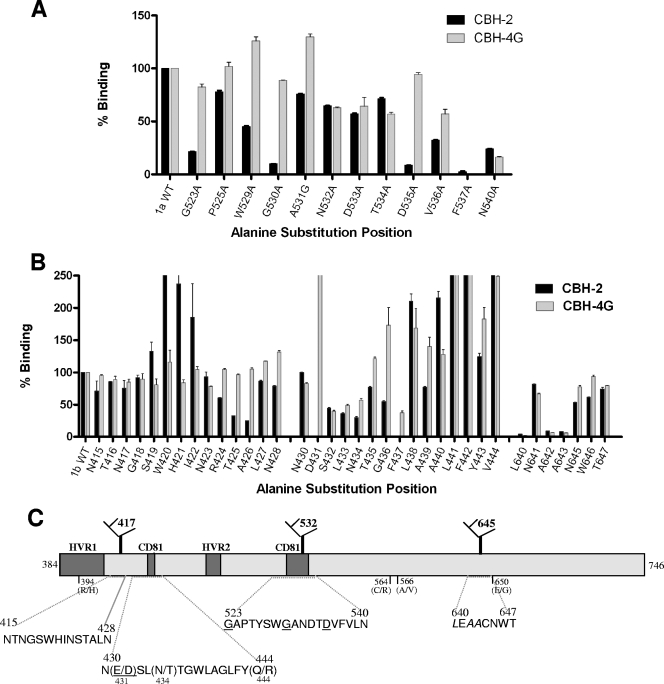
We next created a series of substitution mutants in order to map the residues contributing to the CBH-2 epitope. Since this antibody inhibits E2 binding to CD81 (5), our initial efforts focused on two regions containing contact residues that are known to be engaged in this interaction (3, 14) and on three E2 glycosylation sites (N417, N532, and N645) that have been shown to modulate the response to neutralizing antibodies (6). These regions of E2 are depicted in Fig. 2C. We created a series of single Ala substitution mutants at positions G523A, P525A, W529A, G530A, A531A, N532A, D533A, T534A, D535A, V536A, F537A, and N540A within a plasmid encoding the Hvar E1E2 proteins, by site-directed mutagenesis as described by the manufacturer (Stratagene). We then assessed CBH-2 binding to these proteins by enzyme-linked immunosorbent assay (ELISA), using as antigen lysates of transiently transfected HEK-293T cells (9) (Fig. 2A). E1E2 proteins present in the lysates were captured onto Galanthus nivalis lectin (GNA)-coated plates and, after blocking, were incubated with the HMAb (10 μg/ml). The bound HMAb was detected by alkaline phosphatase-conjugated goat anti-human immunoglobulin G (Promega, Madison, WI), followed by color development. The results were normalized according to the E2 abundance in each lysate, which was determined by measuring the binding of a non-neutralizing HMAb, CBH-17, that recognizes a linear HCV E2 epitope (5). We also assessed binding to CBH-4G, since this antibody recognizes an E2 epitope within domain A that elicits non-neutralizing antibodies (10). Since this epitope is outside the region being mutated, substitutions that result in a reduction in binding to both CBH-2 and CBH-4G may be interpreted as having a global effect on E2 structure instead of being CBH-2 epitope specific. As shown in Fig. 2A, the binding of CBH-2 was reduced by >90% by mutations G530A and D535A, approximately 80% by G523A, and 60% by W529A. Since the binding of CBH-4G to these mutants was relatively unchanged from the wild type (wt), these substitutions are likely to be within the CBH-2 epitope. On the other hand, the F537A and N540A substitutions resulted in reduced binding to both CBH-2 and CBH-4G. F537A substitution probably leads to a global change on the E2 structure since the two antibodies recognize nonoverlapping epitopes. Asn540 is modified by N-linked glycosylation, and substitution at this residue alters the folding of E2 (4). Thus, N540 is not likely to be part of an epitope. These experiments demonstrated that residues G530 and D535 are critical, and that G523 is also likely needed, for CBH-2 binding. The results are in agreement with prior mapping of the CBH-5 epitope (13) and suggest that these residues form the core for all domain B epitopes. Although N532 was not identified as contributing to the epitope by this approach, its proximity to G530 and D535 provides an explanation for how glycosylation at N532 may lessen neutralization by domain B-reactive HMAbs (6).
FIG. 2.
