Abstract
Simian immunodeficiency virus (SIV) persistence in wild populations of African nonhuman primates (NHPs) may occur through horizontal and vertical transmission. However, the mechanism(s) and timing of the latter type of transmission have not been investigated to date. Here we present the first study of SIV transmissibility by breast-feeding in an African NHP host. Six mandrill dames were infected with plasma containing 300 50% tissue culture infective doses of SIVmnd-1 on the day after delivery. All female mandrills became infected, as demonstrated by both plasma viral loads (VLs) and anti-SIVmnd-1 seroconversion. Neither fever nor lymphadenopathy was observed. At the peak of SIVmnd-1 viral replication (days 7 to 10 postinoculation), plasma VLs were high (8 × 106 to 8 × 108 RNA copies/ml) and paralleled the high VLs in milk (4.7 × 104 to 5.6 × 105 RNA/ml). However, at the end of the breast-feeding period, after 6 months of follow-up, no sign of infection was observed for the offspring. Later on, during a 4-year follow-up examination, two of the offspring showed virological evidence of SIVmnd-1 infection. Both animals seroconverted at least 6 months after the interruption of lactation. In conclusion, despite extensive viral replication in mandrill mothers and high levels of free virus in milk, no SIVmnd-1 transmission was detectable at the time of breast-feeding or during the following months. Since we observed a markedly lower expression of CCR5 on the CD4+ T cells of young mandrills and African green monkeys than on those of adults, we propose that low levels of this viral coreceptor on CD4+ T cells may be involved in the lack of breast-feeding transmission in natural hosts of SIVs.
Pathogenic human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infections of humans and macaques are characterized by the invariable progression to AIDS in a variable time frame (25). The hallmarks of this infection are as follows: (i) continuous depletion of CD4+ T cells in peripheral blood (6, 23) and at the mucosal sites (7, 34); (ii) continuous viral replication (26, 51, 68), in which set point viral load (VL) levels are predictive of the progression to AIDS (24, 32, 35-37); and (iii) high levels of immune activation (20, 64), the magnitude of which is also predictive of disease progression (20, 64).
In contrast, natural SIV infection of numerous African nonhuman primate (NHP) hosts, including mandrills, African green monkeys (AGMs), and sooty mangabeys (SMs), usually does not progress to AIDS and is characterized by (i) high prevalences in the wild for most species (1, 4, 29, 50, 52, 57); (ii) an active viral replication, with set point levels similar to or even higher than those reported for pathogenic infection (40, 41, 44, 47-49, 59-61); (iii) a transient depletion of CD4+ T cells in peripheral blood during the primary infection, with a rebound to near preinfection levels during the chronic stage (41, 44, 47, 59); (iv) a significant CD4+ T-cell depletion in the intestine that can be partially restored during the chronic infection in the presence of an important viral replication (21, 46); (iv) low levels of immune activation and apoptosis (8, 13, 22, 40, 41, 44, 46-49, 59, 61); and (v) low levels of CD4+ CCR5+ T cells in blood and tissues (45).
Another major difference between pathogenic and nonpathogenic models of HIV/SIV infections concerns the vertical transmission of the viruses. In HIV-1-infected patients, the risk of HIV-1 perinatal transmission varies widely, ranging from 13 to 48% (15, 19, 69). The timing and mechanisms of transmission have been intensively studied, with somewhat discrepant results, possibly due to the largely correlative nature of these studies. A substantial proportion of infants acquire HIV infection during the peripartum period (5, 11, 30, 31, 56). Breast-feeding is independently associated with a doubling of transmission risk (33, 65), and this risk appears to be related to the maternal levels of plasma VLs (17). This finding is particularly significant, as it is estimated that in developing countries, 95% of babies are initially breastfed and most children continue to receive some breast-feeding until 6 months of age (39). The mechanisms regulating HIV-1 transmission through breast-feeding are not yet fully understood; factors such as colostrum intake, feeding practices, nipple pathology, and the presence of mastitis have been proposed, but their roles in virus transmission require further analysis (19). If the mother seroconverts during postpartum, the transmission rates are particularly high (29%), probably due to high levels of HIV in the breast milk during the primary infection (12). Recent studies in SIV-infected macaques have demonstrated the importance of viral dynamics for breast-feeding transmission during early primary HIV/SIV infections (2, 3).
In African NHPs that are natural hosts of SIV, however, the vertical transmission of the virus appears to be negligible. This conclusion is supported by two lines of evidence: the incidence of SIV infection in captive and wild monkeys appears to dramatically increase after the onset of sexual maturity (29, 52); and in captive SMs and AGMs, there was no evidence for vertical transmission during prospective studies (16, 43). However, sequence analyses of wild SMs and captive chimpanzees recently suggested that mother-to-offspring transmission may occur in some rare cases (4, 10, 57).
Longitudinal studies of SIV transmission in a semifree colony of mandrills in Franceville, Gabon, revealed some intriguing features. First, in this colony, no sexual transmission was found after 16 years of follow-up (9, 14, 18, 38), which is different from what has been seen with wild mandrills from central Gabon, where cases of SIVmnd-1 infection could be diagnosed in both sexes (63). Two of the founders of the CIRMF colony had been infected with two different virus types (SIVmnd-1 and SIVmnd-2) (63, 66). Interestingly, two of the dominant males in the colony became infected with SIVmnd-2, with no observable evidence of sexual transmission of this virus type. Phylogenetic analyses showed that the virus infecting the alpha males was transmissible, since SIVmnd-2 had been transmitted from the infected founder male to four other males, following aggressive contacts for dominance (38, 63). The other SIV-infected founder was a female naturally infected with an SIVmnd-1 strain that was the source of SIVmnd-1 princeps isolate (strain GB1) (66, 67). Over a follow-up period of 20 years, SIVmnd-1 GB1 had been transmitted to 4/10 offspring (males and females) of the infected female founder. Moreover, one of the female offspring of the founder females also transmitted SIVmnd-1 to 2/8 (male and female) of her offspring. Altogether, these data suggest that, unlike the findings previously reported for other African NHP hosts of SIV infections, for which vertical transmission does not appear to account for a significant number of cases (16, 43), vertical transmission may account for some cases of SIVmnd-1 infection in mandrills.
In order to determine if mother-to-offspring transmission of SIVmnd-1 can occur by breast-feeding in mandrills, we performed experimental SIVmnd-1 infections of female mandrills on the day after delivery to facilitate SIVmnd-1 transmission through breast-feeding. In spite of massive viral replication during the primary infection and the detection of high levels of SIVmnd-1 RNA in milk, none of the offspring showed any sign of viral replication after 6 months of follow-up, which corresponds to the lactation period in mandrills.
MATERIALS AND METHODS
Animals and infections.
