Abstract
Control of human immunodeficiency virus type 1 (HIV-1) by HLA-B27-positive subjects has been linked to an immunodominant CD8+ cytotoxic T-lymphocyte (CTL) response targeting the conserved KK10 epitope (KRWIILGLNK263-272) in p24/Gag. Viral escape in KK10 typically occurs through development of an R264K substitution in conjunction with the upstream compensatory mutation S173A, and the difficulty of the virus to escape from the immune response against the KK10 epitope until late in infection has been associated with slower clinical progression. Rare alternative escape mutations at R264 have been observed, but factors dictating the preferential selection of R264K remain unclear. Here we illustrate that while all observed R264 mutations (K, G, Q, and T) reduced peptide binding to HLA-B27 and impaired viral replication, the replicative defects of the alternative mutants were actually less pronounced than those for R264K. Importantly, however, none of these mutants replicated as well as an R264K variant containing the compensatory mutation S173A. In assessing the combined effects of viral replication and CTL escape using an in vitro coculture assay, we further observed that the compensated R264K mutant also displayed the highest replication capacity in the presence of KK10-specific CTLs. Comparisons of codon usage for the respective variants indicated that generation of the R264K mutation may also be favored due to a G-to-A bias in nucleotide substitutions during HIV-1 replication. Together, these data suggest that the preference for R264K is due primarily to the ability of the S173A-compensated virus to replicate better than alternative variants in the presence of CTLs, suggesting that viral fitness is a key contributor for the selection of immune escape variants.
CD8+ cytotoxic T lymphocytes (CTL) play a critical role in the immune response against the human immunodeficiency virus type 1 (HIV-1) and against the simian immunodeficiency virus (SIV) in the macaque model. This assertion is supported by accumulating evidence that links effective CTL responses with viral containment (1, 6, 9, 36, 50) and ascribes a protective phenotype to particular major histocompatibility complex (MHC) class I alleles, such as human leukocyte antigen (HLA)-B57 and -B27 in HIV-1 infection (20, 25, 32, 41, 46, 57) or Mamu-A01 and -B17 in SIV infection (43, 61). Conversely, depletion of CD8+ cells results in impaired control of SIV infection (31, 40, 51), and in several reports, mutational escape in prominent CTL epitopes has been associated with disease progression for HIV-1 (1, 5, 11, 17, 26, 33, 35, 47, 52) and for SIV/SHIV (6).
HLA-B27-positive (HLA-B27+) individuals infected with HIV-1 clade B mount an immunodominant CTL response targeting the KK10 epitope (KRWIILGLNK263-272) in p24 Gag (3, 26, 45, 56). Viral escape from this conserved epitope is reported to arise late in infection and to be associated with progression to AIDS (1, 17, 26, 33). We have recently demonstrated that the predominant CTL escape mutation R264K in KK10 dramatically compromises in vitro viral replication capacity and impacts the interaction between the viral capsid and the host protein cyclophilin A (CypA) (52), highlighting the vulnerability of this region in p24 to host immune pressures. Replication of the mutated virus is restored to wild-type (WT) levels through incorporation of a rare upstream compensatory mutation observed in vivo, S173A, which functions by restoring the normal CypA-dependent phenotype (52). These data suggest a significant impact of the respective mutations on the overall structure and/or function of HIV-1 capsid.
Besides R264K, three alternative mutations at codon 264 are described to occur infrequently in HLA-B27+ individuals, namely, R264T, R264Q, and R264G (4, 8, 17, 24, 33, 48, 52) (Table 1). As each of these substitutions also facilitates escape from the B27-KK10 response (4, 24, 26, 33), we sought to examine why the R264K mutation is selected preferentially despite its dramatic impact on viral replication. Factors that might account for the predominant selection of R264K include potential differences in the abilities of the escape mutations at codon 264 to impair HLA binding, their respective impacts on viral replication capacity in the absence and/or presence of CTL, and biased nucleotide substitution rates such that some escape variants are biologically favored during error-prone viral replication.
TABLE 1.
Reported CTL escape mutations in KK10 epitope
| Viral variant or WT | Mutations for indicated positions and amino acid and nucleotide sequencesa | Frequencyb | Reference(s) | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 170 | (…) | 260 | 270 | ||||||||||||||||||||||||||
| WT | P | M | F | S | A | L | S | P | V | G | E | I | Y | K | R | W | I | I | L | G | L | N | K | I | V | R | |||
| CCC | ATG | TTT | TCA | GCA | TTA | TCA | CCA | GTA | GGA | GAA | ATC | TAT | AAA | AGA | TGG | ATA | ATC | CTG | GGA | TTA | AAT | AAA | ATA | GTA | AGA | ||||
| SARKLM | A | K | M | 22/707 | 1,c,d22,c,d24,c,d | ||||||||||||||||||||||||
| GCA | AAA | ATG | 26,c,d33,c52 | ||||||||||||||||||||||||||
| RTLM | T | M | 0/707 | 17,c,d24,c,d | |||||||||||||||||||||||||
| ACA | ATG | ||||||||||||||||||||||||||||
| RT | T | 0/707 | 17,c,d52 | ||||||||||||||||||||||||||
| ACA | |||||||||||||||||||||||||||||
| RQLM | Q | M | 1/707 | 4,c48c,d | |||||||||||||||||||||||||
| CAA | ATG | ||||||||||||||||||||||||||||
| EDRG | D | G | 1/707 | 33c | |||||||||||||||||||||||||
| GAC | GGA | ||||||||||||||||||||||||||||
| RG | G | 0/707 | 8c | ||||||||||||||||||||||||||
| GGA | |||||||||||||||||||||||||||||
The numbering of the amino acids is according to NL4-3 Gag (GenBank accession no. AF324493). The amino acid and nucleotide sequences displayed are identical in the WT NL4-3 and B-clade consensus sequences (www.hiv.lanl.gov). Boldfaced amino acids indicate the KK10 epitope (amino acids 263 to 272).
Frequency, the number of sequences in which the mutation is present/total number of sequences. Values represent frequency in LANL HIV sequence database (www.hiv.lanl.gov) for sequences covering Gag amino acids 152 to 300, as of October 2007.
Published sequences do not cover Gag codon 173.
No accession numbers available.
