Abstract
Vesicular stomatitis virus (VSV) is a candidate oncolytic virus that replicates and induces cell death in cancer cells while sparing normal cells. Although defects in the interferon antiviral response facilitate VSV oncolysis, other host factors, including translational and growth regulatory mechanisms, also appear to influence oncolytic virus activity. We previously demonstrated that VSV infection induces apoptosis in proliferating CD4+ T lymphocytes from adult T-cell leukemia samples but not in resting T lymphocytes or primary chronic lymphocytic leukemia cells that remain arrested in G0. Activation of primary CD4+ T lymphocytes with anti-CD3/CD28 is sufficient to induce VSV replication and cell death in a manner dependent on activation of the MEK1/2, c-Jun NH2-terminal kinase, or phosphatidylinositol 3-kinase pathway but not p38. VSV replication is specifically impaired by the cell cycle inhibitor olomoucine or rapamycin, which induces early G1 arrest, but not by aphidicolin or Taxol, which blocks at the G11S or G21M phase, respectively; this result suggests a requirement for cell cycle entry for efficient VSV replication. The relationship between increased protein translation following G0/G1 transition and VSV permissiveness is highlighted by the absence of mTOR and/or eIF4E phosphorylation whenever VSV replication is impaired. Furthermore, VSV protein production in activated T cells is diminished by small interfering RNA-mediated eIF4E knockdown. These results demonstrate that VSV replication in primary T lymphocytes relies on cell cycle transition from the G0 phase to the G1 phase, which is characterized by a sharp increase in ribogenesis and protein synthesis.
Oncolytic viruses constitute a promising novel therapeutic approach for cancer (reviewed in references 9, 10, and 47). Vesicular stomatitis virus (VSV), an RNA virus belonging to the Rhabdoviridae family, possesses intrinsic oncolytic properties that permit cancer cell destruction while sparing normal cells (8, 53). VSV is exquisitely sensitive to the antiviral effects of the interferon (IFN) pathway and therefore fails to replicate efficiently in primary cells that contain a functional IFN system (6, 73, 74). However, VSV replicates to high titers in transformed cells in which aspects of the IFN signaling or downstream effectors including translational control are compromised (4, 21, 27, 72). The oncolytic capacity of VSV has been established in vitro and in vivo; VSV infection selectively killed a large panel of human tumor cell lines, including 80% of the NCI 60 tumor cell bank, cleared bone marrow of leukemic AML cells, and effectively arrested metastatic spread of CT26 lung metastases in immunocompetent animals (5, 25, 27, 48). However, 20% of tumor cells tested were partially or completely refractory to VSV oncolysis, suggesting that in the clinical setting many primary cancers may not respond to VSV treatment. For example, although VSV efficiently induced oncolysis of chronic lymphocytic leukemia (CLL) cell lines, primary ex vivo CLL samples were not permissive to VSV replication (17). To date, few studies have addressed the issue of VSV resistance from a mechanistic perspective. While defects in the host antiviral response provide one explanation for VSV-mediated oncolysis, additional regulatory alterations in tumors also facilitate VSV oncolysis; for example, defective control of mRNA translation initiation plays an important role in cell permissiveness to VSV (4, 6, 7, 21, 24).
Ligation of the T-cell receptor (TCR) and CD28 in a naive T lymphocyte rapidly leads to activation of distinct but interactive signaling cascades (reviewed in references 52 and 79). The Ras pathway activates the mitogen-activated protein kinases (MAPKs) extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun NH2-terminal kinases (JNK), and p38, whereas the calcium pathway activates phosphatidylinositol-3 kinase (PI3K), leading to Akt phosphorylation (18, 68). Such signals culminate in the activation of the transcription factor families NF-AT, AP-1, and NF-κB, leading to the upregulation of genes involved in protein translation and cell cycle progression.
To exit quiescence, D-type cyclins (CycD) are synthesized de novo (reviewed in reference 69). CycD-cdk4/6 complexes accumulate in early G1 phase and promote cell division by phosphorylating retinoblastoma protein (Rb) and sequestering cdk inhibitory proteins (Cip/Kip family). The cdk inhibitor p27Kip1 is an important regulator of T-cell cycle progression: high levels of the p27kip1 protein are present in resting T cells, preventing G1- to S-phase transition by inhibiting the cyclin E/cdk2 complex (41). The activity of p27Kip1 is regulated at two levels, acting in early G1 and in G1/S transition: p27Kip1 is sequestered by CycD/cdk complexes, and free p27Kip1 is degraded via the proteasome pathway by cyclin E/cdk2-dependent and -independent mechanisms that require MEK and PI3K activation (reviewed in references 20, 32, 50, and 62). Once free from p27Kip1, newly synthesized cyclin E and cyclin A along with cdk2 orchestrate the G1/S-phase transition. The mitogen stimulated kinase Akt controls the stability of cyclin E as well as the subcellular localization of Cip/Kip proteins (46, 70). It is now evident that activation of the MAPK and PI3K cascades following TCR/CD28 stimulation regulates the cell cycle by acting at both at the transcriptional and posttranslational levels on cdk inhibitory protein and cyclin molecules (2, 14).
Cell cycle progression is tightly linked to protein synthesis and ribogenesis, since optimal cell size is required to enter and successfully complete the cell division process. Mammalian target of rapamycin (mTOR) is a central coordinator of protein synthesis and cell cycle progression (34, 61), although the mechanisms by which mTOR mediates these events are not fully understood. It has been proposed that mTOR controls protein translation through two distinct mechanisms that in turn are crucial for cell cycle progression (28, 29). One branch is regulated by phosphorylation of eIF4E binding protein by mTOR, leading to its dissociation from eIF4E (37, 63). Free eIF4E can then be phosphorylated by MAPK-interacting protein 1 (Mnk1), resulting in the activation of eIF4E and enhanced ribosome recruitment to the translational start site. In T cells, extracellular signal-regulated kinase (ERK) is an upstream regulator of Mnk1 and therefore directly involved in the activation of eIF4E throughout the cell cycle. The second branch relies on phosphorylation of the ribosomal S6 kinase 1 (S6K1) by mTOR: translation initiation, elongation, and/or ribosome biogenesis is stimulated by as yet poorly defined mechanisms involving S6K1-mediated phosphorylation of the 40S ribosomal protein S6 and eIF3 (translation initiation factor 3) (3, 65). The relationship between protein translation and cell cycle control is highlighted by the fact that rapamycin, the best-characterized mTOR inhibitor, causes cell cycle arrest in early G1 phase and reportedly inactivates cdk2 kinase by favoring formation of the cyclin E/cdk2-p27 complex (15, 35).
We previously demonstrated that ex vivo primary adult T-cell leukemia (ATL) cells—rapidly proliferating CD4+/CD25+ T-lymphocytes—are permissive to VSV infection and undergo rapid oncolysis, whereas neither viral replication nor oncolysis was observed in resting T cells or in ex vivo cells from patients with CLL, corresponding to B lymphocytes arrested in the G0 phase (17, 23, 42). In this report, we demonstrate that T-cell activation with anti-CD3 and anti-CD28 antibodies renders primary CD4+ T lymphocytes permissive to VSV replication with a concomitant induction of apoptosis. Similarly, activation of primary CLL cells with phorbol myristate acetate (PMA)-ionomycin, causing them to exit from G0 and enter the cell cycle, renders them susceptible to VSV infection and oncolysis. As demonstrated in CD4+ T cells, activation of the ERK, JNK, or AKT pathway, leading to G0- to G1-phase transition, is crucial for VSV replication in primary lymphocytes due to a global increase in protein translation mediated by the activation of mTOR and eIF4E.
MATERIALS AND METHODS
Isolation of CD4+ T cells and B-CLL cells.
Peripheral blood mononuclear cells from CLL patients were obtained at the Jewish General Hospital, Montreal, Quebec, Canada, with informed consent, in agreement with the Jewish General Hospital and McGill University Research Ethics Committee. Leukophoresis from healthy donors was obtained at the Royal Victoria Hospital, Montreal, Quebec, Canada, with informed consent, in agreement with the Royal Victoria Hospital, the Jewish General Hospital, and McGill University Research Ethics Committee.
Peripheral blood mononuclear cells were isolated by centrifugation (400 × g at 20°C for 25 min) of blood on a Ficoll-Hypaque gradient (GE Healthcare Bio-Sciences Inc., Oakville, Ontario, Canada). B cells or CD4+ T lymphocytes were isolated using the CD4 or CD19 enrichment cocktail by negative selection with the high-speed autoMACS system (kit no. 130-050-301 or 130-091-894, respectively; Miltenyi Biotec) according to the manufacturer's instructions. In all cases, the purity of B cells or CD4+ T lymphocytes was between 90 and 95% as determined by flow cytometry. Cells were cultured in RPMI 1640 medium (Wisent Inc., San Diego, CA) supplemented with 15% heat-inactivated fetal bovine serum and 100 U/ml penicillin-streptomycin.