In order to investigate the possibility of SIVmnd-1 transmission through breast-feeding, six pregnant female mandrills (5I, 12A7, 5J, 12A3, 16C, and 6B) were included in this study. SIV and simian T-lymphotropic virus seronegativity prior to inoculation was demonstrated by Western blotting (New Lav Blot II; Diagnostics Pasteur, Marne-la-Coquette, France; and human T-lymphocyte virus [HTLV] Blot 2.3; Genelabs Diagnostic). All animals were young adults between the ages of 6 and 8 years when the protocol began. Housing and handling were carried out in accordance with Gabonese national guidelines and institutional policies. The day after the delivery, all dames were inoculated with SIVmnd-1. Animals were anesthetized with 10 mg/ml ketamine-HCl and were inoculated intravenously with 1 ml of plasma from an acutely SIVmnd-1-infected mandrill, which was adjusted to 300 50% tissue culture infective doses. Stock preparation has been described previously (40).
Offspring were females (12A7A, 5J1, and 10A1D) and males (5I1, 12A3D, and 16C4). They were monitored monthly by serology and PCR during the follow-up and every 3 months after the completion of this study (at day 180 postinoculation). As dames were infected after the delivery and therefore there was no passive transmission of anti-SIVmnd-1 antibodies from dames to offspring, serology could be used as a diagnostic tool in infant mandrills.
To study CCR5 expression on CD4+ T cells, we included six uninfected infant mandrills from the CIRMF colony in addition to the six infant mandrills from this study. A comparison was done with 23 uninfected adult mandrills from the colony. In addition, samples from uninfected AGMs housed at the Tulane National Primate Research Center were also tested for CCR5 expression on CD4+ T cells: 12 AGMs were less than 4 years old, and 18 AGMs were over 4 years old.
Specimen collection.
Whole blood (7 to 10 ml) was collected in EDTA-K2 tubes from dames before they were infected (at days −30, −15, and 0) and then weekly throughout the first month postinfection (p.i.; days 7, 10, 14, 21, and 28) and monthly for up to 2 months p.i. (at days 28, 32, and 60 p.i.) and then every 2 months (days 120 and 180 p.i.). The same sampling schedule was followed for the offspring, but the volumes were significantly lower (1 to 2 ml). Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque gradient. Fresh PBMC were used for flow cytometry staining. Plasma aliquots were stored at −70°C.
Milk was collected from dames at days 7, 10, 14, 21, 28, and 180 for VL quantification. The aqueous supernatant fraction of breast milk was prepared by centrifugation of freshly expressed milk at 710 × g for 20 min. The lipid layer was removed and discarded. The remaining supernatant was stored at −80°C until used. Prior to RNA extraction, the breast milk supernatant was centrifuged at 10,000 × g for 5 min to remove any remaining cells.
Plasma VL quantification.
SIVmnd-1 RNA was extracted from 560 μl of plasma and 280 to 560 μl of breast milk supernatant, using a QIAamp viral RNA mini-kit (Qiagen, Valencia, CA). RNA quantification was performed by a limiting dilution reverse transcription (RT)-PCR assay specific for SIVmnd-1, which was described previously (40), using primers MP5 and MP6, allowing amplification of a 476-bp region within the SIVmnd-1 GB1 pol gene, as previously described (38). Since the virus used in this study was derived from the SIVmnd-1 GB1 strain, the possibility of mismatches is minimal. SIVmnd-1 RNAs extracted from plasma and milk were subjected to VL quantification using seven 10-fold serial dilutions and RT-PCR (one-step RT-PCR kit; Qiagen, Courtaboeuf, France). RT was performed at 50°C for 30 min in the presence of the primers MP5 and MP6 (38), followed by 47 cycles of 95°C for 15 s, 55°C for 45 s, and 72°C for 1 min, with a final extension of 7 min at 72°C, as previously described (40). Sensitivity of the limiting dilution RT-PCR assay was 500 copies of SIVmnd-1 per ml.
Nested PCR for SIVmnd-1.
In addition to semiquantitative PCR, samples collected from offspring were also tested by nested PCR in order to confirm the lack of infection. DNA was extracted from the PBMCs of the offspring, collected at all the time points using a QIAamp kit (Qiagen, Valencia, CA). RNA was extracted from 280 to 560 μl plasma, as described above. Nested PCR was then performed with both DNA and RNA. A 451-bp pol fragment of SIVmnd-1 was obtained by a nested PCR protocol, using primers MP2/MP3 and MP5/MP6, as described previously (38).
Antibody detection.
The dynamics of anti-SIVmnd-1 GB1 antibodies were monitored by an HIV-1/HIV-2 enzyme-linked immunosorbent assay (ELISA) (Genelavia Mixt, Sanofi-Diagnostics Pasteur) and confirmed by a primate immunodeficiency virus (PIV)-specific enzyme immunoassay (PIV-EIA) designed to detect antibodies directed toward the immunodominant region of the gp36 protein and the highly variable region of the V3 loop of SIVmnd-1 GB1 (62). Reactivity was also confirmed by Western blotting (New Lav Blot, Sanofi Diagnostics Pasteur). Antibody titers to both gp41 and V3 peptide were measured by using a PIV-enzyme-linked immunosorbent assay, as previously described (40, 62). Twofold serial dilutions from 1/100 to 1/12,800 and from 1/100 to 1/43,200 were done for each sample tested against the gp36 and V3 peptide, respectively. The cutoff was established at 0.1.
Flow cytometry analysis.
EDTA-treated whole blood (100 ml) was stained for flow cytometry analysis employing a whole-blood lysis technique, as previously described (46), using staining combinations with monoclonal antibodies (MAbs): CD3 (clone SP34)-fluorescein isothiocyanate (FITC) or CD3-peridinin chlorophyll A protein (PerCP), CD20-phycoerythrin (PE), CD8 (clone Leu2a)-PerCP or CD8-PE, CD4-allophycocyanin (APC) or CD4 (clone L200)-PerCP, HLA-DR (clone L243)-PerCP, CD28 (clone Leu28)-APC or CD28-PE, CCR5 (clone 3A9)-PE, Ki-67 (clone B56)-FITC (BD Biosciences Pharmingen, San Diego, CA). Cells were incubated with an excess amount of MAbs at 4°C for 30 min, followed by a phosphate-buffered saline wash, centrifugation (400 × g, 7 min), and fixation in 2% paraformaldehyde. CD4+ and CD8+ T-cell percentages were obtained by first gating on lymphocytes and then on CD3+ T cells. Memory, activation, proliferation, and apoptosis markers were determined by gating on lymphocytes and then on CD3+ T cells and finally on CD4+ CD3+ or CD8+ CD3+ T cells. Flow cytometric acquisition and analysis of samples were performed with at least 50,000 acquired events, gated on lymphocytes, on a FACScalibur flow cytometer driven by CellQuest software (BD-Pharmingen). Data analysis was performed using Cell Quest software (Immunocytometry System, Becton Dickinson).
Statistical analysis and data presentation.