CBH-2 epitope mapping. (A) Alanine scanning of the CBH-2 epitope from residues 523 to 540, a CD81 binding region that includes the glycosylation site N532. 293T cells transfected with Hvar wt and E2 mutants were lysed and analyzed by ELISA as described previously (9) The individual protein expression level was normalized by CBH-17 binding, an HCV E2 HMAb to a linear epitope (5). On the x axis, the mutated amino acids are depicted. The numbers correspond to their relative positions in the polyprotein of reference strain H (GenBank accession no. AF009606). The y axis shows the mean optical density values for triplicate values, and the error bars represent one standard deviation from the mean. The binding was normalized to the nonsubstituted sequence (Hvar) that is set at 100%. CBH-4G binding was used as an indicator for specific CBH-2 binding sites. (B) Substitution mutagenesis of the CBH-2 epitope from residues 430 to 444, a second CD81 binding region, and surrounding glycosylation sites N417 (residues 415 to 428) and N645 (residues 640 to 647). (C) Conformational epitope mapping strategy is shown as a linear schematic of HCV 1a E2 with labeled regions that are associated with CD81 binding, glycosylation sites that affect domain B HMAb neutralization, and hypervariable regions. The underlined residues are in the CBH-2 epitope. L640, A642, and A643 in italics are necessary for overall E2 structure. Residue differences between H77c and Hvar are shown as “(H77c/Hvar)”.
A comparison of the amino acid sequences of the E2 proteins of Hvar and H77c revealed seven differences: H394R, D431E, T434N, R444Q, R564C, V566A, and G650E (shown as Hvar__H77c), none of which were identified in these initial epitope-mapping experiments. Thus, the different susceptibilities of Hvar and H77c to neutralization by CBH-2 and CBH-5 infer that additional contact residues are involved. More CBH-2 mapping studies were carried out with a genotype 1b E2 (GenBank accession no. AF348705), since both antibodies bound and neutralized HCVpp bearing this E2 protein. To interrogate the glycosylation sites at N417 and N645 (6), 14 Ala substitution mutants spanning positions N415 to N428 and 7 mutants spanning positions L640 to T647 were generated. To examine a second proposed CD81 binding region (3) located between the two E2 hypervariable domains, HVR1 and HVR2, 15 mutants spanning positions D430 to V444 were constructed. Importantly, this region contains three residues that differ between Hvar and H77c, namely, D431E, T434N, and R444Q.
As shown in Fig. 2B, the binding of CBH-2 was eliminated by the D431A and F437A mutations and reduced approximately 70% by T425A and A426G. CBH-4G binding to these mutants was minimally affected, with the exception of F437A that was reduced by 60%. Substitutions at S432, L433, and N434 reduced CBH-2 binding by 50 to 60% but similarly reduced the binding of CBH-4G, suggesting that they induced global changes in the conformation of E2. Substitutions at N417 or N645 did not directly affect CBH-2 or CBH-4G binding. However, changes in the residues in close proximity to N645 (L640A, A642G, and A643G mutations) resulted in near elimination of binding by both CBH-2 and CBH-4G, suggesting that this region is essential to the native E2 structure, possibly modulated by the glycan at N645 (6). These data indicate that the critical contact residues for CBH-2 include D431, G523, G530, and D535. F437 may also be involved in the epitope, but E2 proteins containing W437, such as Hvar, are also bound by CBH-2 (see below). The data also indicate that the CBH-2 epitope also involves L640, A642, and A643, all of which are in close proximity to glycan N645. Overall, when taken collectively, they suggest that there is spatial proximity of the two CD81-binding regions, and two of the three glycosylation sites, on the surface of the folded E2 glycoprotein. In addition, the findings of contact residues at distant sites confirm that CBH-2 recognizes a conformational epitope.