Flow cytometry for surface markers.
After two washes with phosphate-buffered saline (PBS), cells were stained with monoclonal allophycocyanin-labeled anti-CD4 (T-cell marker), phycoerythrin (PE)-labeled anti-CD19 (B-cell marker), fluorescein isothiocyanate-labeled anti-CD5 (B-cell marker), and PE-labeled anti-CD25 or PE-labeled anti-CD69 (T-cell activation markers) for 30 min in PBS-1% fetal calf serum. After a final wash with ice-cold PBS, cells were resuspended in 400 μl fluorescence-activated cell sorter (FACS) buffer (PBS-Cytofix; (BD Pharmingen). Flow cytometric analyses (1 × 104 cells/measurement) were performed using a FACScalibur flow cytometer with CELLQuest software (Becton Dickinson). All antibodies were purchased from BD Biosciences (Mississauga, Ontario, Canada).
CD4+ T-cell and B-CLL cell activation.
Freshly isolated CD4+ T lymphocytes (106/ml) were mock activated or activated for 12 to 48 h with 5 μg/ml of immobilized anti-CD3 monoclonal antibody and 1 μg/ml of immobilized anti-CD28 monoclonal antibody (BD Biosciences, Mississauga, Ontario, Canada).
Resting T cells were mock treated or treated with pharmacological inhibitors 1 h prior to their activation, as appropriate. The inhibitors used in this study were LY294002 (PI3K inhibitor; 20 μM), SB203580 (p38 MAPK inhibitor; 12 μM), SP600125 (JNK inhibitor; 20 μM), UO126 (MEK1/2 inhibitor; 10 μM), rapamycin (25 nM), olomoucine (100 μM), Taxol (200 nM), aphidicolin (3 μg/ml), and nocodazole (1 μg/ml). All were purchased from Calbiochem (San Diego, CA) except UO126, which was purchased from Cell Signaling Technologies (Danvers, MA).
B cells were stimulated with PMA (25 ng/ml; Santa Cruz Biotechnology, Santa Cruz, CA) and ionomycin (1 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA) for 24 h.
Cell cycle analysis by flow cytometry.
Enriched human primary T cells, untreated or treated for 1 h with kinase inhibitors, were activated for 48 h with anti-CD3/CD28. Cells were washed with cold PBS-5 mM EDTA and ethanol fixed (70% in PBS) overnight at −20°C. Fixed cells were washed and incubated in PBS containing 2.5 μg/ml propidium iodide (PI) (Sigma-Aldrich) and 50 μg/ml RNase A for 30 min at 37°C. Samples were subjected to FACS (FACScalibur) analysis using CELLQuest software (Becton Dickinson) to determine percentages of cells in G0/G1, S, and G2/M phases of the cell cycle based on DNA content.
VSV infection of primary CD4+ T lymphocytes and CLL samples.
Wild-type VSV (VSV-HR; Indiana serotype) and VSV-AV1 were propagated in Vero cells as described previously (74). Viruses were obtained from cell supernatants and titrated on Vero cells by standard plaque assay. T or B cells were mock infected or infected with VSV at 1 multiplicity of infection (MOI) for 12 to 48 h. Virus infection was performed in RPMI in the absence of serum for 1 h, after which it was replaced with fully supplemented growth medium. Cells were incubated at 37°C for the specified times, washed twice in PBS, and stored at −80°C for further use. Quantification of virus in supernatants was performed in duplicate with log dilutions on 100%-confluent Vero cells plated in six-well dishes.
Plaque assay.
Confluent monolayers of Vero cells in six-well plates were infected with 0.1 ml of serially diluted samples; after 1 h of infection, at 37°C, medium was removed and replaced with complete medium containing 0.5% methyl cellulose (Sigma Aldrich, Oakville, Ontario, Canada) for 48 h. Vero cells were fixed in 4% formaldehyde and stained with crystal violet. Plaques were counted, and titers were calculated in PFU per milliliter.
siRNAs and primary human CD4+ T-lymphocyte transfection.
Control and eIF4E-specific RNA interference sequences were described previously (75). Transfection of purified activated T cells was carried out by electroporation using the Nucleofection system (Amaxa, Köln, Germany), according to the protocols proposed by the furnisher. Briefly, CD4+ T cells were activated with an anti-CD3/28 monoclonal antibody combination for 48 h. T cells (10 × 106) were resuspended in 100 μl of T-cell Nucleofector solution (human T-cell Nucleofector kit) containing 300 pmol of double-stranded small interfering RNAs (siRNAs). After electroporation, 400 μl of prewarmed cultured medium was added to the cuvette and the cells were transferred into cultures plates containing prewarmed culture medium. At the optimal time of gene silencing (usually 48 h posttransfection), T cells were mock infected or infected with VSV at 1 MOI for 24 h.
Immunoblot analysis.
Cells destined for immunoblotting were washed with PBS and lysed in lysis buffer (0.05% NP-40, 1% glycerol, 30 mM NaF, 40 mM β-glycerophosphate, 10 mM Na3VO4, 10 ng/ml of protease inhibitors cocktail [Sigma Aldrich, Oakville, Ontario, Canada]). The protein concentration was determined by using the Bradford assay (Bio-Rad). Whole-cell extracts (30 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 10%-acrylamide gel and transferred to a nitrocellulose membrane (Hybond C Super; GE Healthcare Bio-Sciences Inc., Oakville, Ontario, Canada). Membranes were blocked in 5% nonfat dried milk in Tris-buffered saline (TBS) plus 0.1% Tween 20 for 1 h at room temperature. Membranes were then probed overnight with antibodies against VSV (1:5,000; a gift from John Bell, Ottawa Cancer Centre), cleaved caspase 3, cyclin D3, cyclin A, phospho-Akt, phospho-p38 MAPK, phospho-JNK, phospho-eIF4E (Ser 209), eIF4E, mTOR, p27kip1, β-actin (1:1,000; Cell Signaling, Danvers, MA), CDK4, phospho-ERK1/2 and ERK2 (1 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA), and Rb (1:5,000; BD PharMingen) in 5% bovine serum albumin and PBS at 4°C. Incubation mixtures were washed in TBS-0.05% Tween 20 five times for a total of 25 min. Following washes, the membrane was incubated with peroxidase-conjugated goat antirabbit or antimouse antibody (Amersham) at a dilution of 1:5,000 for 1 h at room temperature. Following the incubation with the secondary antibody, membranes were washed again (5 times, 5 min each) and then visualized with an enhanced chemiluminescence (detection system as recommended by the manufacturer (ECL; GE Healthcare Bio-Sciences Inc., Oakville, Ontario, Canada).
RNA extraction and semiquantitative RT-PCR.
Whole RNA from treated cells was extracted using an RNase extraction kit (Qiagen, Mississauga, Ontario, Canada) according to the manufacturer's instructions. Reverse transcription (RT)-PCR was performed using 1 μg of RNA resuspended in RNase-free double-distilled H2O and an oligo(dT12-18) primer (Invitrogen Canada Inc., Burlington, Ontario, Canada) according to the manufacturer's conditions. RT was performed using Superscript II (Invitrogen Canada Inc., Burlington, Ontario, Canada) at 42°C for 1 h. Following the RT reactions, cDNA samples were brought to 100-μl final volumes, of which 5 μl was used as a template for each PCR with Taq polymerase (Invitrogen Canada Inc., Burlington, Ontario, Canada). The primer sequences used in this study for PCR were as follows: L protein, forward (5′-AAGTTATCAAACGGCCCAGTG-3′) and reverse 5′-ACA AAC TCG TTG GGA GGT TG-3′; and M protein, forward (5′-GCG AAG GCA GGG CTT ATT TG3′) and reverse (5′-CTT TTT CTC GAC AAT CAG GCC-3′). PCR fragments were amplified at an annealing temperature of 55°C for 35 cycles. Products were run on a 2% agarose gel and revealed through use of a Typhoon 9400 phosphorimager (GE Healthcare Bio-Sciences Inc., Oakville, Ontario, Canada).
Statistical analysis.
The data are presented as means ± standard deviations. The statistical significance was estimated with the Student t test. P values of ≤0.005 were considered statistically significant.
RESULTS
Activation renders CD4+ T lymphocytes susceptible to VSV replication.