The flow cytometry parameters of the groups were analyzed for significant differences (P < 0.05) by using the Mann-Whitney test. Correlations were calculated and expressed as the Spearman coefficient of correlation.
RESULTS
SIVmnd-1 infection in female mandrills.
In this study, the animals were followed until day 180 p.i., which corresponds to the lactation period in mandrills. After dames were inoculated with SIVmnd-1 GB1, they all became infected. None of them developed fever, weight loss, lymphadenopathy, or opportunistic infections.
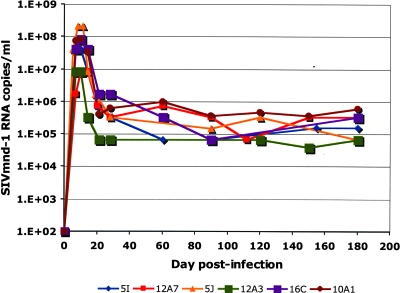
The dynamics of primary SIVmnd-1 infection observed with the dames were very similar to the dynamics of primary SIVmnd-1 infection reported in our previous study (40). A peak of plasma viral RNA was apparent at day 7 or 10 and ranged from 8 × 106 to 108 copies per ml of plasma (Fig. 1), with a rapid post-peak decline of 100- to 1,000-fold and the establishment of a set point for all animals between days 28 and 60 p.i. (Fig. 1). No significant variations in plasma VLs were noted during the chronic phase. Thus, plasma VLs remained around 5 × 104 to 6 × 105 RNA copies/ml in all animals between day 60 and 180 p.i., with slight variations (Fig. 1). These VL levels observed during the chronic phase of experimental infection are similar to those observed in naturally SIVmnd-1-infected mandrills in the wild (48).
FIG. 1.
Viral RNA copy numbers in plasma of SIVmnd-1-infected mandrill dames. The detection limit of the assay was 5 × 102 copies/ml.
As expected, all animals infected with SIVmnd-1 showed detectable anti-SIV antibodies starting from days 28 and 32 p.i. and maintained a sustained antibody response (data not shown). Similar patterns of reactivity (the appearance of anti-p26 at day 28 p.i. and the appearance of anti-gp105 by day 32 p.i.) were also observed by using the HIV-2 Western blot antibody detection assay (data not shown). The reactivity against p26 was similar to the anti-gp105 reactivity in this assay. In order to test reactivity directed specifically against SIVmnd antigens, we used the PIV-EIA (62). Antibodies directed against gp36 of SIVmnd-1 were detected from day 28 to 32 on (data not shown), while anti-SIVmnd-1 V3 antibodies were detected starting from day 32 (data not shown).
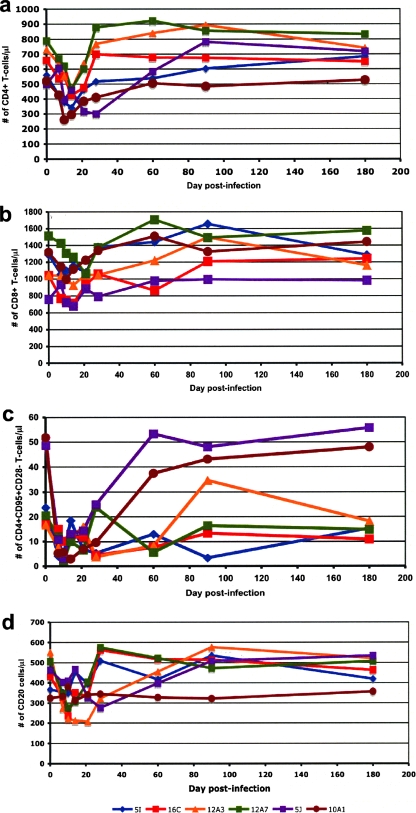
Quantitative changes in major lymphocyte subsets were evaluated over time in SIVmnd-1-infected dames. Similar to the results from our previous report (40), SIVmnd-1 replication during acute infection induced a moderate but statistically significant (P < 0.05) peripheral CD4+ T-cell depletion. An average of 35% CD4+ T-cell depletion was recorded after the peak of viral replication (day 14 p.i. average CD4+ T cells, 405 ± 30 cells/μl; range, 286 to 464 CD4+ T cells/μl) compared to baseline (day zero average CD4+ T cells, 626 ± 49 cells/μl; range, 497 to 789 CD4+ T cells/μl) (Fig. 2a). CD4+ T cells were then rapidly restored, returning to baseline levels by day 60 p.i. (average, 677 ± 70 CD4+ T cells/μl; range, 505 to 920 CD4+ T cells/μl). While all CD4+ T-cell subsets were somewhat depleted during acute infection (data not shown), significant depletion (P < 0.0005) occurred only in the effector memory CD4+ T-cell subset (defined as CD4+ CD28− T cells) (Fig. 2b). At the peak of viral replication, the average effector memory CD4+ T cells were 5 ± 1.15 cells/μl (range, 2 to 10 cells/μl), which corresponds to more than 80% depletion compared to baseline (average, 30 ± 7 cells/μl; range, 17 to 52 cells/μl) (Fig. 2b). During the chronic infection stage, the restoration of peripheral effector memory CD4+ T cells was observed. Thus, at day 180 p.i., the effector memory CD4+ T cells were at the baseline levels (average, 27 ± 8 cells/μl; range, 11 to 56 cells/μl) (Fig. 2b). No significant changes were observed for CD8+ T cells (Fig. 2c) and for CD20 cells (Fig. 2d).
FIG. 2.
Kinetics of major lymphocyte populations and subsets (absolute counts) in peripheral blood from SIVmnd-1-infected mandrill dames. SIVmnd-1 infection induced significant depletion at the peak of viral replication of CD4+ T cells (a). During chronic infection, the quantity of CD4+ T cells rebounded to near preinfection values (a). At the peak of viral replication, the effector memory CD4+ T cells (defined as CD4+ CD28−) were significantly depleted in peripheral blood (b). No significant changes were observed for the kinetics of CD8+ T cells (c) and CD20 cells (d).