Here we illustrate that despite the infrequent selection of these alternative mutations, substitutions R264T, R264G, and R264Q were nonetheless better able to abrogate binding to HLA-B27 than R264K. Similarly, while each of these mutations impaired viral replication, the R264K mutation exhibited the most pronounced impact on viral replication. However, viral replication in both the absence and presence of KK10-specific CTL was compromised to a greater degree for the alternative escape mutations than for the R264K virus containing the upstream S173A compensatory mutation, suggesting that a minimal threshold of viral replication may be required to allow for selection of the dominant variant in the quasispecies. Notably, in analyzing codon usage of the respective variants, we also found that generation of the predominant R264K mutation was favored over others due to a bias in the frequency of G-to-A nucleotide substitutions during the replicative cycle of HIV-1. Taken together, these data suggest that the preferential selection of R264K in vivo is strongly influenced by structural and functional constraints on this region of p24/Gag and by the ability of the S173A substitution to restore viral replication capacity. Despite the capability of the alternative escape variants R264T, R264G, and R264Q to effectively impair HLA binding, their substantial impact on viral replication without sufficient compensation limits the possibility of these mutations to emerge.
MATERIALS AND METHODS
Peptide binding studies.
Quantitative assays to measure the binding of peptides to purified HLA B*2705 class I molecules are based on the inhibition of the binding of a radiolabeled standard peptide (human 60s rL28 38-46, sequence FRYNGLIHR; 50% inhibitory concentration [IC50], 6.7 nM) and were performed as previously described (54, 55). Briefly, 0.1 to 1 nM of radiolabeled peptide was coincubated at room temperature with 1 nM to 1 μM of purified MHC in the presence of 1 to 3 μM human beta2-microglobulin (Scripps Laboratories, San Diego, CA) and a cocktail of protease inhibitors. Following a 2-day incubation, the percentage of MHC-bound radioactivity was determined by capturing MHC/peptide complexes on W6/32 antibody-coated Lumitrac 600 microplates (Greiner Bio-One) and measuring bound counts per minute using the TopCount microscintillation counter (Packard Instrument Co.). Peptides were tested at six different concentrations covering a 100,000-fold dose range and in three or more independent assays. Under the conditions utilized, where [label] < [MHC] and IC50 ≥ [MHC], the measured IC50 values are reasonable approximations of KD (equilibrium dissociation constant) (14, 27).
Variant NL4-3 constructs.
HIV-1 strain NL4-3 was modified to express one or more mutations in p24 using the GeneTailor site-directed mutagenesis system (Invitrogen). A SacI-SbfI fragment (residues 491 to 2844) was isolated from pNL4-3 and ligated into pUC19. Mutations S173A, R264K, and L268M were introduced as previously described (52). Mutagenesis of the other variants was performed using 5′ oligonucleotide primers R264T F (CCAGTAGGAGAAATCTATAAAACATGGATAATCCTG; nucleotide [nt] 1593), R264T on LM F (CCAGTAGGAGAAATCTATAAAACATGGATAATCATG; nt 1593), R264X R-1 (TTTTATAGATTTCTCCTACTGGGATAGGTGG; nt 1549), R264X R-2 (TTTATAGATTTCTCCTACTGGGATAGGTGG; nt 1549), R264Q F (CCAGTAGGAGAAATCTATAAACAATGGATAATCCTG; nt 1593), R264Q on LM F (CCAGTAGGAGAAATCTATAAACAATGGATAATCATG, nt 1593), E260D F (CCACCTATCCCAGTAGGAGACATCTATAAAA; nt 1578), E260D R (TCTCCTACTGGGATAGGTGGATTATGTGTC; nt 1539), R264G F (CCAGTAGGAGAAATCTATAAAGGATGGATAATCCTG; nt 1593), R264G on LM F (CCAGTAGGAGAAATCTATAAAGGATGGATAATCATG, nt 1593), S173T F (GAAGTAATACCCATGTTTACAGCATTATCAG; nt 1317), and S173T R (AAACATGGGTATTACTTCTGGGCTGAAAG; nt 1277). Mutated codons are underlined, and primer positions are numbered according to pNL4-3 (GenBank accession no. AF324493). Mutated SacI-SbfI fragments were then isolated from pUC19 and cloned back into pNL4-3. The complete HIV-1 coding region of the variant proviruses was sequenced using published primers (2) on an ABI 3730 XL DNA analyzer. Escherichia coli One Shot Stbl3 cells (Invitrogen) were used to propagate full-length proviral plasmids, and stocks were prepared using a QIAprep spin miniprep kit or HiSpeed plasmid midi kit (Qiagen).
Cells.
HEK293T cells were cultured at 37°C in a 5% CO2 humidified incubator in Dulbecco's modified Eagle medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (Atlantic Biologicals), penicillin (50 IU/ml), and streptomycin (50 μg/ml). CEM-GXR cells (12) were grown in R10+ medium (RPMI 1640 medium [Sigma] supplemented with 10% fetal bovine serum, 2 mM l-glutamine, penicillin [50 IU/ml], and streptomycin [50 μg/ml]) at 37°C and 5% CO2. Peripheral blood mononuclear cells (PBMC) were separated from whole blood using Histopaque-1077 (Sigma) and then stimulated with a CD3:CD8-bispecific monoclonal antibody at a concentration of 0.5 μg/ml in R10-50 medium (R10+ medium containing 50 U/ml human interleukin-2 [Hoffmann-La Roche]) for 4 days prior to infection. A KK10-specfic CTL clone was generated using PBMC isolated from a B27+ HIV controller subject by peptide stimulation and limiting dilution using previously described methods (60). CTL were restimulated periodically using anti-CD3 antibody (12F6 clone) plus irradiated allogeneic feeder PBMC and maintained in R10-50 medium prior to use. Peptide-specific lytic activity was verified using chromium-51 release assays, and clonality was confirmed by sequencing of the T-cell receptor (TCR) alpha and beta chains (not shown) using standard methods (62).
Viral stocks.
Viral stocks were generated by transfection of HEK293T cells with 5 μg of plasmid DNA in antibiotic-free DMEM using Lipofectamine 2000 (Invitrogen). Pseudotyping with the vesicular stomatitis virus glycoprotein G (VSV-g) was performed by cotransfecting with 300 ng of pHEF-VSVG (13). Supernatants were harvested 48 h after transfection, and frozen aliquots stored at −80°C. The capsid concentration of the viral stocks was quantified by p24 enzyme-linked immunosorbent assay (ELISA) using the Alliance HIV-1 p24 ELISA kit (Perkin-Elmer). Initial titers were determined by flow cytometry using CEM-GXR cells.
Viral replication assays.