VSV replicates and induces cell death in primary CD4+ T lymphocytes from ATL samples (17). To determine whether VSV susceptibility requires T-cell activation, VSV replication was monitored in resting and CD3/CD28-activated CD4+ T lymphocytes. Immunoblot analysis using VSV antiserum revealed that viral protein synthesis was not detectable in resting CD4+ T lymphocytes, whereas production of VSV proteins—L, G, N, P, M—was detected at 24 h postinfection in T lymphocytes preincubated for 12 h with anti-CD3/CD28 (Fig. 1A). Apoptosis occurred concomitantly with viral replication as indicated by cleavage of caspase 3 (Fig. 1A). Quantification of virus titers by plaque assay revealed that T-cell activation led to a 4-log increase in the virus titer at 24 h postinfection, compared to that for resting T cells (Fig. 1B). To determine if VSV replication was directly proportional to the level of CD4+ T-cell activation, lymphocytes were stimulated with CD3/CD28 for 12, 24, or 48 h and subsequently infected with VSV for 12 or 24 h (Fig. 1C). VSV protein production was detected as early as 12 h postactivation and increased as the activation time was prolonged (Fig. 1C, lanes 4 to 9). In cells activated with CD3/CD28 for 48 h, VSV G protein levels were ∼3.6-fold higher than those in cells activated for 12 h (Fig. 1C, compare lanes 5 and 9). In agreement with viral protein levels, cells activated for 48 h produced ∼2-fold more virus than nonstimulated cells at 24 h (Fig. 1D). Furthermore, VSV replication in activated primary CD4+ T lymphocytes led to induction of apoptosis, as demonstrated by immunoblotting for cleaved caspase 3 (Fig. 1C, lanes 6 to 9).
FIG. 1.
VSV replicates and induces apoptosis in activated CD4+ T lymphocytes. (A) Kinetics of VSV replication and caspase 3 cleavage in resting and activated CD4+ T lymphocytes. Resting CD4+ T lymphocytes were activated with anti-CD3/CD28 for 24 h. Activated and resting CD4+ T lymphocytes were mock infected or infected with VSV (1 MOI). At the indicated times postinfection, VSV replication and caspase 3 cleavage were analyzed in 20 μg of total cell lysates by SDS-PAGE followed by immunoblotting with anti-cleaved caspase 3, anti-VSV, and anti-β-actin as a loading control. (B) Infectious VSV was measured at 12, 24, and 48 h postinfection by plaque assay in Vero cells. Virus titers were determined in triplicate; each bar indicates mean ± standard deviation. (C) VSV protein levels are proportional to T-cell activation time. Purified primary T lymphocytes were incubated with anti-CD3/CD28 monoclonal antibodies for 12 to 48 h and then infected with VSV (1 MOI). At the indicated times postinfection, VSV replication and caspase 3 cleavage were assessed in 20 μg of whole-cell lysates by immunoblot analysis. The VSV N/P protein expression level was quantified and normalized to the β-actin level using the Scion Image 4.0 software program. (D) Supernatants of cells activated for 24 or 48 h were collected 12 or 24 h postinfection and virus titers determined by standard plaque assay. Assays were performed in triplicate; each bar indicates mean ± standard deviations.
Since VSV is exquisitely sensitive to IFN-mediated inhibition and therefore fails to replicate efficiently in primary cells that contain a functional IFN system, we tested the possibility that anti-CD3/CD28 treatment interfered with type I IFN (alpha/beta IFN) production. PCR analysis of type I IFN mRNA demonstrated that treatment of CD4+ T lymphocytes with anti-CD3/CD28 did not inhibit the IFN response, thus ruling out this possibility as a cause for the observed increase in viral replication (see Fig. S1 in the supplemental material). These results indicate that triggering the CD3/CD28 activation pathways is sufficient to render normal CD4+ T lymphocytes permissive to VSV replication, suggesting that downstream effectors of CD3/CD28 are critical for VSV replication and induction of apoptosis.
VSV requires ERK, JNK, and AKT but not p38 activation for replication in T lymphocytes.
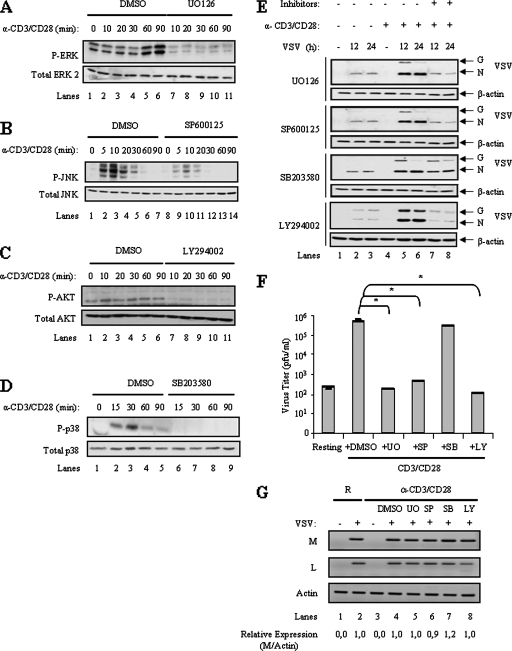
Since MAPK and PI3K signaling pathways are downstream of CD3/CD28, we postulated that activation of these pathways is required for VSV replication and oncolysis. CD4+ T-cell stimulation with CD3/CD28 antibodies resulted in enhanced ERK, JNK, Akt, and p38 phosphorylation as determined by immunoblotting with phospho-specific antibodies, while total protein levels remained unchanged (Fig. 2A, lanes 1 to 6, B, lanes 1 to 7, C, lanes 1 to 6, and D, lanes 1 to 5). To examine the essential signaling pathways downstream of CD3/CD28 required for VSV replication in T cells, small-molecule inhibitors specific for PI3K (LY294002), MEK1/2 (U0126), stress-activated protein kinase (SAPK)/JNK (SP600125), or p38 (SB203580) were used to block distinct pathways. The concentration for each of these inhibitors (20 μM, 10 μM, 20 μM, or 12 μM, respectively) was chosen based on values reported to be specific and sufficient to inhibit each kinase (2, 26, 55, 76). CD4+ T lymphocytes were pretreated for 1 h with each individual inhibitor, activated with the combination of anti-CD3/CD28 monoclonal antibodies for 12 h, and subsequently infected with VSV for 12 or 24 h. The effectiveness of the drug treatment was confirmed by the disappearance of phosphorylated substrates by immunoblotting (Fig. 2A to D). Thus, U0126 inhibited phosphorylation of ERK by MEK1/2 (Fig. 2A, compare lanes 1 to 6 to lanes 7 to 11), SP600125 inhibited phosphorylation of JNK (Fig. 2B, compare lanes 1 to 7 to lanes 8 to 14), LY294002 inhibited phosphorylation of Akt by PI3K (Fig. 2C, compare lanes 1 to 6 to lanes 7 to 11), and SB203580 inhibited phosphorylation of p38 (Fig. 2D, compare lanes 1 to 5 to lanes 6 to 9). VSV replication was monitored by viral protein immunoblotting (Fig. 2E). Specific inhibition of the MEK1/2, JNK, or PI3K pathway impaired VSV replication, whereas VSV protein synthesis was not blocked by SB203580 at concentrations that effectively inhibited p38 phosphorylation. Interestingly, the MEK1/2, PI3K, and JNK inhibitors had no effect on VSV replication when added subsequent to CD4+ T-cell activation (data not shown). To confirm the suppressive effect of the MEK1/2, JNK, and PI3K inhibitors on VSV replication, virus titers were assessed by plaque assay (Fig. 2F); CD4+ T-lymphocyte activation led to a 4-log increase in virus titer compared to that for resting T cells, while inhibition of MEK1/2, JNK or PI3K signaling effectively prevented VSV virion production (P ≤ 0.0003). In agreement with the VSV protein synthesis results, inhibition of the p38 pathway had no effect on the viral titer in activated CD4+ T lymphocytes. These results demonstrate that VSV replication in primary T lymphocytes depends on the activation of the ERK, JNK, and PI3K signaling pathways.
FIG. 2.
Activation of the MAPK and PI3K pathways is required for VSV replication. (A to D) CD3/CD28 stimulation induces PI3K and MAPK signaling in CD4+ T lymphocytes. Resting primary CD4+ T lymphocytes were mock treated (dimethylsulfoxide [DMSO]) or treated with the MEK1/2 inhibitor UO126 (A), the JNK inhibitor SP600125 (B), the PI3K inhibitor LY294002 (C), or the p38 kinase inhibitor SB203580 (D) before activation with anti-CD3/CD28. At the indicated times, cells were lysed and analyzed by immunoblotting with antibodies specific for total or phosphorylated forms of p38 MAPK, ERK1/2, Akt, and JNK. (E) Effect of MAPK and PI3K pathway inhibitors on VSV replication in T lymphocytes. Mock- and inhibitor-treated activated T lymphocytes were infected with VSV (1 MOI) for 12 h or 24 h, lysed, and subjected to immunoblot analysis with an anti-VSV antibody. Data are representative of three different experiments. (F) Effect of various MAPK and PI3K inhibitors on virus yield. CD4+ T lymphocytes pretreated with UO126 (UO), SB203580 (SB), SP600125 (SP), or LY294002 (LY) and activated as above were infected with VSV (1 MOI) for 24 h. Supernatants were collected and viral titers determined by standard plaque assay. The data shown are the means ± standard deviations (n = 3). Statistically significant differences are indicated by asterisks (*, P ≤ 0.00003). (G) Effect of various MAPK and PI3K inhibitors on VSV mRNA transcripts. Cells were treated as described for panel E and collected 24 h after infection for total RNA extraction; resting T cells are designated R. One microgram of RNA from each sample was subjected to RT-PCR for selective amplification of specific VSV mRNAs (M or L) and the constitutively expressed actin, as a control. PCR products were separated on a 2% agarose gel and visualized with ethidium bromide staining. The VSV M mRNA expression levels were quantified and normalized to actin levels using the Scion Image 4.0 software program.