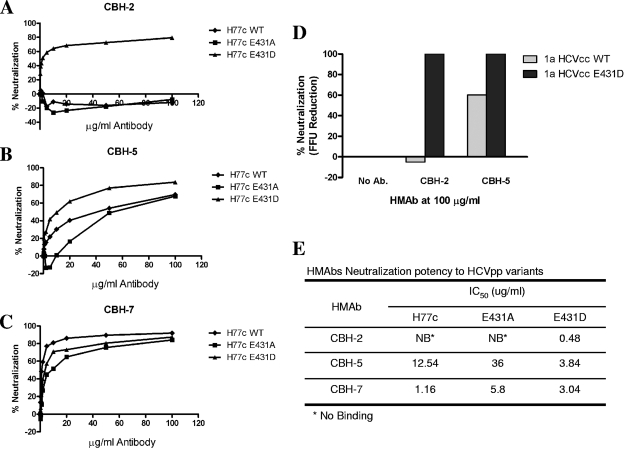
Since the substitution of D431 with Ala completely eliminated CBH-2 binding, it seemed likely that the presence of E431 in H77c (rather than D431, as in Hvar) might account for the escape of H77c from neutralization by CBH-2. To assess whether the different side chains residing at position 431 in the two H strains are in fact responsible for the differences in neutralization by CBH-2, we replaced the E431 residue in the H77c HCVpp with Asp (as found in Hvar) or Ala. As shown in Fig. 3A, wt H77c and E431A mutant HCVpp were not neutralized by CBH-2 at concentrations up to 100 μg/ml. However, the E431D H77c HCVpp mutant showed dose-dependent CBH-2 neutralization with a 50% inhibitory concentration (IC50) of 0.48 μg/ml (Fig. 3E), thereby proving that residue 431 is critical for CBH-2 neutralization activity. Interestingly, CBH-2 showed higher neutralizing activity against E431D HCVpp than wt Hvar, as CBH-2 was unable to reach 50% of the Hvar HCVpp at 20 μg/ml (compare Fig. 3A and 1A). It is thus possible that other residues that differ between the two E2 sequences may contribute to differences in the susceptibility of Hvar and H77c HCVpp to neutralization by CBH-2. The E431A and E431D mutant HCVpp remained sensitive to CBH-5 and CBH-7 (Fig. 3B and C), as expected. However, the IC50 for CBH-5 against the E431A mutant was 36 μg/ml compared to 3.84 μg/ml for the E431D mutant, suggesting that the nature of the side chain on residue 431 influences the CBH-5 epitope in a discrete conformational manner leading to altered neutralizing potencies with this antibody. This could explain why CBH-5 neutralized wt H77c at 40% but Hvar at 60%, as shown in Fig. 1A. To confirm these observations with infectious virus, we also constructed an infectious E431D HCVcc variant. The methods of mutagenesis, HCVcc virus stock production, infection, and neutralization have been described previously (18). As shown in Fig. 3D, whereas the parental HCVcc with the wt H77c E2 protein was resistant to neutralization, the mutated chimeric HCVcc became sensitive to CBH-2 neutralization. The mutant also showed enhanced susceptibility to neutralization with CBH-5 compared to the wt chimeric HCVcc, confirming the observations made with HCVpp.
FIG. 3.
Neutralization of H77c and mutants E431A and E431D. HCVpp containing either H77c (WT), mutant E431A, or mutant E431D envelope proteins were preincubated for 1 h with each HMAb—CBH-2 (A), CBH-5 (B), and CBH-7 (C)—at the indicated concentrations for 1 h at 37°C before infection of Huh7 cells. Neutralization of test antibody is expressed as a percentage of luciferase activity reduction compared to RO4. (D) Inoculum of infectious genotype 1a HJ3-5 chimeric virus (WT) or E431D mutant was incubated with each HMAb at different concentrations for 1 h at 37°C prior to inoculation onto Huh7.5 cells preseeded into eight-well tissue culture chamber slides. Cells were fixed and stained with MAb to core antigen at day 4 postinoculation, and the focus-forming units (FFU) were enumerated. (E) HMAb neutralization potency. The table summarizes the IC50 of each HMAb against wt and mutant HCVpp.
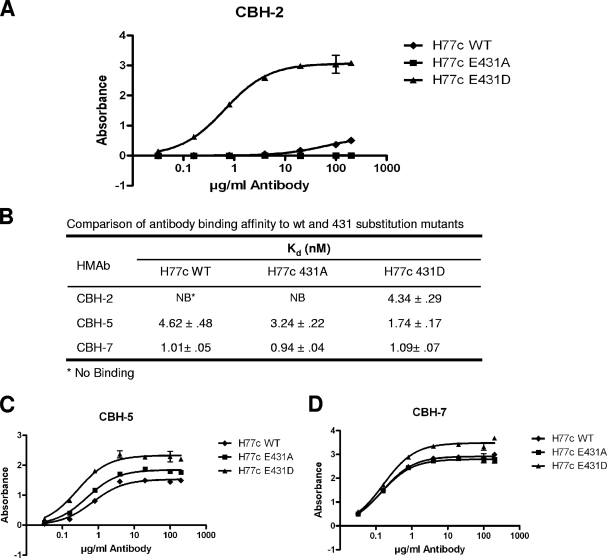
To determine whether the absence of CBH-2 neutralization with H77c and its reconstitution by the E431D substitution correlated with the binding affinity of the antibody, we carried out E2 binding assays using the ELISA method described above for Fig. 2A (9). As shown in Fig. 4A, CBH-2 bound to the mutant E431D with a Kd of 4.34 nM but failed to bind to either the wt H77c or the E431A mutant. This result shows that restoration of binding to the CBH-2 epitope was the mechanism underlying the gain in susceptibility to neutralization accompanying the E431D substitution. In contrast, the Kd for the binding of CBH-7 was similar (approximately 1 nM) for wt H77c and E431A and E431D mutants (Fig. 4B and D), demonstrating that CBH-7 binds and neutralizes HCV by interacting with an epitope distinctly different from that recognized by CBH-2, as proposed previously (10). There were modest differences in the Kd for CBH-5, which demonstrated a slight increase in affinity for the E431A mutant (Kd = 3.2 nM), and a nearly threefold increase in affinity (Kd = 1.7 nM) for the E431D mutant compared to wt H77c (Kd = 4.6 nM) (Fig. 4B and C). The similarity in the changes in CBH-2 and CBH-5 binding affinity, albeit on different scales, is consistent with the concept that their epitopes overlap each other within the dominant immunogenic domain B (10).