One million CEM-GXR cells were pelleted and resuspended with WT or variant virus at a multiplicity of infection (MOI) of 0.0015 in a total volume of 3 ml R10+ medium. Five hundred-microliter aliquots of the culture were harvested at the indicated times, and the volume was replaced with fresh R10+ medium. The proportion of green fluorescent protein (GFP)-expressing cells was determined by fluorescence-activated cell sorter (FACS). For the analyses of primary cells, one million stimulated PBMC were infected with inocula of WT, SARKLM, RTLM, RQLM, and EDRG viral variants, leading to similar fractions of CD4-positive cells with intracellular p24, as detected by FACS 72 h postinfection. Infection with RK and RKLM viruses was performed using 300 μl inocula. Day 3 infectious rates for WT, SARKLM, RTLM, RQLM, and EDRG viruses varied for the different PBMC samples between 2 and 10% of CD4-positive cells. Rates for RK and RKLM variants were consistently 1 log lower. PBMC were infected in a total volume of 1.5 ml R10-50 medium. Aliquots of 250 μl of the culture were harvested at the indicated times, and the volume was replaced with fresh R10-50 medium.
Staining and flow cytometry.
CEM-GXR cells were fixed in phosphate-buffered saline containing 2% paraformaldehyde, and GFP expression was determined using FACS. A signal 10-fold above the median fluorescence index of uninfected cells was considered positive, excluding greater than 99.95% of uninfected cells. PBMC were permeabilized using the Cytofix/Cytoperm kit (BD Biosciences) and stained for CD4 (CD4-allophycocyanin; BD Pharmingen) and intracellular p24/Gag (KC57-RD1; Beckman Coulter) to identify the proportion of infected CD4+ T cells in the cultures with the different viral variants. FACS analysis was done using a FACSCalibur flow cytometer (Becton Dickinson) and FlowJo software (TreeStar). A minimum of 25,000 cells was analyzed for each sample.
CTL coculture assays.
CEM-GXR cells were transduced with a murine stem cell virus-derived retroviral vector (Clontech) encoding HLA-B2705 and selected in R10 medium containing 5 μg/ml puromycin. Cells were inoculated with WT or variant viruses at an MOI of 0.0015, and the proportion of GFP-expressing cells was verified by FACS on day 2. Virus-infected cells were then washed and incubated in R10-50 medium in the absence or presence of a B27-KK10-restricted CTL clone mixed at an effector-to-target cell ratio of 1:10. To measure viral spread, the proportion of GFP-expressing CEM-GXR cells was assessed by FACS at days 3 through 7.
CsA experiments.
Single-cycle infectivity was determined by inoculation of CEM-GXR reporter cells with VSV-g pseudotyped virus in the presence or absence of cyclosporine A (CsA) (Sigma Aldrich). Cells were preincubated in R10+ medium containing 0.5 μM of drug for 30 min and then inoculated with WT or viral variants. Viral inocula were normalized to 50 ng p24 determined by ELISA. Infected cells were quantified using FACS to measure GFP expression at 48 h postinfection.
Nucleotide substitution rates.
Full-length HIV-1 sequences derived from seven subjects with acute HIV-1 infection and 6 months thereafter (37) were analyzed to determine the relative rates of each specific type of synonymous nucleotide substitution. All sequences were derived from proviral DNA, with hypermutated sequences excluded. Sequences correspond to accession numbers DQ676870 to DQ676875, DQ676877, DQ676878, DQ676880, DQ676881, DQ676883, DQ676884, DQ676886, and DQ676887.
Statistical analysis.
All analyses were performed using Prism 4.0 (GraphPad).
RESULTS
Alternative escape mutations in the B27-KK10 epitope impair viral replication to a lesser degree than R264K.
Viral escape from the immunodominant HLA-B27-restricted KK10 epitope (KRWIILGLNK) in Gag has been described to typically consist of an early L268M mutation arising at position 6 (P6) of the epitope, which is followed many years later by an R264K mutation at the anchor residue P2 (26, 39). The L268M mutation can partially impair TCR recognition (38), while R264K imparts a substantial impact on binding to HLA-B27 and epitope presentation (4, 24, 26, 44). R264T, R264Q, and R264G have been described as alternative escape mutations at P2 of KK10 (Table 1), although they occur at much lower frequencies than R264K (4, 8, 17, 24, 33, 48). In a recent study of chronic clade B-infected subjects, we confirmed these results, with R264K selected in 13 of 15 (87%) HLA-B27+ subjects developing mutations at P2 of KK10 (52) and with the R264T mutation observed in the remaining two cases. The predominant R264K mutation results in a significant defect in HIV-1 replication capacity, which can be restored through the generation of a compensatory substitution, S173A (52).
We therefore hypothesized that differences in the replicative capacities of the respective escape variants may contribute to the overall frequencies of the mutations observed in vivo. To examine this, variant NL4-3 proviruses were constructed, (Table 2) containing either the R264T (RT) or the R264Q (RQ) mutation, as well as both in combination with the L268M substitution observed in vivo (RTLM and RQLM) (4, 17, 24, 48). Since the R264G escape mutation has been associated in some cases with an upstream E260D mutation (ED) (33), this escape variant was engineered as single (RG), double (EDRG), and triple mutant (EDRGLM) forms. Replicative capacities of these variants were compared to WT NL4-3 and to viruses containing the predominant R264K and L268M escape mutations (RKLM). In addition, the compensated form of RKLM containing S173A was included (SARKLM) (52). Viral particles were produced by transfection of HEK293T cells, and the kinetics of replication were evaluated by measuring the percentage of infected cells over 1 week using a CEM-based GFP reporter T-cell line, CEM-GXR (12).
TABLE 2.
Viral constructs and characteristicse
| Variant or WT | Mutation(s) | Observed in vivo | Replicative capacitya | HLA-B27 bindingb | % Inhibition by CTLc | Influence of CsA on infectivity | P2 substitution favored by mutational bias |
|---|---|---|---|---|---|---|---|
| WT | Yes | ++ | ++ | 97.5 | ↓ | NA | |
| LM | L268M | Yes | ++ | ++ | ND | ↓ | NA |
| RK | R264K | No | − | +/− | 1 | ↑ | Yes |
| RKLM | R264K, L268M | Yesd | − | +/− | 34.6 | ↑ | Yes |
| SARKLM | S173A, R264K, L268M | Yes | + | +/− | 37.8 | ↓ | Yes |
| RT | R264T | Yes | + | − | 39.8 | ↓ | No |
| RTLM | R264T, L268M | Yes | + | − | 20 | ↓ | No |
| STRTLM | S173T, R264T, L268M | Yes | + | − | ND | ND | No |
| RQ | R264Q | No | + | − | 73.1 | ↓ | No |
| RQLM | R264Q, L268M | Yes | + | − | 34.4 | ↓ | No |
| RG | R264G | Yes | + | − | 33.8 | ↓ | No |
| ED | E260D | Yes | ++ | ++ | 95.4 | ↓ | NA |
| EDRG | E260D, R264G | Yes | + | − | 23.7 | ↓ | No |
| EDRGLM | E260D, R264G, L268M | No | − | ND | ND | ND | No |
| EDRK | E260D, R264K | No | − | +/− | ND | ND | Yes |
Measured as viral spread in CEM-GXR cell culture; ++, replicates at WT level or more than WT level; +, replicates at 50 to 100% of WT level; −, replicates at <10% of WT level.