Previous studies have demonstrated that VSV can infect both permissive and nonpermissive cells to produce viral mRNA, yet VSV protein synthesis occurs only in permissive cells (64), suggesting that cell permissiveness depends on posttranscriptional regulation of viral protein synthesis. To determine if VSV permissiveness in primary T cells is governed by a similar mechanism, the level of individual viral mRNAs in CD4+ T cells following VSV infection was investigated (Fig. 2G). Total RNA was extracted from resting or activated CD4+ T cells challenged with VSV for 24 h. RT-PCR analysis of VSV mRNAs demonstrated that both resting and activated CD4+ T lymphocytes supported VSV M and L mRNA production (Fig. 2G, lanes 2 and 4). The level of viral mRNA production remained unchanged in activated CD4+ T lymphocytes pretreated with the MEK1/2, SAPK/JNK, PI3K, or p38 inhibitor (Fig. 2G, compare lanes 2 and 4 to lanes 5 to 8). Thus, the blockade in VSV replication imposed by MEK1/2, JNK, or PI3K pharmacological inhibitors occurs at the level of viral protein synthesis and not at the level of virus entry or mRNA generation.
Inhibition of ERK, JNK, or PI3K leads to cell cycle arrest in primary T lymphocytes.
Because anti-CD3/CD28 stimulation of T lymphocytes induces cell proliferation through the activation of the MEK1/2, JNK, and PI3K pathways, we sought to examine the relationship between VSV replication and induction of the cell cycle by MAPK. Treatment of T lymphocytes with anti-CD3/CD28 for 48 h resulted in exit from G0 phase and cell cycle entry as demonstrated by FACS analysis of PI-labeled cells; T-cell activation led to a 30% increase in cells in S+G2/M phases of the cell cycle (Fig. 3A, compare panels a and b; Fig. 3B). As expected, addition of MEK1/2 or PI3K inhibitor for 1 h prior to CD3/CD28 stimulation decreased the percentage of cells in S+G2/M phases to background levels (3 to 5%) (P ≤ 0.007 or P ≤ 0.006, respectively) (Fig. 3A, compare panel b to panels c and f; Fig. 3B). Surprisingly, inhibition of the JNK pathway by SP600125 prevented T-lymphocyte cell cycle entry/progression, resulting in G0/G1 arrest (2%) (P ≤ 0.004) (Fig. 3A, panel d, and B). Simultaneous assessment of apoptosis by DNA content analysis showed that these inhibitors did not affect cell survival (data not shown). To our knowledge, this constitutes the first report that JNK activation is specifically required for T-lymphocyte G0/G1-phase transition. Inhibition of p38 had no effect on T-cell proliferation (Fig. 3A, panel e, and B).
FIG. 3.
Proliferation and cell cycle progression of primary T cells is dependent on MEK1/2, JNK, and PI3K activation. (A and B) Resting CD4+ T cells were left untreated (a), treated with anti-CD3/CD28 (b), or pretreated with MEK1/2 inhibitor UO126 (UO) (c), JNK inhibitor SP600125 (SP) (d), P38 inhibitor SB203580 (SB) (e), or PI3K inhibitor LY294002 (LY) (f) before stimulation with anti-CD3/CD28. Cell cycle analysis was performed by flow cytometry on PI-labeled cells (A), and percentages of cells in S, G2 and M phase were determined as means of triplicate measurements (B). Statistically significant differences are indicated by asterisks (*, P < 0.007; **, P < 0.004; ***, P < 0.006). Results are representative of more than three independent experiments using separate donors. (C) CD3/CD28 stimulation leads to G1 progression, as demonstrated by immunoblots showing down-regulation of P27Kip1 and synthesis of CycD3 and Cdk4. Cell lysates were prepared at different times, and equal amounts of protein (30 μg) were resolved by SDS-PAGE followed by immunoblotting with antiserum specific for p27kip1, CycD3, or cdk4. Immunoblotting against β-actin was performed as a loading control. (D) CD3/CD28 stimulation leads to G1/S-phase transition: immunoblotting for Rb and cyclin A proteins. Cells were treated and cultured as for panel A. Top panel, line indicates hyperphosphorylated Rb. An immunoblot against β-actin was used as a loading control.
To confirm that CD3/CD28-stimulated T lymphocytes underwent G0/G1 transition, the induction or degradation of proteins controlling cell cycle progression was assessed by immunoblotting. High levels of the cdk inhibitor p27kip1 were present in resting cells, whereas cyclin D3 (CycD3) and cdk4 were undetectable, thus confirming G0 arrest (Fig. 3C, lane 1). CD3/CD28 stimulation resulted in rapid degradation of p27kip1, concomitant with induction of CycD3 and cdk4 expression (Fig. 3C, lanes 2 to 5). p27kip1 downregulation and CycD3/cdk4 induction were inhibited by pretreatment with LY294002, SP600125, or UO126 but not with SB203580 (Fig. 3C). Entry into S phase was monitored by measuring hyperphosphorylation of Rb (a CycD/cdk4 substrate) and cyclin A protein expression. As demonstrated in Fig. 3D, CD3/D28 stimulation increased Rb phosphorylation as well as cyclin A expression, whereas pretreatment with LY294002, SP600125, and UO126—but not SB203580—abrogated both Rb hyperphosphorylation and cyclin A expression following T-cell activation. These results confirm that inhibition of MEK1/2, SAPK/JNK, or PI3K activity arrests CD4+ T lymphocytes in G0/G1 phase by preventing the expression of positive regulators of cell cycle entry, such as CycD3 and cdk4, or preventing the downregulation of cell cycle inhibitors, such as p27Kip1.
VSV replication is dependent on cell cycle entry and increased protein translation.
Activation of the ERK, JNK, or PI3K pathway appears to confer VSV permissiveness to activated CD4+ T cells by mediating cell cycle entry, which is characterized by a sharp increase in protein translation. To test this hypothesis, activated CD4+ T lymphocytes were arrested at different stages of the cell cycle using the pharmacological inhibitors olomoucine, rapamycin, aphidicolin, nocodazole, and Taxol before challenge with VSV. FACS analysis of PI-labeled cells confirmed that CD3/CD28 stimulation induced cell cycle progression (Fig. 4A, panel b, and B). Olomoucine, a purine derivative drug that inhibits different cdk's (36, 38), and rapamycin, an mTOR-targeting, immunosuppressive drug that blocks the elimination of p27Kip1 and inactivates the kinase activity of the G1 cyclin/cdk complex (31, 40), arrested T lymphocytes in G0/G1 (97.5% and 95.9%, respectively). Aphidicolin, an inhibitor of DNA polymerase α (44, 54, 66), blocked the cell cycle between the G1 and S phases (92.3%). Taxol and nocodazole block the cell cycle in G2/M (30.3% and 15%, respectively) by modulating microtubule polymerization (16, 39) (Fig. 4A, panels c to g, and B). Simultaneous assessment of apoptosis by DNA content analysis showed that these inhibitors did not affect cell survival (data not shown). To investigate if cell cycle arrest in G0/G1 specifically impaired VSV replication, CD4+ T lymphocytes were pretreated or not with inhibitors and activated with anti-CD3/CD28 for 12 h prior to VSV infection. VSV protein synthesis was decreased by olomoucine or rapamycin (Fig. 4C, compare lanes 4 and 5 to lanes 6 and 7, and D, compare lanes 2 and 3 to lanes 4 and 5) but not with aphidicolin, nocodazole, or Taxol (Fig. 4C, compare lanes 4 and 5 to lanes 8 to 13), demonstrating that VSV replication in primary T lymphocytes is exclusively dependent on G0/G1-phase transition.
FIG. 4.
VSV protein synthesis requires cell cycle entry. (A) Effect of pharmacological inhibitors of the cell cycle on CD3/CD28-stimulated primary T cells. Resting CD4+ T cells were left untreated (a) or pretreated with olomoucine (c), aphidicolin (d), Taxol (e), nocodazole (f), or rapamycin (g) for 1 h before stimulation with anti-CD3/CD28 for 48 h. Activated T cells are shown in panel b. Cell cycle analysis was performed by flow cytometry on PI-labeled cells. In panels A and B, percentages of cells in various phases of the cell cycle are indicated. The data shown represent the means ± standard deviations (n = 3). (C and D) Effect of rapamycin, olomoucine, aphidicolin, Taxol, and nocodazole on VSV protein synthesis. Human CD4+ T cells were treated as for panel A and then infected with VSV (1 MOI). Cells were collected 12 h or 24 h postinfection, lysed, and subjected to immunoblot analysis with anti-VSV antisera. Results are representative of more than three independent experiments using separate donors.