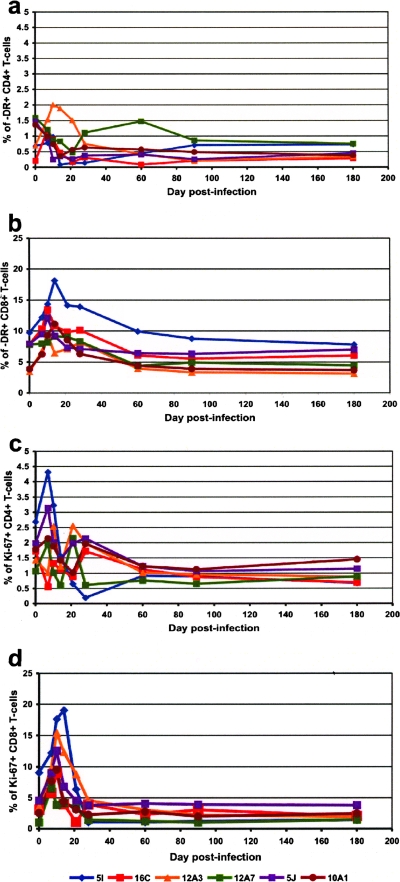
During our previous study of primary SIVmnd-1 infection, only a limited immunophenotypic analysis was conducted (40); therefore, here we measured some immune activation parameters. We found that high viral replication during acute SIVmnd-1 infection did not induce any significant increase in the immune activation of CD4+ T cells but was associated with a transient but significant (P < 0.05) increase in the activation status of CD8+ T cells, as demonstrated by the dynamics of CD4+ DR+ (Fig. 3a) and CD8+ DR+ T cells (Fig. 3b). Similarly, a study of T-cell proliferation dynamics did not show a significant increase in the proliferation of peripheral CD4+ T cells and showed only a transient increase in CD8+ T-cell proliferation, as illustrated by the dynamics of CD4+ Ki-67+ (Fig. 3c) and CD8+ Ki-67+ cells (Fig. 3d).
FIG. 3.
Kinetic expression immune activation, expressed as the percentage of HLA-DR MAbs upon CD4+ (a) and CD8+ T lymphocytes (b) and of cell proliferation, expressed as the percentage of Ki-67 upon CD4+ (c) and CD8+ T lymphocytes (d) in peripheral blood of SIVmnd-1-infected mandrill dames.
High levels of SIVmnd-1 RNA in breast milk during primary infection of dames.
A limited number of samples were available for this analysis, but we collected at least one milk sample from each female mandrill at around the peak of viral replication (days 7 to 14 p.i.). SIVmnd-1 RNA VL in milk ranged from 3 × 104 copies (mandrill dame 16C) to 4 × 105 copies (mandrill dame 12A7) (Table 1). However, during the chronic phase of SIVmnd-1 infection, VL in milk was below the detection limit (Table 1). We therefore demonstrated that high levels of cell-free SIVmnd-1 were present in the breast milk from acutely infected female mandrills and that the mandrill offspring were exposed to significant amounts of SIVmnd-1.
TABLE 1.
Dynamics of SIVmnd-1 RNA in milk in female mandrills
| Day p.i. | SIVmnd-1 RNA (copies/ml) in the milk of female mandrill subjects
|
|||||
|---|---|---|---|---|---|---|
| 5I | 12A7 | 5J | 12A3 | 16C | 10A1 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 300,00a | 30,000 | ||||
| 10 | 75,000 | 375,000 | 75,000 | 75,000 | 47,000 | 234,000 |
| 14 | 46,875 | 75,000 | 75,000 | |||
| 21 | <500 | <500 | ||||
| 28 | <500 | <500 | <500 | <500 | ||
| 180 | <500 | <500 | <500 | <500 | <500 | <500 |
Value was determined by semiquantitative (qc) RT-PCR. The limit of detection of the qc RT-PCR was 500 copies/ml of milk.
Lack of SIVmnd-1 transmission through breast-feeding.
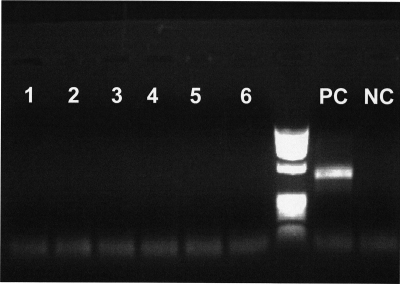
No evidence of early SIVmnd-1 breast-feeding transmission from acutely infected mandrill females to offspring was observed during the first 6 months of the study, which roughly corresponds to the lactation period in mandrills, despite the high levels of SIVmnd-1 in milk and the prolonged exposure via breast-feeding. Both serology (data not shown) and nested RT-PCRs with plasma and nested DNA-PCR with PBMCs using different sets of primers failed to provide any evidence of SIVmnd-1 in the offspring (Fig. 4). Therefore, we concluded that even in the context of high maternal VLs in both plasma and milk, the risk of SIVmnd-1 transmission through breast-feeding is very small in SIVmnd-1-infected mandrills. At the end of the study, the whole group of female and babies were mixed in a large cage for socialization, and then, 6 months later, both females and offspring were released into a semifree enclosure with SIVmnd-1- and SIVmnd-2-infected mandrills. At the time of release, one of the offspring (female 10A1D) seroconverted for SIVmnd-1. During the second year of follow-up, a second juvenile mandrill (female 12A7A) seroconverted. These two seroconversions might have occurred as a result of offspring being subjected to aggressive behavior from infected females (Jean Wickings, personal communication). Due to the sampling design, very limited information about the dynamics of infection in these two offspring is available.
FIG. 4.
SIVmnd-1 pol nested PCR results for offspring of experimentally infected mandrill dames at the end of the lactation period (day 180 p.i.). None of the nested PCRs from offspring samples was positive (lanes 1 to 6). Positive (PC) and negative (NC) controls of the PCR are also shown.
Lack of CCR5 expression on CD4+ T cells may represent a potential mechanism behind the lack of breast-feeding transmission during SIVmnd-1 primary infection.
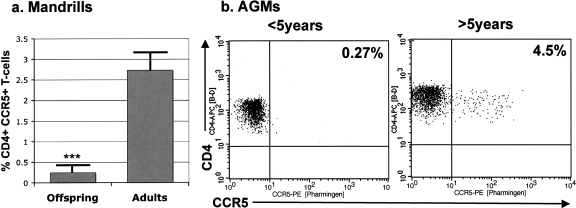
Previous studies by our group reported a paucity of CCR5+ CD4+ T cells in natural NHP hosts of SIV (45). Therefore, we determined if low levels of CCR5+ CD4+ T cells in offspring might be associated with this lack of vertical transmission in natural hosts. We compared the levels of CCR5 expression on CD4+ T cells among uninfected young and adult mandrills and AGMs. As shown in Fig. 5, we found a significantly lower expression of CCR5 on the CD4+ T cells of suckling mandrills and young AGMs than on those of adults (Fig. 5a). Thus, the CCR5 expression on CD4+ T cells was significantly lower in suckling mandrills (0.249% ± 0.13%) than in adult animals (2.73% ± 0.31%; P < 0.0005). During the breast-feeding period, CCR5 levels in the two offspring that were infected after the completion of this study were in the same ranges as those of uninfected offspring (data not shown). In order to confirm that this lower CCR5 expression on CD4+ T cells of young African NHPs is a general feature of natural hosts of SIVs, we performed this analysis with another host that is naturally infected with SIV, the AGMs. Again, the CD4+ T cells from adult AGMs had higher expression levels of CCR5 (4.9% ± 2%) than those of younger AGMs (1.7% ± 0.8%; P < 0.01) (Fig. 5b). Conversely, although in newborn macaques the levels of CCR5 expression on CD4+ T cells is very low, by 6 months of age, there are no significant differences in CCR5 expression on CD4+ T cells between the infant and adult macaques (Ronald Veazey, personal communication). It should be noted that the CCR5+ CD4+ T-cell levels in adult NHP African hosts of SIVs are already low compared to that of species that are progressing to AIDS, such as rhesus macaques (45). We therefore suggest that this low expression of target cells might be responsible for the observed lack of breast-feeding transmission of SIV in natural hosts.