Measured by binding of peptide to HLA-B27 molecules; ++, binding capacity same as WT; +/−, binding capacity 1 to 2 log lower than WT; −, binding capacity >2 log lower than WT.
Measured as percent reduction in GFP-positive cells compared to no CTL at day 7 after infection.
Only in association with compensatory mutation.
ND indicates “not determined”; NA indicates “not applicable”; ↓, decreased infectivity; ↑, increased infectivity.
The R264T substitution appears to represent the second most frequent variant at P2 of KK10, according to an analysis of several HLA-B27+ subjects (52), although at the moment, it is not observed in sequences available in the LANL HIV sequence database (www.hiv.lanl.gov) (Table 1). Both RT and RTLM displayed a reduction in replicative capacity, having infected 10 times (RTLM) and 3 times (RT) fewer cells than WT NL4-3 after 7 days in culture (Fig. 1A). In contrast to RK (and RKLM; data not shown), which failed to replicate, RT and RTLM were capable of spreading in the culture. Notably, however, both RT and RTLM replicated slower than the SARKLM variant, which nearly achieved WT levels of replication. While not observed in vivo, we also combined the upstream S173A mutation with the RT and RTLM variants, but no improvement in replication was observed for these viruses (data not shown). In previously published sequences, one instance with a combination of an S173T mutation with R264T was described (52), but in our assay S173T did not compensate for the fitness defect of the RT, RTLM, or RK variant (data not shown). Therefore, the R264T mutation impaired viral replication to a lesser degree than R264K; however, the defect for R264T was more severe than for the compensated R264K escape variant SARKLM. Similar to RT and RTLM, both the RQ and RQLM variants displayed lower rates of viral replication than WT NL4-3 (Fig. 1B), and these variants replicated substantially better than viruses encoding R264K, but less well than the compensated SARKLM variant.
FIG. 1.
Alternative escape mutations in KK10 impact viral replication in vitro to a lesser extent than R264K. (A to C) One million CEM-GXR cells were incubated with WT or variant virus, and viral spread over 8 days was determined by flow cytometry. In all assays, the proportion of cells infected by RK declined over time, while SARKLM spread efficiently. RT and RTLM (A) as well as RQ and RQLM (B) replication was reduced by up to 1 log compared to the WT and by up to 0.5 log compared to SARKLM. Replication of RG (C) was reduced 1.5 logs compared to the WT and combining RG with E260D (EDRG) compensated for this impairment. EDRGLM as well as EDRK failed to spread throughout the culture. Results shown are representative for three independent experiments. (D) Replication of KK10 variant viruses was examined using primary cells. PBMC from a healthy HIV-negative individual were isolated and CD4+ T cells were stimulated for 4 days. Cells were inoculated with WT NL4-3 or KK10 variants (MOI = 0.02), and viral replication was measured by detection of intracellular p24. The proportion of CD4+ p24+ cells at each time is displayed. As seen using CEM-GXR cells, the RK and RKLM variants were the most defective strains tested, while the SARKLM variant replicated better than the alternative escape mutants. Results are representative of three independent assays using PBMC from different donors.
Viral escape in KK10 through an R264G mutation has also been described, although it is consistently in the absence of the early L268M mutation (4, 8, 33). Furthermore, the neighboring mutation E260D has been proposed to function as a compensatory mutation for R264G (33). In examining these variants, we observed that replication of RG was more impaired than that of either RT or RQ, but that this variant still spread substantially better than RK (Fig. 1C). Incorporation of E260D into RG (EDRG) improved its replicative capacity, although this variant still did not spread as efficiently as SARKLM or WT NL4-3. Notably, combining the E260D mutation with the RK variant (EDRK) did not restore the replicative defect for RK. Therefore, E260D functions to compensate only for the R264G escape mutation, not for R264K. Finally, a severe replication defect was observed when we combined L268M with E260D/R264G (EDRGLM), which might explain the absence of this particular combination of mutations in vivo.
To ensure that these differences in replication capacities were not merely due to the reporter cell system used for these studies, we assessed the ability of each variant to spread in primary T-cell cultures. As shown in Fig. 1D, we observed a similar hierarchy of viral replication of the variants in primary T cells, verifying results from the reporter cell assays. Namely, the RTLM, RQLM, and EDRG variants spread more efficiently than RK and RKLM viruses, but each appeared less fit than the WT or the SARKLM variant.
Together, these results indicate that each of these CTL escape mutations in KK10 (R264K, R264T, R264Q, and R264G) imparts a defect in viral replicative capacity. Notably, each of the less frequently observed variants at this residue replicated better than the predominant escape mutation R264K, but none of these alternative variants spread as well as the compensated SARKLM virus, which appeared similar to the WT in our assays. Overall, in vitro replication capacities corresponded well with the observed in vivo frequencies of the particular KK10 epitope variants, suggesting that viral fitness may be a key determinant during in vivo selection of CTL escape mutants.
Alternative CTL escape mutations in KK10 are dependent upon CypA for replication.
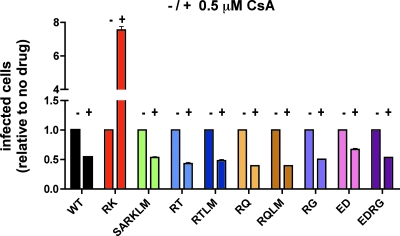
The severe fitness defect of the R264K mutant is due in part to its altering of critical interactions between HIV-1 capsid and the host cellular protein CypA (52), where, unlike with wild-type HIV-1, the R264K mutant is unable to replicate in the presence of CypA. Binding of CypA to the viral capsid is normally necessary for efficient infection by HIV-1 and is believed to regulate the uncoating process or to impair recognition of the capsid by host restriction factors (10, 19, 21, 58). The replication competence of the R264K variant is partially recovered by treating target cells with CsA, a drug that binds to CypA and competes with HIV-1 for this interaction, or through replication in cell lines deficient in CypA (52). Notably, the normal CypA-dependent phenotype could be restored by introduction of the S173A compensatory mutation into the R264K variant virus (52). In order to determine whether a similar CypA-mediated impact on viral replication was induced in the RT, RQ, or RG escape variant, CEM-GXR cells were treated with 0.5 μM CsA and then infected with the respective viral variants. As expected, addition of 0.5 μM CsA increased the infectivity of the RK variant more than sevenfold (Table 2 and Fig. 2). In contrast, we observed declines in infectivity levels between two- and 2.5-fold for all of the alternative escape variants, which were similar to that of the WT virus, suggesting that only the R264K mutation has a significant impact on CypA interactions (Table 2 and Fig. 2). Together with the replication data presented in Fig. 1, these results suggest that the less frequent selection of the alternative escape mutations at residue 264 is not due to a greater impact of these substitutions on p24 function than that of R264K.