The effect of rapamycin on the cell cycle is largely dependent on its ability to inhibit mTOR, a regulator of 5′-cap-dependent translation and ribogenesis (reviewed in references 49 and 61). mTOR regulates cell growth by phosphorylating S6K and 4EPB1, and its activity is controlled through phosphorylation by the serine/threonine kinase Akt. Phosphorylation of 4EBP1 leads to its dissociation from the translation initiation factor eIF4E, which can then be phosphorylated by Mnk1 (reviewed in reference 49), resulting in activation of eIF4E and enhanced ribosome recruitment to the translational start site. Thus, eIF4E activity is regulated through expression, phosphorylation, and interaction with eIF4BP1 (37, 58). We therefore investigated the phosphorylation status of mTOR (3, 78) and the levels of total and phosphorylated eIF4E (11, 59) as readouts for 5′-cap dependent translation and ribogenesis. CD3/CD28 stimulation of CD4+ T lymphocytes induced mTOR phosphorylation in a time-dependent manner (Fig. 5A, lanes 1 to 2, and B, lanes 1 to 5); eIF4E expression and phosphorylation were also induced following T-cell activation. Addition of the PI3K inhibitor LY294002 1 h prior to CD3/CD28 stimulation abolished mTOR and eIF4E phosphorylation (Fig. 5A, lane 6, and B, lanes 18 to 21), whereas the ERK inhibitor UO126 abrogated eIF4E phosphorylation only (Fig. 5A, lane 3, and B, lanes 6 to 9). A modest decrease in the total level of eIF4E was also observed in activated T cells pretreated with the ERK (UO126) or PI3K (LY294002) inhibitor, respectively. This result is in agreement with previous reports on the role of PI3K and ERK1/2 signaling in the control of protein translation (51). Surprisingly, inhibition of the JNK pathway by SP600125 inhibited mTOR phosphorylation-activated T lymphocytes, as well as eIF4E phosphorylation and expression (Fig. 5A, lane 4, and B, lanes 10 to 13). To our knowledge, this represents the first report that JNK activation following TCR stimulation is necessary for translational control via mTOR and eIF4E. As expected, SB203850 had no effect on mTOR and eIF4E phosphorylation (Fig. 5A, lane 5, and B, lanes 14 to 17). These results suggest that the dependence of VSV on G0/G1-phase transition in primary lymphocytes is related to a global enhancement in protein translation that depends on mTOR and eIF4E activity.
FIG. 5.
mTOR and eIF4E phosphorylation requires ERK1/2, JNK, and Akt activation in T lymphocytes. (A and B) Isolated CD4+ T lymphocytes were mock treated (DMSO) or treated with the MEK1/2 inhibitor UO126 (UO), the JNK inhibitor SP600125 (SP), the p38 kinase inhibitor SB203580 (SB), or the PI3K inhibitor LY294002 (LY) 1 h prior to activation with anti-CD3/CD28 for 0 to 2 days. Cell lysates (30 μg) were resolved by SDS-page followed by immunoblotting with antibody specific for mTOR phosphorylation (A) or eIF4E phosphorylation as well as total eIF4E protein (B). The mTOR phosphorylation level in panel A was quantified and normalized to β-actin levels using the Scion Image 4.0 software program.
siRNA against eIF4E inhibits VSV replication in activated T lymphocytes.
Because it is generally accepted that VSV protein synthesis requires the cap-dependent translational machinery (8), RNA interference was next used to examine whether the high level of eIF4E found in activated T lymphocytes was required for VSV protein synthesis. Cells were stimulated with anti-CD3/CD28 treated for 48 h with siRNA directed against eIF4E mRNA (Fig. 6A). A 50% decrease in the level of the eIF4E protein compared to the control siRNA was observed (Fig. 6A, compare lane 1 to lanes 2 and 3); simultaneously, siRNA directed against eIF4E induced cell cycle arrest in G0/G1 (89.5%; P ≤ 0.005), as demonstrated by PI staining (Fig. 6B, compare panels c and d; Fig. 6C). VSV infection was performed on siRNA-treated and preactivated T lymphocytes (Fig. 6D), and by 12 h postinfection, VSV protein production was detected in control siRNA cells but a 50% decrease in the VSV N/P protein level was observed in eIF4E siRNA-expressing cells. These results demonstrate that eIF4E plays an important role in regulating VSV replication in CD4+ T lymphocytes, either through a global regulation of cellular protein synthesis that leads to G0/G1 transition or specifically through translation initiation of viral 5′-capped mRNAs.
FIG. 6.
siRNA directed against eIF4E inhibits VSV replication in primary activated CD4+ T lymphocytes. (A) Primary CD4+ T lymphocytes were stimulated with anti-CD3/CD28 for 48 h before electroporation with eIF4E-specific siRNA or scrambled siRNA. After an additional 48-h incubation period, cells were lysed and analyzed by immunoblotting with an antibody against total eIF4E. “−” denotes cells electroporated without siRNA. (B) Cell cycle analysis was performed on cells described in legend to panel A by PI-labeling and flow cytometry analysis. In panels B and C, the percentages of cells in various phases of the cell cycle are indicated. The data shown represent the means ± standard deviations (n = 3). Statistically significant differences are indicated by asterisks (P < 0.005). (D) eIF4E knockdown and control cells were infected with VSV (1 MOI) for 12 h. Cells were lysed and subjected to immunoblotting with anti-VSV antisera. The eIF4E and VSV N/P protein expression levels in panels A and B were quantified and normalized to β-actin levels using the Scion Image 4.0 software program. Levels of eIF4E were expressed as percentages of levels for mock-infected cells.
Cell cycle entry and translation initiation renders CLL cells susceptible to VSV replication.
Cell cycle transition from G0 to G1 phase—concomitant with an increase in protein translation—is essential for VSV replication in lymphocytes. Primary ex vivo CLL cells are resistant to VSV oncolysis due to a Bcl-2-mediated arrest in G0 phase (30, 43, 45, 56) and thus represent an interesting model for investigation of whether cell cycle induction is sufficient to confer VSV susceptibility to primary CLL cells. Ex vivo CLL samples were stimulated with PMA-ionomycin for 12 to 72 h prior to VSV infection; FACS analysis with PI-labeled cells demonstrated that treatment of CLL cells with PMA-ionomycin induced their progression through the cell cycle in a time-dependent manner, leading to an increase of cells in S+G2/M phases from 5% to 18% (Fig. 7A). Cell cycle entry was further confirmed by immunoblotting for the p27Kip1 protein, which was degraded in a time-dependent manner (Fig. 7B, compare lanes 1 to 4 to lanes 5 to 8). Interestingly, treatment of CLL cells with PMA-ionomycin induced eIF4E phosphorylation in a time-dependent manner (Fig. 7C, compare lanes 1 to 4 to lanes 5 to 8). VSV protein levels and caspase 3 cleavage were also monitored at 12 and 72 h postinfection (Fig. 7D); VSV protein decreased in unstimulated primary CLL cells (Fig. 7D, lanes 2 to 5), and no caspase 3 cleavage was observed 24 h postinfection (Fig. 7D, lanes 2 to 5). In agreement with this result, activated CLL cells produced ∼3-fold more virus than unstimulated CLL cells at 48 h postinfection (Fig. 7E). Furthermore, PMA-ionomycin treatment of CLL cells stimulated VSV replication, concomitant with a time-dependent increase in the cleaved forms of caspase 3 (Fig. 7D, lanes 7 to 10). Thus, using the example of G0/G1-arrested primary CLL cells, resistance to VSV oncolysis can be overcome by pretreatment with pharmacological compounds that induce cell cycle entry and translation initiation.
FIG. 7.
Pharmacological induction of cell cycle entry of primary CLL cells is sufficient to overcome VSV resistance. (A) PMA-and-ionomycin treatment induces cell cycle entry of CLL cells. Isolated CLL cells were mock treated (DMSO) or treated with 25 ng PMA and 1 μg ionomycin. After 48 h, cell cycle analysis was performed by flow cytometry on PI-labeled cells. The percentages of cells in various phases of the cell cycle are indicated. Cell lysates from CLL samples were prepared at 12, 24, 48, or 72 h after PMA-ionomycin stimulation, and equal amounts of protein (20 μg) were resolved by SDS-PAGE followed by immunoblotting with antibody specific for P27kip1 (B) or eIF4E phosphorylation and total eIF4E protein (C). (D) Effect of PMA-ionomycin on VSV replication. CLL cells were treated as for panel A for 12 h prior to infection with VSV (1 MOI). (D) Cells lysates were prepared at 12, 24, 48, or 72 h postinfection, and equal amounts of protein (20 μg) were resolved by SDS-PAGE followed by immunoblotting with anti-VSV antisera. Immunoblotting against β-actin is shown as a loading control. (E) Supernatants were collected at 48 h postinfection and virus titers determined by standard plaque assay. Images of the assay plate are also shown. Assays were performed in triplicate.