FIG. 5.
CCR5 expression on CD4+ T cells of uninfected baby (n = 12; aged >6 months) versus adult (n = 23) mandrills (a) and representative flow cytometry plots of CCR5 expression on CD4+ T cells in a young AGM versus that in an adult AGM (b). Significantly lower CCR5 expression was observed with young versus adult animals. Note that CCR5 expression is already low in African NHPs compared to that in rhesus macaques or in humans (45).
DISCUSSION
In this study we report that in SIVmnd-1-infected mandrills, there is no evidence of breast-feeding transmission. Our experimental results are in agreement with previous epidemiological data reporting a lack of vertical transmission of SIVs in their natural African NHP hosts (16, 42). We designed our study to maximize the opportunity of mother-to-offspring transmission of SIVmnd-1 by inoculating the dames after the delivery and observing the effects of high levels of virus replication (in both plasma and milk) on the risk of transmission via breast-feeding. As such, our experimental system created the worst-case scenario, with ample opportunities for transmission. However, at the end of lactation period, we did not observe SIV infection in any of the offspring of dames included in this study. This result is surprising for two reasons. First, by quantifying the SIVmnd-1 RNA loads in milk, we have shown that high amounts of replicative virus were present in milk concomitantly with the primary infection. Second, VL levels during the chronic infection were also of orders of magnitude higher than in HIV-1-infected patients, in whom breast-feeding transmission occurred in a high proportion of cases. Thus, in HIV-1-infected patients, the rates of breast-feeding transmission are related to the VLs, with transmission rates particularly high (29%), when primary infection occurs during the lactation period (12).
Due to the very low amounts of collected milk, we were unable to quantify the amounts of cell-associated SIVmnd-1 provirus in milk. The same limiting factor prevented us from testing whether or not the virus present in milk (and quantified in this study) was infectious. This is a major limitation of our study, since previous studies reported that there is a direct correlation between the amount of cell-associated virus and the rates of breast-feeding transmission in humans (28, 54, 55).
Why these high levels of SIV replication in natural African NHP hosts do not result in breast-feeding transmission is not known. However, one of the potential explanations relies on the low number of target cells observed with these species. We have recently shown that natural hosts for SIV infection express remarkably low levels of CCR5 on CD4+ T cells isolated from blood, lymph nodes, and mucosal tissues (45). As this immunological feature is found in all of the African natural NHP hosts of SIV but absent in four nonnatural/recent hosts (i.e., humans, macaques, and baboons), we proposed that the low levels of CD4+ CCR5+ T cells in natural SIV hosts represent a key feature of the coevolution between the virus and its natural hosts that led to a nonpathogenic infection (45). In our original publication, we concluded that beneficial effects of low CCR5 expression on CD4+ T cells might include the reduction of target cells for viral replication and/or a decreased homing of activated CD4+ T cells to the mucosal tissue. Our present data suggest that the reduced number of target cells may also help to prevent the breast-feeding transmission and possibly mucosal transmission in general. This conclusion is supported by observations with another African NHP species, the African green monkeys, in which there is a statistically significant lower expression of CCR5 on CD4+ T cells in juvenile monkeys than in adults (Fig. 5b). In AGMs, there is a significant increase in CCR5+ CD4+ T-cell expression at sexual maturity, which is strongly associated with increases in SIVagm prevalence (29, 52). In humans and rhesus macaques, the percentage of CD4+ CCR5+ cells is also very low in cord blood and infants but increases significantly with age, to levels comparable to those observed with adults by the end of the lactation period (58) (R. Veazey, personal communication). This observation may explain recent studies of macaques in which breast-feeding transmission occurred late during the lactation period (27), in agreement with the observation that human cord blood cells are preferentially infected by R5 isolates (53). We concluded that this low expression of target cells might be responsible for the observed lack of breast-feeding transmission in mandrills.
Our previous report of primary SIVmnd-1 infection investigated only the dynamics of major T-cell populations (40). Since recent data suggested that one of the most significant differences between pathogenic and nonpathogenic lentiviral infections in humans and NHPs may depend on the levels of immune activation (41, 44, 48, 60, 61), in the current study, we extended our previous data on the dynamics of primary SIVmnd-1 infection in mandrills (40). We confirmed that SIVmnd-1 replicated at high levels during the chronic phase of infection in all infected dames and that the active viral replication does not result in a chronic loss of CD4+ T cells. Moreover, here we have extended the previous data and showed that chronic SIVmnd-1 infection is characterized by a limited immune activation and T-cell proliferation in peripheral blood.
In conclusion, our study shows that in spite of the presence of large amounts of SIVmnd-1 RNA in milk and therefore of a large exposure of offspring to SIVmnd-1, no transmission was detected in mandrills during the breast-feeding. As we observed very low levels of CCR5+ CD4+ T cells that are maintained throughout the lactation period in young African NHPs, we propose that this immunological feature may represent a factor that protects these animals from breast-feeding transmission of SIV. In this regard, the current work supports the idea that previously described low levels of CCR5+ CD4+ T cells in natural hosts of SIV may represent a major host adaptation to prevent vertical transmission, in spite of high prevalence levels of SIV infection in the wild. These results are highly significant for the control of mother-to-infant transmission of HIV, pointing to a major prevention approach of one of the main mechanisms of HIV transmission.
Acknowledgments
We thank Mary Barnes for critical reading of the manuscript and Jean K. Wickings for information on mandrill behavior.
CIRMF is funded by the Gabonese government and French cooperation.
This study was funded by an Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS) grant to F.S. and P.R. and by grants RO1 AI064066 and R21AI069935 (to I.P.) and RO1 AI065325 (to C.A.) from the National Institutes of Health.
Footnotes
Published ahead of print on 2 April 2008.