FIG. 2.
Infectivity of the alternative escape variants is reduced in the presence of CsA. CEM-GXR cells were infected with VSV-g-pseudotyped WT, RK, SARKLM, ED, RG, EDRG, RT, RTLM, RQ, and RQLM viruses (50 ng p24 equivalent each) in the presence (+) or absence (−) of 0.5 μM CsA. The percentage of GFP-expressing cells 48 h postinfection is shown. The percentage of infected cells for RK increased 7.5-fold, while the infectivity levels of all other viruses were reduced between 1.5- and 2.5-fold in the presence of the drug. Results displayed are mean values for duplicate parallel cultures representative of two independent experiments.
Rare alternative CTL escape mutations in KK10 efficiently impair binding to HLA-B27.
We next hypothesized that differences in the relative ability of KK10 mutations to escape CTL-mediated immune pressure might contribute to their selection frequencies in vivo. Since R264 serves as the P2 binding site for the HLA-B27 molecule, we compared the HLA binding capacities of KK10 epitope variants to that of the WT peptide. As a control, we examined an HLA-B27-restricted influenza nucleoprotein epitope (SRYWAIRTR; positions 383 to 391; SR9) (29). HLA-B27 binding capacities were determined using a competition assay in which a radiolabeled standard peptide-HLA-B27 complex was incubated in the presence of increasing concentrations of unlabeled variant peptide, as described previously (54, 55). As expected, the L268M mutation had little impact on HLA binding, while the R264K mutation, alone or in combination with L268M, impaired binding by nearly 2 logs (Fig. 3, Table 2, and Table 3) (26). Binding capacities for the less frequent escape mutants (R264G, R264T, and R264Q) were reduced even more substantially, by nearly 3 logs or greater, and similar results were observed for R264T and R264Q in combination with L268M, which have been associated in vivo (4, 24, 48). Given that an IC50 of 500 nM is generally considered a physiological cutoff for peptide binding to MHC class I (53), these data indicate that all P2 mutations in KK10 substantially abrogated binding to HLA-B27. Notably, however, the less frequent mutations at P2 had an even greater impact on binding than R264K in this assay. The results of this direct comparison of epitopes with all known mutations in KK10 agree with HLA binding capacities for the R264T, R264G, and R264Q variant peptides which have previously been documented independently using differing assays (4, 24, 44). Therefore, an ability of the alternative escape mutations in KK10 to bind HLA did not appear to account for their decreased frequency.
FIG. 3.
Escape mutations at the P2 anchor residue abrogate binding to HLA-B27. A radiolabeled standard peptide (FRYNGLIHR) and variant KK10 peptides at different concentrations were incubated with purified HLA-B2705 molecules for 48 h, and binding capacity was determined by analyzing the percentage of MHC-bound radioactivity. The IC50 in nM is displayed for the different peptides (influenza-SR9, SRYWAIRTR; WT-KK10, KRWIILGLNK; LM, KRWIIMGLNK; RK, KKWIILGLNK; RKLM, KKWIIMGLNK; RG, KGWIILGLNK; RT, KTWIILGLNK; RTLM, KTWIIMGLNK; RQ, KQWIILGLNK; and RQLM, KQWIIMGLNK [boldface indicates differences in residues compared to WT KK10]). Binding capacities for WT-KK10, influenza-SR9, and LM were not significantly different, while the binding of RK and RKLM was reduced by 2 logs. Peptides with alternative mutations without LM (RG, RT, and RQ) or with LM (RTLM and RQLM) reduced binding capacities by nearly 3 logs or greater. Raw binding capacity values (IC50) are shown in Table 3.
TABLE 3.
HLA-binding capacities of variant HIV-1 B27-KK10 peptides and influenza SR9
| Variant peptide, SR9, or WT | Peptide sequencea | IC50 (nM) |
|---|---|---|
| SR9 | SRYWAIRTR | 15 |
| WT | KRWIILGLNK | 8.3 |
| LM | KRWIIMGLNK | 13 |
| RK | KKWIILGLNK | 886 |
| RKLM | KKWIIMGLNK | 741 |
| RG | KGWIILGLNK | 6,816 |
| RT | KTWIILGLNK | 36,644 |
| RTLM | KTWIIMGLNK | 32,287 |
| RQ | KQWIILGLNK | 5,462 |
| RQLM | KQWIIMGLNK | 67,680 |
Boldfaced amino acids indicate differences in residues compared to WT KK10.
In vitro inhibition of R264 variants by KK10-specific CTL.
Despite the low HLA-B27 binding affinities for KK10 peptides with mutations at the P2 anchor position, it is possible that differences in the levels of residual CTL recognition and/or processing efficiency of these epitopes could contribute to their propensity to be selected in vivo. To address these issues directly, a CEM-GXR cell line was generated that stably expressed the HLA-B2705 molecule. We infected these cells with variant viruses and measured viral replication capacity in the absence or presence of a highly active CTL clone recognizing the WT KK10 epitope. KK10-restricted CTL inhibited WT virus replication significantly during the 7-day coculture assay (Fig. 4A), while the compensated SARKLM variant was largely resistant to the effects of CTL (Fig. 4B). Similar assays were conducted for the alternative KK10 variants, and a wide range of viral replication capacities was observed in the absence and presence of CTL (Fig. 4C and Table 2). Notably, the compensated SARKLM virus appeared to be the most fit escape variant in the absence and presence of CTL, followed by the R264T-containing variants. In addition, the remaining CTL-mediated inhibition among the KK10 variants in these coculture assays suggested that at least some CTL recognition still took place for the KK10 mutants tested. Together with the data represented in Fig. 1 and 3, our results indicate that viral replication capacity and the degree of residual CTL recognition are major driving forces during in vivo selection of KK10 escape variants.