DISCUSSION
The emergence of VSV as a potential oncolytic virus has made dissection of the molecular determinants of host cell permissiveness to VSV an important objective. Although promising results have been obtained with cell lines and murine cancer models (25, 27, 48), accumulating evidence indicates that certain types of cancer are partially or completely resistant to VSV oncolysis. In a previous study, we demonstrated that CD4+ T lymphocytes from proliferating primary ATL samples were susceptible to VSV oncolysis while resting primary T cells were restricted for VSV replication (17). In this study, we demonstrate that stimulation of primary human CD4+ T lymphocytes with anti-CD3/CD28 allows VSV replication and VSV-induced cell death by triggering the MAPK, PI3K, and mTOR/eIF4E pathways and cell cycle entry (Fig. 8).
FIG. 8.
Schematic diagram illustrating the signaling pathways that impact VSV replication in activated T cells. The point of action of pharmacological inhibitors is indicated. Following CD3/CD28 stimulation, activation of the ERK, JNK, or AKT pathways—leading to G0/G1-phase transition—is crucial for VSV replication in primary lymphocytes due to a global increase in protein translation mediated by the activation of mTOR and eIF4E.
Stimulation of T cells by TCR plus coreceptor engagement leads to the activation of two main signaling pathways: Ras and PI3K. The Ras pathway signals through the MAPKs ERK, JNK, and p38, whereas PI3K activates Akt. It is generally accepted that ERK, JNK, and Akt play a role in cell cycle control, but not p38 (1). Similarly, by using the pharmacological inhibitor U0126, SP600125, LY-294002, or SB203580, we demonstrated that VSV replication in primary T lymphocytes requires activation of MEK1/2, JNK, and PI3K but not p38. These inhibitors had no effect on the production of VSV mRNA but led to a decrease in viral protein levels and the virus titer, indicating that these pathways affect VSV mRNA translation but not virus entry or mRNA production. Since inhibition of any one of the kinases blocked VSV replication, it appears that the activities of MEK1/2, JNK, and PI3K are nonredundant in regard to VSV permissiveness.
VSV viral protein synthesis appears to depend on cell cycle entry, because inhibitors that blocked VSV protein synthesis also resulted in G0/G1 arrest. Indeed, pretreatment of naive T cells with U0126, SP600125, or LY294002 but not SB203580 prior to activation led to arrest in G0/G1, preventing degradation of p27kip1 and induction of CycD3/cdk4. Remarkably, the inhibitors had no effect on VSV replication if added to preactivated T lymphocytes, suggesting that VSV infection is dependent on global activation events and not on the direct action of these kinases or their targets in viral replication. The use of pharmacological inhibitors to specifically block cell cycle progression independently of MAPK or PI3K activity confirmed that entry into the G1 phase but not progression through S or G2/M allowed VSV replication. G0/G1 cell cycle arrest induced by rapamycin or olomoucine treatment abrogated VSV replication, as did the inhibitors of CD3/CD28 signaling. In contrast, robust VSV protein expression was observed in the presence of aphidicolin or Taxol, which induced cell cycle arrest in G1/S or G2/M phase, respectively. Therefore, entry in G1 phase of the cell cycle is sufficient to render normal primary human CD4+ T lymphocytes susceptible to VSV replication.
T cells arrested in G0 phase of the cell cycle have low rates of mRNA and protein synthesis. Phosphorylation of mTOR and the translation initiation factor eIF4E is observed in T cells following CD3/CD28 stimulation, which leads to a surge in protein synthesis that allows cell cycle progression (reviewed in references 49 and 61). Our data demonstrate that the Ras pathway through ERK and the PI3K pathway through Akt regulate translation initiation and/or ribosome biogenesis, since their inhibition by U0126 and LY294002 prevented eIF4E and mTOR phosphorylation (49, 60, 63). Interestingly, JNK is also required for mTOR and eIF4E activation in T lymphocytes, since the presence of the inhibitor SP600125 prevented mTOR phosphorylation as well as eIF4E protein induction and phosphorylation following CD3/CD28 stimulation. This is to our knowledge the first report indicating that in primary T cells, JNK activation leads to mTOR and eIF4E phosphorylation. As expected, p38 inhibition had no effect on mTOR and eIF4E phosphorylation following CD3 and CD28 engagement.
Rapamycin induces G1 cell cycle arrest and blocks mRNA translation by binding to and inhibiting mTOR. This prevents phosphorylation of eIF4-BP1 and therefore blocks the release of the translation initiation factor eIF4E. Rapamycin also inhibits the phosphorylation of ribosomal S6K1, a downstream target of mTOR, further blocking translation initiation of 5′-capped and/or 5′-TOP mRNAs (13, 57, 77). In this study, we demonstrate that rapamycin blocks VSV protein production in primary activated CD4+ T lymphocytes, suggesting that mTOR-mediated translation initiation and ribosome biogenesis during G1 phase are crucial for VSV replication in primary human CD4+ T lymphocytes. In contrast, Connor et al. (22) concluded that rapamycin had no effect on VSV protein synthesis in HeLa cells, although this study did not address the cell cycle status of HeLa cells following rapamycin treatment. The discrepancy might be explained by the fact that the antiproliferative effect of rapamycin is maximal in quiescent cells that express high levels of p27kip1 but not in exponentially growing cells (12, 71). In primary T cells, rapamycin induced a strong G1-phase arrest that was concomitant with inhibition of VSV replication.
eIF4E is a downstream target of mTOR that regulates 5′-cap-dependent translation (33). Considering that VSV mRNA is capped and polyadenylated, the possibility that eIF4E is directly involved in VSV replication in primary activated T cells was explored using siRNA directed against eIF4E. VSV protein synthesis was indeed inhibited in activated T lymphocytes expressing eIF4E siRNA, suggesting that this host translational factor is required for VSV replication. However, treatment of activated CD4+ T lymphocytes with eIF4E siRNA led to cell cycle arrest in G0/G1. Therefore, it was not possible to ascribe a direct role to eIF4E in VSV replication, since it is not clear whether the observed effect is a direct consequence of eIF4E inhibition or an indirect consequence of a delay in cell cycle progression.
The results obtained with primary T cells demonstrate that VSV replication in lymphocytes depends on G0/G1-phase transition. We postulated that resistance to VSV-induced oncolysis observed in ex vivo CLL samples is due to the low proliferative index of CLL cells, which are arrested in G0 phase (67). Indeed, treatment of ex vivo primary CLL samples with PMA-ionomycin to induce G0/G1 transition was sufficient to restore VSV sensitivity: PMA-ionomycin stimulation led to cell cycle entry, as demonstrated by a 13% increase in cells in S+G2/M phase, and this was sufficient to restore VSV replication. Induction of eIF4E phosphorylation was also observed in CLL cells activated with PMA-ionomycin, although—in contrast to primary T-cell samples—total levels of eIF4E remained constant. This and previous reports argue that eIF4E phosphorylation constitutes a limiting step in the induction of translation initiation (3, 11, 19, 49). Induction of apoptosis was also confirmed by increased caspase 3 cleavage compared to results obtained in untreated cells. This result constitutes the first evidence that VSV oncolysis in G0-arrested CLL cells can be augmented by pharmacological agents that stimulate exit from G0/G1.
The current study illustrates that VSV replication in primary lymphocytes is dependent on cell cycle entry and global enhancement of protein synthesis via mTOR and eIF4E activity. The results of this study may provide an incentive to explore other combinatorial approaches to overcome the resistance of different cancers to oncolytic virus therapy.
Supplementary Material
Acknowledgments
We thank Nathalie Grandvaux, Yan Long, Romieu Raphaëlle, Lézin Agnès, Césaire Raymond, and the members of the Molecular Oncology Group, Lady Davis Institute, McGill University, for helpful discussions.
This research was supported by grants from the National Cancer Institute with funds from the Terry Fox Foundation to J.H. and J.B., Canadian Institutes of Health Research. S.O. and P.N. are supported by fellowships from the FRSQ, V.T. is supported by a studentship from NSERC, and J.H. is the recipient of a CIHR Senior Investigator award.