FIG. 4.
Binding of HMAbs to E2 glycoproteins of H77c (WT) and mutants (E431A and E431D) by ELISA. Antibody affinity measurements were performed with E1E2 expressed in 293T cells containing 1 μg of E2/ml essentially as described previously (9). E1E2 was captured by precoated GNA on the plate and later bound by a range of 0.01 to 200 μg of each HMAb/ml (x axis): CBH-2 (A), CBH-5 (C), and CBH-7 (D). The y axis shows the mean optical density values for triplicate wells. Error bars represent one standard deviation from the mean. (B) Comparison of antibody binding affinity to wt and 431 substitution mutants. The data were analyzed by nonlinear regression to calculate the antibody disassociation constant (Kd) using Prism software (GraphPad).
Collectively, these results indicate that the neutralization escape phenotype displayed by H77c against the CBH-2 HMAb is due to a loss of the CBH-2 epitope on E2 envelope protein and that this is conferred by a single amino acid substitution at residue 431. The aspartic acid residue at 431 is highly conserved, with tabulated percentages of different genotypes and subtypes as follows: 44%, 1a; 95%, 1b; 90%, 2a; 100%, 2b; 50%, 3; 95%, 4; 100%, 5; and 94%, 6 (http://hcv.lanl.gov). The fact that CBH-5 and CBH-7 retain binding and the ability to neutralize H77c confirms that its ability to escape CBH-2 neutralization is due to an epitope-specific change that does not have a global impact on E2 conformation.
In summary, our findings show how an amino acid substitution at one residue within a conformation-dependent epitope can result in escape from neutralization by a MAb, leaving the mutant virus sensitive to other related and unrelated neutralizing antibodies. CBH-2 is one of a series of antibodies that have been described as recognizing overlapping epitopes that comprise a discreet antigenic domain (domain B). The conserved nature of this domain makes it of interest in terms of vaccine development. However, the results reported here indicate that additional studies are required to assess the likelihood that other substitutions in E2 may similarly lead to escape from neutralization by other domain B HMAbs without loss of envelope function or viral fitness. While the identification of neutralizing epitopes that are broadly conserved among different HCV genotypes and subtypes is important, an effective vaccine will need to induce antibodies that ideally are directed at more invariant epitopes in order to minimize the possibility of virus escape. Further, our findings are relevant to antibody-based therapy and suggest that such therapies should consist of two or more antibodies recognizing different and nonoverlapping epitopes to lessen the chances of virus escape.
Acknowledgments
This study was supported in part by National Institutes of Health grants HL079381 to S.K.H.F. and U19-AI40035 to S.M.L. M.G.-T. was supported by a McLaughlin Postdoctoral Research Fellowship.
Footnotes
Published ahead of print on 2 April 2008.