FIG. 4.
The SARKLM variant displays the highest replication capacity in the presence of KK10-specfic CTL. CEM-GXR cells stably expressing HLA-B2705 were inoculated with WT NL4-3 or KK10 variants (MOI = 0.0015). KK10-specific CTL were added to one replicate well at an effector-to-target cell ratio of 1:10. Viral replication was measured using FACS at days 2 through 7, as described in the legend for Fig. 1, and the proportion of GFP+ CEM-GXR cells at each time is shown. Replication of WT virus was substantially inhibited in the presence of CTL (A), but the SARKLM variant was largely resistant to the effects of CTL (B). Replication capacity was highly variable for the different KK10 variants in the absence and presence of KK10-specific CTL, as demonstrated by the proportion of virus-infected cells present at day 7 using this in vitro coculture assay. Overall, however, the SARKLM virus exhibited the highest level of replication in either the absence or presence of CTL (C).
Mutational bias preferentially selects for the R264K mutation.
Our results for viral replication and CTL recognition demonstrate that the compensated SARKLM is likely to be the most fit KK10 escape variant among those observed in vivo. However, the alternative escape variants also appeared to retain a relatively high replication capacity, particularly for the R264T mutants, in the absence and presence of KK10-specific CTL (Fig. 4C). Nevertheless, the alternative escape mutants are observed less frequently in vivo than would be suggested from our functional studies. We therefore examined whether additional factors might directly influence the underlying evolutionary process at P2 of KK10 toward the R264K mutation by analyzing differences in the rates of nucleotide substitutions that may affect codon 264.
It is known that the genomes of retroviruses vary considerably in their nucleotide composition, with a high content of adenosine (A) in the RNA of HIV-1 (7). This imbalance might result from the error proneness of HIV-1 reverse transcriptase, which has been described to preferentially incorporate G-to-T mismatches during minus-strand cDNA synthesis (59). Recently, it has also been suggested that HIV-1 sequence evolution may be influenced by the mutagenic potential of the APOBEC3 family of host proteins, which have been linked to the phenomenon of retroviral G-to-A hypermutation (28, 30). We hypothesized that the rates of the 12 possible nucleotide substitutions that occur during the replicative process of HIV-1 could additionally bias the mutational pathway during escape from CTL pressure against KK10 and hence contribute to the greater in vivo frequency of R264K. To determine the rate of accumulation of each nucleotide change, we used previously published longitudinal full-length HIV-1 sequences from seven individuals collected during acute infection and 6 months thereafter (37). To avoid possible influences by particular selection pressures of the host, we disregarded all nonsynonymous mutations and calculated the number of synonymous substitutions that accumulated during this interval, relative to the total number of synonymous mutations possible. Using this approach, the most frequent changes observed during the first 6 months of infection were G-to-A mutations, which accumulated at approximately one mutation per 100 opportunities. The next most frequent synonymous nucleotide change was an A-to-G transition, which was observed at about half that rate (Table 4) (A. Schneidewind and Y. E. Wang, unpublished data).
TABLE 4.
Rates of nucleotide substitutions required for evolution of KK10 escape mutations at position P2
| Viral variant or consensus | Nucleotide sequence at codon 264 | Required nucleotide substitution(s) | Rate(s) of nucleotide substitution(s)a |
|---|---|---|---|
| Consensus R264 | AGA | ||
| R264K | AAA | G to A | 0.0096 |
| R264G | GGA | A to G | 0.0051 |
| R264T | ACA | G to C | 0.0000 |
| R264Q | CAA | G to A and A to C | 0.0096 (G to A) and 0.0006 (A to C) |
In vivo rates of synonymous substitutions as determined by analysis of pairs of longitudinal sequences derived from seven subjects over an interval of 6 months (37).
Of the four different amino acid mutations at P2 of KK10, only the predominant R264K mutation derives from the relatively more common transition of G to A (Table 4). In contrast to R264K, the R264T mutation is generated by transversion from G to C, which happens very rarely according to our analysis. The R264G mutation requires a transition from A to G that is only slightly less frequent than the G-to-A substitution, but this escape pathway may be hindered by the typically early generation of the L268M substitution, which is incompatible with R264G replication in our assays (Fig. 1C). Finally, the R264Q substitution could arise through two pathways. The first would require the frequent G-to-A transition (AGA→AAA) followed by an additional A-to-C transversion event (AAA→CAA), which was observed to occur at a rate 16 times lower than that of G to A. Obviously, the occurrence of the AAA intermediate, which codes for lysine (K), might be limited due to the poor replication capacity of the R264K variant. Alternatively, the R264Q mutation could also arise through an initial A-to-C substitution (AGA→CGA) that would remain synonymous on the amino acid level, followed by a G-to-A substitution (CGA→CAA) generating R264Q. However, here the CGA intermediate is rarely observed in vivo (0.11% of sequences in the LANL HIV sequence database; www.hiv.lanl.gov), which might further explain the limited frequency of this particular escape mutation. Therefore, mutational bias at the P2 position of KK10 during evolution of the HIV-1 quasispecies may preferentially select for the R264K substitution (Table 2), which could also contribute to the higher observed frequency of this escape mutation.
DISCUSSION
Viral escape in the B27-KK10 epitope in Gag typically occurs through development of an R264K mutation. Three alternative escape mutations at the same codon, R264T, R264Q, and R264G, have been reported at lower frequencies (4, 8, 17, 24, 33, 48, 52), although the factors contributing to the preferential selection of R264K remain unclear. Here our data illustrate that each of the alternative escape mutations at residue R264 was capable of abrogating peptide binding to HLA-B27 to an even greater degree than R264K, despite their infrequent selection in vivo. The alternative mutations also impaired viral replication capacity, but surprisingly, each had a less severe effect than the predominant R264K mutation, and only the RK variant altered the critical interaction between the capsid and the host protein CypA. Notably, however, the S173A-compensated R264K variant demonstrated the highest level of replication capacity among known escape variants and restored normal CypA dependence. Moreover, while all of the escape variants successfully evaded inhibition by KK10-specific CTL in coculture, the S173A-compensated R264K variant replicated most efficiently. Therefore, in vitro replication capacities of KK10 variants in the presence of CTL correlated well to the relative frequencies of escape mutants in B27+ individuals in vivo, with SARKLM being selected most often.