Footnotes
Published ahead of print on 16 April 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Alberola-Ila, J., and G. Hernandez-Hoyos. 2003. The Ras/MAPK cascade and the control of positive selection. Immunol. Rev. 19179-96. [DOI] [PubMed] [Google Scholar]
- 2.Appleman, L. J., A. A. van Puijenbroek, K. M. Shu, L. M. Nadler, and V. A. Boussiotis. 2002. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J. Immunol. 1682729-2736. [DOI] [PubMed] [Google Scholar]
- 3.Averous, J., and C. G. Proud. 2006. When translation meets transformation: the mTOR story. Oncogene 256423-6435. [DOI] [PubMed] [Google Scholar]
- 4.Balachandran, S., and G. N. Barber. 2004. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 551-65. [DOI] [PubMed] [Google Scholar]
- 5.Balachandran, S., and G. N. Barber. 2000. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life 50135-138. [DOI] [PubMed] [Google Scholar]
- 6.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13129-141. [DOI] [PubMed] [Google Scholar]
- 7.Baltzis, D., L. K. Qu, S. Papadopoulou, J. D. Blais, J. C. Bell, N. Sonenberg, and A. E. Koromilas. 2004. Resistance to vesicular stomatitis virus infection requires a functional cross talk between the eukaryotic translation initiation factor 2α kinases PERK and PKR. J. Virol. 7812747-12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber, G. N. 2005. VSV-tumor selective replication and protein translation. Oncogene 247710-7719. [DOI] [PubMed] [Google Scholar]
- 9.Bell, J. C., K. A. Garson, B. D. Lichty, and D. F. Stojdl. 2002. Oncolytic viruses: programmable tumour hunters. Curr. Gene Ther. 2243-254. [DOI] [PubMed] [Google Scholar]
- 10.Bell, J. C., B. Lichty, and D. Stojdl. 2003. Getting oncolytic virus therapies off the ground. Cancer Cell 47-11. [DOI] [PubMed] [Google Scholar]
- 11.Beretta, L. 2004. Translational control in T lymphocytes. Int. Rev. Immunol. 23347-363. [DOI] [PubMed] [Google Scholar]
- 12.Beretta, L., A. C. Gingras, Y. V. Svitkin, M. N. Hall, and N. Sonenberg. 1996. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 15658-664. [PMC free article] [PubMed] [Google Scholar]
- 13.Bilanges, B., R. Argonza-Barrett, M. Kolesnichenko, C. Skinner, M. Nair, M. Chen, and D. Stokoe. 2007. Tuberous sclerosis complex proteins 1 and 2 control serum-dependent translation in a TOP-dependent and -independent manner. Mol. Cell. Biol. 275746-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonni, A., A. Brunet, A. E. West, S. R. Datta, M. A. Takasu, and M. E. Greenberg. 1999. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 2861358-1362. [DOI] [PubMed] [Google Scholar]
- 15.Boyer, D., R. Quintanilla, and K. K. Lee-Fruman. 2008. Regulation of catalytic activity of S6 kinase 2 during cell cycle. Mol. Cell Biochem. 30759-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunz, F., A. Dutriaux, C. Lengauer, T. Waldman, S. Zhou, J. P. Brown, J. M. Sedivy, K. W. Kinzler, and B. Vogelstein. 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 2821497-1501. [DOI] [PubMed] [Google Scholar]
- 17.Cesaire, R., S. Oliere, E. Sharif-Askari, M. Loignon, A. Lezin, S. Olindo, G. Panelatti, M. Kazanji, R. Aloyz, L. Panasci, J. C. Bell, and J. Hiscott. 2006. Oncolytic activity of vesicular stomatitis virus in primary adult T-cell leukemia. Oncogene 25349-358. [DOI] [PubMed] [Google Scholar]
- 18.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 41037-40. [DOI] [PubMed] [Google Scholar]
- 19.Clemens, M. J. 2004. Targets and mechanisms for the regulation of translation in malignant transformation. Oncogene 233180-3188. [DOI] [PubMed] [Google Scholar]
- 20.Cobrinik, D. 2005. Pocket proteins and cell cycle control. Oncogene 242796-2809. [DOI] [PubMed] [Google Scholar]
- 21.Colina, R., M. Costa-Mattioli, R. J. Dowling, M. Jaramillo, L. H. Tai, C. J. Breitbach, Y. Martineau, O. Larsson, L. Rong, Y. V. Svitkin, A. P. Makrigiannis, J. C. Bell, and N. Sonenberg. 2008. Translational control of the innate immune response through IRF-7. Nature 452323-328. [DOI] [PubMed] [Google Scholar]
- 22.Connor, J. H., and D. S. Lyles. 2002. Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1. J. Virol. 7610177-10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danilov, A. V., A. K. Klein, H. J. Lee, D. V. Baez, and B. T. Huber. 2005. Differential control of G0 programme in chronic lymphocytic leukaemia: a novel prognostic factor. Br. J. Haematol. 128472-481. [DOI] [PubMed] [Google Scholar]
- 24.Durbin, R. K., S. E. Mertz, A. E. Koromilas, and J. E. Durbin. 2002. PKR protection against intranasal vesicular stomatitis virus infection is mouse strain dependent. Viral Immunol. 1541-51. [DOI] [PubMed] [Google Scholar]
- 25.Ebert, O., K. Shinozaki, T. G. Huang, M. J. Savontaus, A. Garcia-Sastre, and S. L. Woo. 2003. Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats. Cancer Res. 633605-3611. [PubMed] [Google Scholar]
- 26.Farassati, F., A. D. Yang, and P. W. Lee. 2001. Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat. Cell Biol. 3745-750. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez, M., M. Porosnicu, D. Markovic, and G. N. Barber. 2002. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J. Virol. 76895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fingar, D. C., and J. Blenis. 2004. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 233151-3171. [DOI] [PubMed] [Google Scholar]
- 29.Fingar, D. C., C. J. Richardson, A. R. Tee, L. Cheatham, C. Tsou, and J. Blenis. 2004. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol. Cell. Biol. 24200-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaddy, D. F., and D. S. Lyles. 2005. Vesicular stomatitis viruses expressing wild-type or mutant M proteins activate apoptosis through distinct pathways. J. Virol. 794170-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Morales, P., E. Hernando, E. Carrasco-Garcia, M. P. Menendez-Gutierrez, M. Saceda, and I. Martinez-Lacaci. 2006. Cyclin D3 is down-regulated by rapamycin in HER-2-overexpressing breast cancer cells. Mol. Cancer Ther. 52172-2181. [DOI] [PubMed] [Google Scholar]
- 32.Garcia, Z., A. Kumar, M. Marques, I. Cortes, and A. C. Carrera. 2006. Phosphoinositide 3-kinase controls early and late events in mammalian cell division. EMBO J. 25655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gebauer, F., and M. W. Hentze. 2004. Molecular mechanisms of translational control. Nat. Rev. Mol. Cell Biol. 5827-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gelinas, J. N., J. L. Banko, L. Hou, N. Sonenberg, E. J. Weeber, E. Klann, and P. V. Nguyen. 2007. ERK and mTOR signaling couple beta-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J. Biol. Chem. 28227527-27535. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez, J., T. Harris, G. Childs, and M. B. Prystowsky. 2001. Rapamycin blocks IL-2-driven T cell cycle progression while preserving T cell survival. Blood Cells Mol. Dis. 27572-585. [DOI] [PubMed] [Google Scholar]
- 36.Gray, N., L. Detivaud, C. Doerig, and L. Meijer. 1999. ATP-site directed inhibitors of cyclin-dependent kinases. Curr. Med. Chem. 6859-875. [PubMed] [Google Scholar]
- 37.Hay, N., and N. Sonenberg. 2004. Upstream and downstream of mTOR. Genes Dev. 181926-1945. [DOI] [PubMed] [Google Scholar]
- 38.Heredia, A., C. Davis, A. Amoroso, J. K. Dominique, N. Le, E. Klingebiel, E. Reardon, D. Zella, and R. R. Redfield. 2003. Induction of G1 cycle arrest in T lymphocytes results in increased extracellular levels of beta-chemokines: a strategy to inhibit R5 HIV-1. Proc. Natl. Acad. Sci. USA 1004179-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jordan, M. A., and L. Wilson. 2004. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 4253-265. [DOI] [PubMed] [Google Scholar]
- 40.Kawamata, S., H. Sakaida, T. Hori, M. Maeda, and T. Uchiyama. 1998. The upregulation of p27Kip1 by rapamycin results in G1 arrest in exponentially growing T-cell lines. Blood 91561-569. [PubMed] [Google Scholar]
- 41.Kim, J. A., S. Hong, B. Lee, J. W. Hong, J. Y. Kwak, S. Cho, and C. C. Kim. 2007. The inhibition of T-cells proliferation by mouse mesenchymal stem cells through the induction of p16INK4A-cyclin D1/cdk4 and p21waf1, p27kip1-cyclin E/cdk2 pathways. Cell Immunol. 24516-23. [DOI] [PubMed] [Google Scholar]