REFERENCES
- 1.Aghokeng, A. F., W. Liu, F. Bibollet-Ruche, S. Loul, E. Mpoudi-Ngole, C. Laurent, J. M. Mwenda, D. K. Langat, G. K. Chege, H. M. McClure, E. Delaporte, G. M. Shaw, B. H. Hahn, and M. Peeters. 2006. Widely varying SIV prevalence rates in naturally infected primate species from Cameroon. Virology 345174-189. [DOI] [PubMed] [Google Scholar]
- 2.Amedee, A. M., N. Lacour, and M. Ratterree. 2003. Mother-to-infant transmission of SIV via breast-feeding in rhesus macaques. J. Med. Primatol. 32187-193. [DOI] [PubMed] [Google Scholar]
- 3.Amedee, A. M., J. Rychert, N. Lacour, L. Fresh, and M. Ratterree. 2004. Viral and immunological factors associated with breast milk transmission of SIV in rhesus macaques. Retrovirology 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apetrei, C., M. J. Metzger, D. Robinson, B. Ling, P. T. Telfer, P. Reed, D. L. Robertson, and P. A. Marx. 2005. Detection and partial characterization of new simian immunodeficiency virus (SIVsm) strains from bush meat samples from rural Sierra Leone. J. Virol. 792631-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertolli, J., M. E. St. Louis, R. J. Simonds, P. Nieburg, M. Kamenga, C. Brown, M. Tarande, T. Quinn, and C. Y. Ou. 1996. Estimating the timing of mother-to-child transmission of human immunodeficiency virus in a breast-feeding population in Kinshasa, Zaire. J. Infect. Dis. 174722-726. [DOI] [PubMed] [Google Scholar]
- 6.Brenchley, J. M., D. A. Price, and D. C. Douek. 2006. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 7235-239. [DOI] [PubMed] [Google Scholar]
- 7.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakrabarti, L. A., S. R. Lewin, L. Zhang, A. Gettie, A. Luckay, L. N. Martin, E. Skulsky, D. D. Ho, C. Cheng-Mayer, and P. A. Marx. 2000. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J. Virol. 741209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper, R., A. Feistner, S. Evans, H. Tsujimoto, and M. Hayami. 1989. A lack of evidence of sexual transmission of a simian immunodeficiency agent in a semifree-ranging group of mandrills. AIDS 3764. [PubMed] [Google Scholar]
- 10.Corbet, S., M. C. Muller-Trutwin, P. Versmisse, S. Delarue, A. Ayouba, J. Lewis, S. Brunak, P. Martin, F. Brun-Vezinet, F. Simon, F. Barre-Sinoussi, and P. Mauclere. 2000. env sequences of simian immunodeficiency viruses from chimpanzees in Cameroon are strongly related to those of human immunodeficiency virus group N from the same geographic area. J. Virol. 74529-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn, D. T., C. D. Brandt, A. Krivine, S. A. Cassol, P. Roques, W. Borkowsky, A. De Rossi, E. Denamur, A. Ehrnst, and C. Loveday. 1995. The sensitivity of HIV-1 DNA polymerase chain reaction in the neonatal period and the relative contributions of intra-uterine and intra-partum transmission. AIDS 9F7-11. [DOI] [PubMed] [Google Scholar]
- 12.Dunn, D. T., M. L. Newell, A. E. Ades, and C. S. Peckham. 1992. Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet 340585-588. [DOI] [PubMed] [Google Scholar]
- 13.Estaquier, J., T. Idziorek, F. de Bels, F. Barre-Sinoussi, B. Hurtrel, A. M. Aubertin, A. Venet, M. Mehtali, E. Muchmore, P. Michel, et al. 1994. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc. Natl. Acad. Sci. USA 919431-9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estaquier, J., M. Peeters, L. Bedjabaga, C. Honore, P. Bussi, A. Dixson, and E. Delaporte. 1991. Prevalence and transmission of simian immunodeficiency virus and simian T-cell leukemia virus in a semi-free-range breeding colony of mandrills in Gabon. AIDS 51385-1386. [DOI] [PubMed] [Google Scholar]
- 15.European Collaborative Study. 1991. Children born to women with HIV-1 infection: natural history and risk of transmission. Lancet 337253-260. [PubMed] [Google Scholar]
- 16.Fultz, P. N., T. P. Gordon, D. C. Anderson, and H. M. McClure. 1990. Prevalence of natural infection with simian immunodeficiency virus and simian T-cell leukemia virus type I in a breeding colony of sooty mangabey monkeys. AIDS 4619-625. [DOI] [PubMed] [Google Scholar]
- 17.Garcia, P. M., L. A. Kalish, J. Pitt, H. Minkoff, T. C. Quinn, S. K. Burchett, J. Kornegay, B. Jackson, J. Moye, C. Hanson, C. Zorrilla, and J. F. Lew for the Women and Infants Transmission Study Group. 1999. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N. Engl. J. Med. 341394-402. [DOI] [PubMed] [Google Scholar]
- 18.Georges-Courbot, M. C., P. Moisson, E. Leroy, A. M. Pingard, E. Nerrienet, G. Dubreuil, E. J. Wickings, F. Debels, I. Bedjabaga, V. Poaty-Mavoungou, N. T. Hahn, and A. J. Georges. 1996. Occurrence and frequency of transmission of naturally occurring simian retroviral infections (SIV, STLV, and SRV) at the CIRMF Primate Center, Gabon. J. Med. Primatol. 25313-326. [DOI] [PubMed] [Google Scholar]
- 19.Gibb, D. M., and B. H. Tess. 1999. Interventions to reduce mother-to-child transmission of HIV infection: new developments and current controversies. AIDS 13(Suppl. A)S93-S102. [PubMed] [Google Scholar]
- 20.Giorgi, J. V., L. E. Hultin, J. A. McKeating, T. D. Johnson, B. Owens, L. P. Jacobson, R. Shih, J. Lewis, D. J. Wiley, J. P. Phair, S. M. Wolinsky, and R. Detels. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179859-870. [DOI] [PubMed] [Google Scholar]
- 21.Gordon, S., N. R. Klatt, J. M. Milush, J. Engram, R. M. Dunham, M. Paiardini, E. A. Strobert, C. Apetrei, I. Pandrea, S. Staprans, D. L. Sodora, and G. Silvestri. 2007. Severe depletion of mucosal CD4+ T cells in AIDS-free SIV-infected sooty mangabeys. J. Immunol. 1793026-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gougeon, M. L., S. Garcia, J. Heeney, R. Tschopp, H. Lecoeur, D. Guetard, V. Rame, C. Dauguet, and L. Montagnier. 1993. Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res. Hum. Retrovir. 9553-563. [DOI] [PubMed] [Google Scholar]
- 23.Grossman, Z., M. Meier-Schellersheim, W. E. Paul, and L. J. Picker. 2006. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 12289-295. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch, V. M., T. R. Fuerst, G. Sutter, M. W. Carroll, L. C. Yang, S. Goldstein, M. Piatak, Jr., W. R. Elkins, W. G. Alvord, D. C. Montefiori, B. Moss, and J. D. Lifson. 1996. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 703741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch, V. M., and P. R. Johnson. 1994. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 32183-203. [DOI] [PubMed] [Google Scholar]