During viral escape from the B27-KK10 response, the L268M substitution develops significantly earlier than mutations at codon 264 (17, 22, 33, 52). Rather than functioning as a compensatory mutation, however, this L268M mutation appears to represent an early partial escape mutation at a TCR contact residue (38, 52) and has little impact on the replicative capacity of HIV-1 (52). In the context of alternative KK10 mutations, R264Q was always associated with L268M (4, 48), and R264T has been described to occur both in the presence and absence of L268M (17, 26, 52). We have observed only minor differences in the replicative capacities for the RK, RQ, and RT variants in the presence of L268M (Fig. 1A and 1B), further supporting the model that L268M functions mainly as an early CTL escape mutation that is recognized by only a subset of KK10-specific TCR clonotypes (38, 52). Indeed, it is likely that a secondary de novo T-cell response against the LM variant drives the subsequent development of mutations at residue R264.
The mutation E260D is associated with R264G and has been postulated to restore a replication defect (33), although in one report CTL escape in KK10 via development of R264G was not associated with E260D (8). We now demonstrate that E260D does indeed compensate for a fitness defect of R264G, although it had no similar effect on R264K (Fig. 1C). Residue E260D lies four amino acids N terminal of residue R264, and simultaneous alanine substitutions at codons 260 and 264 have been shown to stabilize the viral core (18) without affecting the rate of capsid assembly (16). In contrast, single alanine substitutions at these charged amino acids led to faster and slower capsid assembly, respectively (16). These data suggest close interactions between residues at codons 260 and 264, whose side chains sit on the same planar face of the molecule, and imply that charge balances in this region might be critical for normal capsid function. Development of the R264G mutation is also notable in that it occurs in the absence of L268M (8, 33), and we observed that the combination of substitutions E260D, R264G, and L268M (EDRGLM) led to a viral variant that was as severely compromised in its replicative capacity as RK (Fig. 1C). Therefore, the ability to generate the R264G escape mutant might also be limited due to the early selection of the LM variant in the majority of HLA-B27+ individuals.
The alternative escape mutants efficiently abrogated HLA binding of the KK10 epitope at least as well as the RK variant (Fig. 3), and we observed that escape from CTL pressure was as complete for the alternative variants as for SARKLM in our coculture assays (Table 2). The in vitro replication kinetics of the various alternative escape mutants, including the compensated EDRG, however, were consistently slower than that of SARKLM in the absence or presence of KK10-specific CTL (Fig. 1 and 4). This suggests that a threshold of replicative capacity may exist that determines whether a mutant can dominate in the quasispecies. Indeed, despite its typically late development in vivo, the nearly WT-level replication capacity and profound CTL escape phenotype of SARKLM in our in vitro assays suggest that this variant would have a significant in vivo replicative advantage over the parental virus in the context of an immunodominant KK10-specific CTL response. In contrast, despite similar or even enhanced CTL evasion capabilities, the more substantially impaired replicative capacities observed for the RT/RTLM, RQ/RQLM, and RG/EDRG variants might still outweigh the selective advantage of a successful CTL escape. A potential model to explain the importance of viral replication capacity and evasion from CTL pressure relative to an in vivo threshold for HIV outgrowth is shown in Fig. 5. We propose that during chronic HIV-1 infection, only the SARKLM variant is suitably fit to escape KK10-specific CTL pressure and establish itself as the predominant viral strain in vivo.
FIG. 5.
Model summarizing forces that influence selection of the escape mutations in the B27-KK10 epitope. The relative influence of CTL escape mutations in the KK10 epitope on HLA-B27 binding, in vitro viral replication, and predicted in vivo viral replication capacity in the presence of KK10-specific CTL is illustrated. Viral sequences in the KK10 epitope are shown on the left, and mutations at residue 264 are indicated in red or blue text. The ability of the WT KK10 peptide (yellow) or KK10 P2 variants (red) to bind to HLA-B27 is classified as strong, weak, or very weak based on data presented in Fig. 3. In the panels on the right, in vitro and putative in vivo replication capacities of WT (black), R264K (red), R264T/G/Q (collectively in blue), and SARKLM (green) are displayed. The dashed line represents the hypothesized in vivo replication “threshold” that may need to be overcome for a given variant to dominate the quasi-species.
Several reports have addressed the question of how frequently each of the 12 possible nucleotide substitutions occurs during sequence evolution in HIV-1. The results of these analyses vary substantially, possibly due to differences in the data sets or in the methodologies applied. Goodenow et al. (23) compared short gag and env fragments of the viral quasispecies from seven subjects and noted a predominance of G-to-A substitutions, assuming that minor sequence populations were derived from the dominant ones. In their data set, 100% of all guanosine alterations in gag showed a G-to-A change. Moriyama et al. (42) estimated the relative frequency of nucleotide substitutions at the third codon positions in gag by analyzing published sequences of 15 independent HIV-1 isolates, proposing that 43.8% of all mutations were G-to-A changes, while only 16.4% were A-to-G changes. G-to-C changes and A-to-C changes were not observed in that study. In contrast, Plikat et al. (49) collected longitudinal nef sequences from one subject and reported relative frequencies for A-to-G and T-to-C transitions twofold higher than for G-to-A changes. Our analysis of synonymous nucleotide changes in seven longitudinal viral sequence pairs supports that the predominant nucleotide substitution introduced during the replicative cycle of HIV-1 is a G-to-A transition, which matches the change required to generate the R264K mutation. Nucleotide substitutions necessary for evolution of the other KK10 escape variants, including R264T (G to C), R264G (A to G), and R264Q (two mutations: A to C and G to A) arise significantly less often (Table 4). Our results correlate well with a recently published estimation of the substitution frequency during HIV evolution in vivo, which was derived from a considerably large data set of over 5,000 pol population sequence pairs (15).