- 42.Klein, A., O. Miera, O. Bauer, S. Golfier, and F. Schriever. 2000. Chemosensitivity of B cell chronic lymphocytic leukemia and correlated expression of proteins regulating apoptosis, cell cycle and DNA repair. Leukemia 1440-46. [DOI] [PubMed] [Google Scholar]
- 43.Kopecky, S. A., and D. S. Lyles. 2003. The cell-rounding activity of the vesicular stomatitis virus matrix protein is due to the induction of cell death. J. Virol. 775524-5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korin, Y. D., and J. A. Zack. 1998. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J. Virol. 723161-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korz, C., A. Pscherer, A. Benner, D. Mertens, C. Schaffner, E. Leupolt, H. Dohner, S. Stilgenbauer, and P. Lichter. 2002. Evidence for distinct pathomechanisms in B-cell chronic lymphocytic leukemia and mantle cell lymphoma by quantitative expression analysis of cell cycle and apoptosis-associated genes. Blood 994554-4561. [DOI] [PubMed] [Google Scholar]
- 46.Liang, J., J. Zubovitz, T. Petrocelli, R. Kotchetkov, M. K. Connor, K. Han, J. H. Lee, S. Ciarallo, C. Catzavelos, R. Beniston, E. Franssen, and J. M. Slingerland. 2002. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat. Med. 81153-1160. [DOI] [PubMed] [Google Scholar]
- 47.Lichty, B. D., A. T. Power, D. F. Stojdl, and J. C. Bell. 2004. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol. Med. 10210-216. [DOI] [PubMed] [Google Scholar]
- 48.Lichty, B. D., D. F. Stojdl, R. A. Taylor, L. Miller, I. Frenkel, H. Atkins, and J. C. Bell. 2004. Vesicular stomatitis virus: a potential therapeutic virus for the treatment of hematologic malignancy. Hum. Gene Ther. 15821-831. [DOI] [PubMed] [Google Scholar]
- 49.Mamane, Y., E. Petroulakis, L. Rong, K. Yoshida, L. W. Ler, and N. Sonenberg. 2004. eIF4E—from translation to transformation. Oncogene 233172-3179. [DOI] [PubMed] [Google Scholar]
- 50.Meloche, S., and J. Pouyssegur. 2007. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 263227-3239. [DOI] [PubMed] [Google Scholar]
- 51.Miron, M., J. Verdu, P. E. Lachance, M. J. Birnbaum, P. F. Lasko, and N. Sonenberg. 2001. The translational inhibitor 4E-BP is an effector of PI(3)K/Akt signalling and cell growth in Drosophila. Nat. Cell Biol. 3596-601. [DOI] [PubMed] [Google Scholar]
- 52.Mustelin, T., and K. Tasken. 2003. Positive and negative regulation of T-cell activation through kinases and phosphatases. Biochem. J. 37115-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakhaei, P. P. S., S. Oliere, V. Tumilasci, J. C. Bell, and J. Hiscott. 2005. Oncolytic virotherapy of cancer with vesicular stomatitis virus. Gene Ther. Mol. Biol. 9269-280. [Google Scholar]
- 54.Nethanel, T., and G. Kaufmann. 1990. Two DNA polymerases may be required for synthesis of the lagging DNA strand of simian virus 40. J. Virol. 645912-5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norman, K. L., K. Hirasawa, A. D. Yang, M. A. Shields, and P. W. Lee. 2004. Reovirus oncolysis: the Ras/RalGEF/p38 pathway dictates host cell permissiveness to reovirus infection. Proc. Natl. Acad. Sci. USA 10111099-11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Otake, Y., S. Soundararajan, T. K. Sengupta, E. A. Kio, J. C. Smith, M. Pineda-Roman, R. K. Stuart, E. K. Spicer, and D. J. Fernandes. 2007. Overexpression of nucleolin in chronic lymphocytic leukemia cells induces stabilization of bcl2 mRNA. Blood 1093069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pende, M., S. H. Um, V. Mieulet, M. Sticker, V. L. Goss, J. Mestan, M. Mueller, S. Fumagalli, S. C. Kozma, and G. Thomas. 2004. S6K1−/−/S6K2−/− mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol. Cell. Biol. 243112-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pestova, T. V., and C. U. Hellen. 2001. Functions of eukaryotic factors in initiation of translation. Cold Spring Harb. Symp. Quant. Biol. 66389-396. [DOI] [PubMed] [Google Scholar]
- 59.Pestova, T. V., V. G. Kolupaeva, I. B. Lomakin, E. V. Pilipenko, I. N. Shatsky, V. I. Agol, and C. U. Hellen. 2001. Molecular mechanisms of translation initiation in eukaryotes. Proc. Natl. Acad. Sci. USA 987029-7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petroulakis, E., Y. Mamane, O. Le Bacquer, D. Shahbazian, and N. Sonenberg. 2007. mTOR signaling: implications for cancer and anticancer therapy. Br. J. Cancer 96(Suppl.)R11-R15. [PubMed] [Google Scholar]
- 61.Proud, C. G. 2007. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem. J. 403217-234. [DOI] [PubMed] [Google Scholar]
- 62.Radimerski, T., J. Montagne, F. Rintelen, H. Stocker, J. van der Kaay, C. P. Downes, E. Hafen, and G. Thomas. 2002. dS6K-regulated cell growth is dPKB/dPI(3)K-independent, but requires dPDK1. Nat. Cell Biol. 4251-255. [DOI] [PubMed] [Google Scholar]
- 63.Richter, J. D., and N. Sonenberg. 2005. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 433477-480. [DOI] [PubMed] [Google Scholar]
- 64.Rose, J. H., and M. A. Whitt 2001. Rhabdoviridae: the viruses and their replication, p. 1121-1244. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 65.Ruvinsky, I., and O. Meyuhas. 2006. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem. Sci. 31342-348. [DOI] [PubMed] [Google Scholar]
- 66.Sampath, D., and W. Plunkett. 2000. The role of c-Jun kinase in the apoptotic response to nucleoside analogue-induced DNA damage. Cancer Res. 606408-6415. [PubMed] [Google Scholar]
- 67.Sanchez-Beato, M., A. Sanchez-Aguilera, and M. A. Piris. 2003. Cell cycle deregulation in B-cell lymphomas. Blood 1011220-1235. [DOI] [PubMed] [Google Scholar]
- 68.Scheid, M. P., and J. R. Woodgett. 2001. PKB/AKT: functional insights from genetic models. Nat. Rev. Mol. Cell Biol. 2760-768. [DOI] [PubMed] [Google Scholar]
- 69.Sherr, C. J., and J. M. Roberts. 2004. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 182699-2711. [DOI] [PubMed] [Google Scholar]
- 70.Shin, I., F. M. Yakes, F. Rojo, N. Y. Shin, A. V. Bakin, J. Baselga, and C. L. Arteaga. 2002. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat. Med. 81145-1152. [DOI] [PubMed] [Google Scholar]
- 71.Slavik, J. M., D. G. Lim, S. J. Burakoff, and D. A. Hafler. 2004. Rapamycin-resistant proliferation of CD8+ T cells correlates with p27kip1 down-regulation and bcl-xL induction, and is prevented by an inhibitor of phosphoinositide 3-kinase activity. J. Biol. Chem. 279910-919. [DOI] [PubMed] [Google Scholar]
- 72.Stojdl, D. F., N. Abraham, S. Knowles, R. Marius, A. Brasey, B. D. Lichty, E. G. Brown, N. Sonenberg, and J. C. Bell. 2000. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J. Virol. 749580-9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stojdl, D. F., B. Lichty, S. Knowles, R. Marius, H. Atkins, N. Sonenberg, and J. C. Bell. 2000. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6821-825. [DOI] [PubMed] [Google Scholar]
- 74.Stojdl, D. F., B. D. Lichty, B. R. tenOever, J. M. Paterson, A. T. Power, S. Knowles, R. Marius, J. Reynard, L. Poliquin, H. Atkins, E. G. Brown, R. K. Durbin, J. E. Durbin, J. Hiscott, and J. C. Bell. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4263-275. [DOI] [PubMed] [Google Scholar]
- 75.Svitkin, Y. V., B. Herdy, M. Costa-Mattioli, A. C. Gingras, B. Raught, and N. Sonenberg. 2005. Eukaryotic translation initiation factor 4E availability controls the switch between cap-dependent and internal ribosomal entry site-mediated translation. Mol. Cell. Biol. 2510556-10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang, F., Y. Ma, J. W. Barrett, X. Gao, J. Loh, E. Barton, H. W. Virgin, and G. McFadden. 2004. Disruption of Erk-dependent type I interferon induction breaks the myxoma virus species barrier. Nat. Immunol. 51266-1274. [DOI] [PubMed] [Google Scholar]
- 77.Wang, X., F. E. Paulin, L. E. Campbell, E. Gomez, K. O'Brien, N. Morrice, and C. G. Proud. 2001. Eukaryotic initiation factor 2B: identification of multiple phosphorylation sites in the epsilon-subunit and their functions in vivo. EMBO J. 204349-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang, Q., and K. L. Guan. 2007. Expanding mTOR signaling. Cell Res. 17666-681. [DOI] [PubMed] [Google Scholar]
- 79.Yokosuka, T., K. Sakata-Sogawa, W. Kobayashi, M. Hiroshima, A. Hashimoto-Tane, M. Tokunaga, M. L. Dustin, and T. Saito. 2005. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat. Immunol. 61253-1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.