- 26.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373123-126. [DOI] [PubMed] [Google Scholar]
- 27.Jayaraman, P., and N. L. Haigwood. 2006. Animal models for perinatal transmission of HIV-1. Front. Biosci. 112828-2844. [DOI] [PubMed] [Google Scholar]
- 28.John, G. C., R. W. Nduati, D. A. Mbori-Ngacha, B. A. Richardson, D. Panteleeff, A. Mwatha, J. Overbaugh, J. Bwayo, J. O. Ndinya-Achola, and J. K. Kreiss. 2001. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J. Infect. Dis. 183206-212. [DOI] [PubMed] [Google Scholar]
- 29.Jolly, C., J. E. Phillips-Conroy, T. R. Turner, S. Broussard, and J. S. Allan. 1996. SIVagm incidence over two decades in a natural population of Ethiopian grivet monkeys (Cercopithecus aethiops aethiops). J. Med. Primatol. 2578-83. [DOI] [PubMed] [Google Scholar]
- 30.Kalish, L. A., J. Pitt, J. Lew, S. Landesman, C. Diaz, R. Hershow, F. B. Hollinger, M. Pagano, V. Smeriglio, and J. Moye for the Women and Infants Transmission Study (WITS). 1997. Defining the time of fetal or perinatal acquisition of human immunodeficiency virus type 1 infection on the basis of age at first positive culture. J. Infect. Dis. 175712-715. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn, L., E. J. Abrams, P. B. Matheson, P. A. Thomas, G. Lambert, M. Bamji, B. Greenberg, R. W. Steketee, and D. M. Thea for the New York City Perinatal HIV Transmission Collaborative Study Group. 1997. Timing of maternal-infant HIV transmission: associations between intrapartum factors and early polymerase chain reaction results. AIDS 11429-435. [DOI] [PubMed] [Google Scholar]
- 32.Lifson, J. D., M. A. Nowak, S. Goldstein, J. L. Rossio, A. Kinter, G. Vasquez, T. A. Wiltrout, C. Brown, D. Schneider, L. Wahl, A. L. Lloyd, J. Williams, W. R. Elkins, A. S. Fauci, and V. M. Hirsch. 1997. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J. Virol. 719508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayaux, M. J., S. Blanche, C. Rouzioux, J. Le Chenadec, V. Chambrin, G. Firtion, M. C. Allemon, E. Vilmer, N. C. Vigneron, J. Tricoire, et al. for the French Pediatric HIV Infection Study Group. 1995. Maternal factors associated with perinatal HIV-1 transmission. The French Cohort Study: 7 years of follow-up observation. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 8188-194. [PubMed] [Google Scholar]
- 34.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mellors, J. W. 1998. Viral-load tests provide valuable answers. Sci. Am. 27990-93. [DOI] [PubMed] [Google Scholar]
- 36.Mellors, J. W., A. Munoz, J. V. Giorgi, J. B. Margolick, C. J. Tassoni, P. Gupta, L. A. Kingsley, J. A. Todd, A. J. Saah, R. Detels, J. P. Phair, and C. R. Rinaldo, Jr. 1997. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann. Intern. Med. 126946-954. [DOI] [PubMed] [Google Scholar]
- 37.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 2721167-1170. [DOI] [PubMed] [Google Scholar]
- 38.Nerrienet, E., X. Amouretti, M. C. Muller-Trutwin, V. Poaty-Mavoungou, I. Bedjebaga, H. T. Nguyen, G. Dubreuil, S. Corbet, E. J. Wickings, F. Barre-Sinoussi, A. J. Georges, and M. C. Georges-Courbot. 1998. Phylogenetic analysis of SIV and STLV type I in mandrills (Mandrillus sphinx): indications that intracolony transmissions are predominantly the result of male-to-male aggressive contacts. AIDS Res. Hum. Retrovir. 14785-796. [DOI] [PubMed] [Google Scholar]
- 39.Nicoll, A., M. L. Newell, C. Peckham, C. Luo, and F. Savage. 2000. Infant feeding and HIV-1 infection. AIDS 14(Suppl. 3)S57-S74. [PubMed] [Google Scholar]
- 40.Onanga, R., C. Kornfeld, I. Pandrea, J. Estaquier, S. Souquiere, P. Rouquet, V. P. Mavoungou, O. Bourry, S. M'Boup, F. Barré-Sinoussi, F. Simon, C. Apetrei, P. Roques, and M. C. Müller-Trutwin. 2002. High levels of viral replication contrast with only transient changes in CD4+ and CD8+ cell numbers during the early phase of experimental infection with simian immunodeficiency virus SIVmnd-1 in Mandrillus sphinx. J. Virol. 7610256-10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onanga, R., S. Souquiere, M. Makuwa, A. Mouinga-Ondeme, F. Simon, C. Apetrei, and P. Roques. 2006. Primary simian immunodeficiency virus SIVmnd-2 infection in mandrills (Mandrillus sphinx). J. Virol. 803303-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otsyula, M., J. Yee, M. Jennings, M. Suleman, A. Gettie, R. Tarara, M. Isahakia, P. Marx, and N. Lerche. 1996. Prevalence of antibodies against simian immunodeficiency virus (SIV) and simian T-lymphotropic virus (STLV) in a colony of non-human primates in Kenya, East Africa. Ann. Trop. Med. Parasitol. 9065-70. [DOI] [PubMed] [Google Scholar]
- 43.Otsyula, M. G., A. Gettie, M. Suleman, R. Tarara, I. Mohamed, and P. Marx. 1995. Apparent lack of vertical transmission of simian immunodeficiency virus (SIV) in naturally infected African green monkeys, Cercopithecus aethiops. Ann. Trop. Med. Parasitol. 89573-576. [DOI] [PubMed] [Google Scholar]
- 44.Pandrea, I., C. Apetrei, J. Dufour, N. Dillon, J. Barbercheck, M. Metzger, B. Jacquelin, R. Bohm, P. A. Marx, F. Barré-Sinoussi, V. M. Hirsch, M. C. Müller-Trutwin, A. A. Lackner, and R. Veazey. 2006. Simian immunodeficiency virus (SIV) SIVagm.sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts. J. Virol. 804858-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandrea, I., C. Apetrei, S. Gordon, J. Barbercheck, J. Dufour, R. Bohm, B. Sumpter, P. Roques, P. A. Marx, V. M. Hirsch, A. Kaur, A. A. Lackner, R. S. Veazey, and G. Silvestri. 2007. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood 1091069-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pandrea, I., R. Gautam, R. Ribeiro, J. M. Brenchley, I. F. Butler, M. Pattison, T. Rasmussen, P. A. Marx, G. Silvestri, A. A. Lackner, A. S. Perelson, D. C. Douek, R. S. Veazey, and C. Apetrei. 2007. Acute loss of intestinal CD4+ T cells is not predictive of SIV virulence. J. Immunol. 1793035-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandrea, I., C. Kornfeld, M. J.-Y. Ploquin, C. Apetrei, A. Faye, P. Rouquet, P. Roques, F. Simon, F. Barré-Sinoussi, M. C. Müller-Trutwin, and O. M. Diop. 2005. Impact of viral factors on very early in vivo replication profiles in simian immunodeficiency virus SIVagm-infected African green monkeys. J. Virol. 796249-6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pandrea, I., R. Onanga, C. Kornfeld, P. Rouquet, O. Bourry, S. Clifford, P. T. Telfer, K. Abernethy, L. T. White, P. Ngari, M. Muller-Trutwin, P. Roques, P. A. Marx, F. Simon, and C. Apetrei. 2003. High levels of SIVmnd-1 replication in chronically infected Mandrillus sphinx. Virology 317119-127. [DOI] [PubMed] [Google Scholar]