REFERENCES
- 1.Bartosch, B., J. Bukh, J. C. Meunier, C. Granier, R. E. Engle, W. C. Blackwelder, S. U. Emerson, F. L. Cosset, and R. H. Purcell. 2003. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc. Natl. Acad. Sci. USA 10014199-14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartosch, B., J. Dubuisson, and F. L. Cosset. 2003. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J. Exp. Med. 197633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drummer, H. E., I. Boo, A. L. Maerz, and P. Poumbourios. 2006. A conserved Gly436-Trp-Leu-Ala-Gly-Leu-Phe-Tyr motif in hepatitis C virus glycoprotein E2 is a determinant of CD81 binding and viral entry. J. Virol. 807844-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goffard, A., N. Callens, B. Bartosch, C. Wychowski, F. L. Cosset, C. Montpellier, and J. Dubuisson. 2005. Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. J. Virol. 798400-8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadlock, K. G., R. E. Lanford, S. Perkins, J. Rowe, Q. Yang, S. Levy, P. Pileri, S. Abrignani, and S. K. Foung. 2000. Human monoclonal antibodies that inhibit binding of hepatitis C virus E2 protein to CD81 and recognize conserved conformational epitopes. J. Virol. 7410407-10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helle, F., A. Goffard, V. Morel, G. Duverlie, J. McKeating, Z. Y. Keck, S. Foung, F. Penin, J. Dubuisson, and C. Voisset. 2007. The neutralizing activity of anti-hepatitis C virus antibodies is modulated by specific glycans on the E2 envelope protein. J. Virol. 818101-8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson, D. X., C. Voisset, A. W. Tarr, M. Aung, J. K. Ball, J. Dubuisson, and M. A. Persson. 2007. Human combinatorial libraries yield rare antibodies that broadly neutralize hepatitis C virus. Proc. Natl. Acad. Sci. USA 10416269-16274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keck, Z., J. Xia, Z. Cai, T. Li, A. M. Owsianka, A. H. Patel, G. Luo, and S. K. H. Foung. 2007. Immunogenic and functional organization of hepatitis C virus (HCV) glycoprotein E2 on infectious HCV virions. J. Virol. 811043-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keck, Z. Y., T. K. Li, J. Xia, B. Bartosch, F. L. Cosset, J. Dubuisson, and S. K. Foung. 2005. Analysis of a highly flexible conformational immunogenic domain a in hepatitis C virus E2. J. Virol. 7913199-13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keck, Z. Y., A. Op De Beeck, K. G. Hadlock, J. Xia, T. K. Li, J. Dubuisson, and S. K. Foung. 2004. Hepatitis C virus E2 has three immunogenic domains containing conformational epitopes with distinct properties and biological functions. J. Virol. 789224-9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krawczynski, K., M. J. Alter, D. L. Tankersley, M. Beach, B. H. Robertson, S. Lambert, G. Kuo, J. E. Spelbring, E. Meeks, S. Sinha, and D. A. Carson. 1996. Effect of immune globulin on the prevention of experimental hepatitis C virus infection. J. Infect. Dis. 173822-828. [DOI] [PubMed] [Google Scholar]
- 12.Law, M., T. Maruyama, J. Lewis, E. Giang, A. W. Tarr, Z. Stamataki, P. Gastaminza, F. V. Chisari, J. M. Jones, R. Fox, J. K. Ball, J. McKeating, N. Kneteman, and D. R. Burton. 2008. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat. Med. 1425-27. [DOI] [PubMed] [Google Scholar]
- 13.Owsianka, A. M., A. W. Tarr, Z. Keck, T. Li, J. Witteveldt, R. Adair, S. Foung, J. K. Ball, and A. H. Patel. 2008. Broadly neutralizing human monoclonal antibodies to hepatitis C virus E2 glycoprotein. J. Gen. Virol. 89653-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owsianka, A. M., J. M. Timms, A. W. Tarr, R. J. Brown, T. P. Hickling, A. Szwejk, K. Bienkowska-Szewczyk, B. J. Thomson, A. H. Patel, and J. K. Ball. 2006. Identification of conserved residues in the E2 envelope glycoprotein of the hepatitis C virus that are critical for CD81 binding. J. Virol. 808695-8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perotti, M., N. Mancini, R. A. Diotti, A. W. Tarr, J. K. Ball, A. Owsianka, A. H. Patel, M. Clementi, and R. Burioni. 2007. Identification of a broadly cross-reacting and neutralizing human monoclonal antibody directed against the HCV E2 protein. J. Virol. 821047-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosa, D., S. Campagnoli, C. Moretto, E. Guenzi, L. Cousens, M. Chin, C. Dong, A. J. Weiner, J. Y. Lau, Q. L. Choo, D. Chien, P. Pileri, M. Houghton, and S. Abrignani. 1996. A quantitative test to estimate neutralizing antibodies to the hepatitis C virus: cytofluorimetric assessment of envelope glycoprotein 2 binding to target cells. Proc. Natl. Acad. Sci. USA 931759-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Von Hahn, T., J. Chun Yoon, H. Alter, C. M. Rice, B. Rehermann, P. Balfe, and J. A. Mckeating. 2007. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology 132667-678. [DOI] [PubMed] [Google Scholar]
- 18.Yi, M., R. A. Villanueva, D. L. Thomas, T. Wakita, and S. M. Lemon. 2006. Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells. Proc. Natl. Acad. Sci. USA 1032310-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]