- 49.Pandrea, I., G. Silvestri, R. Onanga, R. S. Veazey, P. A. Marx, V. M. Hirsch, and C. Apetrei. 2006. Simian immunodeficiency viruses replication dynamics in African non-human primate hosts: common patterns and species-specific differences. J. Med. Primatol. 35194-201. [DOI] [PubMed] [Google Scholar]
- 50.Peeters, M., V. Courgnaud, B. Abela, P. Auzel, X. Pourrut, F. Bibollet-Ruche, S. Loul, F. Liegeois, C. Butel, D. Koulagna, E. Mpoudi-Ngole, G. M. Shaw, B. H. Hahn, and E. Delaporte. 2002. Risk to human health from a plethora of simian immunodeficiency viruses in primate bushmeat. Emerg. Infect. Dis. 8451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perelson, A. S., A. U. Neumann, M. Markowitz, J. M. Leonard, and D. D. Ho. 1996. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 2711582-1586. [DOI] [PubMed] [Google Scholar]
- 52.Phillips-Conroy, J. E., C. J. Jolly, B. Petros, J. S. Allan, and R. C. Desrosiers. 1994. Sexual transmission of SIVagm in wild grivet monkeys. J. Med. Primatol. 231-7. [DOI] [PubMed] [Google Scholar]
- 53.Reinhardt, P. P., B. Reinhardt, J. L. Lathey, and S. A. Spector. 1995. Human cord blood mononuclear cells are preferentially infected by non-syncytium-inducing, macrophage-tropic human immunodeficiency virus type 1 isolates. J. Clin. Microbiol. 33292-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rousseau, C. M., R. W. Nduati, B. A. Richardson, G. C. John-Stewart, D. A. Mbori-Ngacha, J. K. Kreiss, and J. Overbaugh. 2004. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J. Infect. Dis. 1901880-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rousseau, C. M., R. W. Nduati, B. A. Richardson, M. S. Steele, G. C. John-Stewart, D. A. Mbori-Ngacha, J. K. Kreiss, and J. Overbaugh. 2003. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J. Infect. Dis. 187741-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rouzioux, C., D. Costagliola, M. Burgard, S. Blanche, M. J. Mayaux, C. Griscelli, and A. J. Valleron for the HIV Infection in Newborns French Collaborative Study Group. 1995. Estimated timing of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission by use of a Markov model. Am. J. Epidemiol. 1421330-1337. [DOI] [PubMed] [Google Scholar]
- 57.Santiago, M. L., F. Range, B. F. Keele, Y. Li, E. Bailes, F. Bibollet-Ruche, C. Fruteau, R. Noe, M. Peeters, J. F. Brookfield, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 2005. Simian immunodeficiency virus infection in free-ranging sooty mangabeys (Cercocebus atys atys) from the Tai Forest, Cote d'Ivoire: implications for the origin of epidemic human immunodeficiency virus type 2. J. Virol. 7912515-12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shalekoff, S., G. E. Gray, and C. T. Tiemessen. 2004. Age-related changes in expression of CXCR4 and CCR5 on peripheral blood leukocytes from uninfected infants born to human immunodeficiency virus type 1-infected mothers. Clin. Diagn. Lab. Immunol. 11229-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silvestri, G. 2005. Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS? J. Med. Primatol. 34243-252. [DOI] [PubMed] [Google Scholar]
- 60.Silvestri, G., A. Fedanov, S. Germon, N. Kozyr, W. J. Kaiser, D. A. Garber, H. McClure, M. B. Feinberg, and S. I. Staprans. 2005. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J. Virol. 794043-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silvestri, G., D. L. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18441-452. [DOI] [PubMed] [Google Scholar]
- 62.Simon, F., S. Souquiere, F. Damond, A. Kfutwah, M. Makuwa, E. Leroy, P. Rouquet, J. L. Berthier, J. Rigoulet, A. Lecu, P. T. Telfer, I. Pandrea, J. C. Plantier, F. Barre-Sinoussi, P. Roques, M. C. Muller-Trutwin, and C. Apetrei. 2001. Synthetic peptide strategy for the detection of and discrimination among highly divergent primate lentiviruses. AIDS Res. Hum. Retrovir. 17937-952. [DOI] [PubMed] [Google Scholar]
- 63.Souquière, S., F. Bibollet-Ruche, D. L. Robertson, M. Makuwa, C. Apetrei, R. Onanga, C. Kornfeld, J. C. Plantier, F. Gao, K. Abernethy, L. J. White, W. Karesh, P. Telfer, E. J. Wickings, P. Mauclere, P. A. Marx, F. Barré-Sinoussi, B. H. Hahn, M. C. Müller-Trutwin, and F. Simon. 2001. Wild Mandrillus sphinx are carriers of two types of lentivirus. J. Virol. 757086-7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sousa, A. E., J. Carneiro, M. Meier-Schellersheim, Z. Grossman, and R. M. Victorino. 2002. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J. Immunol. 1693400-3406. [DOI] [PubMed] [Google Scholar]
- 65.Tess, B. H., L. C. Rodrigues, M. L. Newell, D. T. Dunn, and T. D. Lago. 1998. Breastfeeding, genetic, obstetric and other risk factors associated with mother-to-child transmission of HIV-1 in Sao Paulo State, Brazil. Sao Paulo Collaborative Study for Vertical Transmission of HIV-1. AIDS 12513-520. [DOI] [PubMed] [Google Scholar]
- 66.Tsujimoto, H., R. W. Cooper, T. Kodama, M. Fukasawa, T. Miura, Y. Ohta, K. Ishikawa, M. Nakai, E. Frost, G. E. Roelants, J. Roffi, and M. Hayami. 1988. Isolation and characterization of simian immunodeficiency virus from mandrills in Africa and its relationship to other human and simian immunodeficiency viruses. J. Virol. 624044-4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsujimoto, H., A. Hasegawa, N. Maki, M. Fukasawa, T. Miura, S. Speidel, R. W. Cooper, E. N. Moriyama, T. Gojobori, and M. Hayami. 1989. Sequence of a novel simian immunodeficiency virus from a wild-caught African mandrill. Nature 341539-541. [DOI] [PubMed] [Google Scholar]
- 68.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373117-122. [DOI] [PubMed] [Google Scholar]
- 69.Working Group on Mother-To-Child Transmission of HIV. 1995. Rates of mother-to-child transmission of HIV-1 in Africa, America, and Europe: results from 13 perinatal studies. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 8506-510. [DOI] [PubMed] [Google Scholar]