The propensity for G-to-A substitutions during HIV-1 replication could therefore additionally hamper the generation of R264T, R264G, or R264Q, while favoring the occurrence of R264K. The significance of a mutational bias during viral replication to select for particular variants with even suboptimal replication capacity is supported by a study demonstrating that the initial selection of the less fit lamivudine drug-resistant mutant M184I could be explained by its underlying G-to-A substitution (34). At later times, the M184V variant displaying an improved replicative capacity becomes dominant, but this delay was attributed to its requirement for the infrequent A-to-G transition. In the context of CTL pressure, therefore, mutational bias may also alter the frequency of escape variants, despite seemingly significant differences in costs to replicative capacity. However, the notion that the nucleotide substitution bias that occurs during viral evolution also influences the preferential selection for R264K is tempered by its escape pathway that requires two independent amino acid substitutions (i.e., R264K and the S173A compensatory change), while any of the alternative escape mutations at the P2 position of KK10 do not absolutely rely on additional mutations in vitro. S173A itself is generated by a T-to-G substitution, which we observed at a rate of 0.0021. Since the S173A mutation in the absence of R264K has a relatively modest impact on replication capacity (52) and is present in 39 out of 707 (5.5%) sequences in the LANL HIV database (www.hiv.lanl.gov), it is likely that it occasionally arises as a minor species and therefore may not provide a substantial barrier to the generation of R264K in the context of HLA-B27. Taking this into consideration, the existence of a particular fitness threshold, which must be surpassed by a viral mutant for it to become the dominant form in the quasispecies, seems even more probable. Viral escape in KK10 might generally be delayed until R264K is generated on a viral backbone with a preexisting S173A mutation, with the alternative escape variants failing to exceed the fitness level required for outgrowth. In this case of a viral backbone incorporating a preexisting S173A mutation, the nucleotide substitution bias would then additionally favor the generation of an R264K mutation over any of the alternative substitutions at the P2 position of the KK10 epitope. Furthermore, it is possible that each of the alternative escape variants also requires particular compensatory mutations in order to establish a variant quasispecies with sufficient replication competence to outcompete viruses with the WT or L268M forms of the KK10 epitope. Analogous to the requirement for S173A, the requirement for such compensatory mutations could then substantially reduce the chance for an alternative escape mutation in KK10 to arise. Unfortunately, due to the limited number of published sequences with R264T and R264Q mutations, we have been unable to identify potential compensatory changes (or in the case of R264G, to identify others besides E260D, which itself requires a rare A-to-C substitution) for the fitness defects associated with these mutations.
Despite the severe replicative defect of R264K in vitro, this mutation is selected as the predominant substitution to escape in vivo immune pressure by the B27-KK10-specific CD8+ T-cell response. We have previously shown that the R264K mutation occurs in nearly all cases concomitantly with the rare compensatory mutation S173A. Therefore, in HLA-B27+ subjects, mutational escape at codon 264 is typically delayed until R264K is generated together with S173A. Presumably, HIV-1 remains under continuous, efficient CTL pressure for this extended length of time, which may contribute to long-term viral suppression (26, 56). We propose that the lower frequency of R264G, R264T, and R264Q versus R264K at P2 of the KK10 epitope can be explained by a combination of several viral constraints. We observed a replicative capacity for all of the alternative escape variants lower than that of the compensated SARKLM mutant in both the absence and the presence of KK10-specific CTL, indicating that a certain fitness threshold may need to be obtained before a variant can outgrow WT virus to become the dominant species. Additionally, we noted that R264G displays a severe replication defect when combined with L268M, which likely limits development of the RG variant in the context of this earlier escape mutation. Finally, we identified a bias in the nucleotide error rate during viral replication that may contribute to the preferential generation of R264K. These observations underscore the notion that the KK10 epitope and especially the Gag codon 264 are located in a highly sensitive area of capsids with structural and functional constraints that limit viral escape from HLA-B27 CTL pressure. This region of Gag might therefore function as an attractive target for efficient immunomodulatory vaccine approaches.
Acknowledgments
The CD3:CD8-bispecific antibody was a kind gift from Johnson T. Wong, Department of Medicine, Massachusetts General Hospital, Boston. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: human rIL-2 from Maurice Gately, Hoffmann-La Roche, Inc.; plasmid pNL4-3 from Malcolm Martin; and plasmid pHEF-VSVg from L. J. Chang. We thank Andreas Meyerhans, Homburg/Saar, Germany, for very helpful discussions of the manuscript. We are grateful to all individuals who enabled this study by donating blood.
This study was supported by the National Institutes of Health, grant R01-AI054178 (T.M.A.).
The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other supporting agencies.
Footnotes
Published ahead of print on 2 April 2008.
REFERENCES
- 1.Almeida, J. R., D. A. Price, L. Papagno, Z. A. Arkoub, D. Sauce, E. Bornstein, T. E. Asher, A. Samri, A. Schnuriger, I. Theodorou, D. Costagliola, C. Rouzioux, H. Agut, A. G. Marcelin, D. Douek, B. Autran, and V. Appay. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 2042473-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altfeld, M., T. M. Allen, X. G. Yu, M. N. Johnston, D. Agrawal, B. T. Korber, D. C. Montefiori, D. H. O'Connor, B. T. Davis, P. K. Lee, E. L. Maier, J. Harlow, P. J. Goulder, C. Brander, E. S. Rosenberg, and B. D. Walker. 2002. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature 420434-439. [DOI] [PubMed] [Google Scholar]
- 3.Altfeld, M., E. T. Kalife, Y. Qi, H. Streeck, M. Lichterfeld, M. N. Johnston, N. Burgett, M. E. Swartz, A. Yang, G. Alter, X. G. Yu, A. Meier, J. K. Rockstroh, T. M. Allen, H. Jessen, E. S. Rosenberg, M. Carrington, and B. D. Walker. 2006. HLA alleles associated with delayed progression to AIDS contribute strongly to the initial CD8(+) T cell response against HIV-1. PLoS Med. 3e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ammaranond, P., J. Zaunders, C. Satchell, D. van Bockel, D. A. Cooper, and A. D. Kelleher. 2005. A new variant cytotoxic T lymphocyte escape mutation in HLA-B27-positive individuals infected with HIV type 1. AIDS Res. Hum. Retrovir. 21395-397. [DOI] [PubMed] [Google Scholar]
- 5.Bailey, J. R., H. Zhang, B. W. Wegweiser, H. C. Yang, L. Herrera, A. Ahonkhai, T. M. Williams, R. F. Siliciano, and J. N. Blankson. 2007. Evolution of HIV-1 in an HLA-B*57-positive patient during virologic escape. J. Infect. Dis. 19650-55. [DOI] [PubMed] [Google Scholar]
- 6.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415335-339. [DOI] [PubMed] [Google Scholar]
- 7.Berkhout, B., and F. J. van Hemert. 1994. The unusual nucleotide content of the HIV RNA genome results in a biased amino acid composition of HIV proteins. Nucleic Acids Res. 221705-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betts, M. R., B. Exley, D. A. Price, A. Bansal, Z. T. Camacho, V. Teaberry, S. M. West, D. R. Ambrozak, G. Tomaras, M. Roederer, J. M. Kilby, J. Tartaglia, R. Belshe, F. Gao, D. C. Douek, K. J. Weinhold, R. A. Koup, P. Goepfert, and G. Ferrari. 2005. Characterization of functional and phenotypic changes in anti-Gag vaccine-induced T cell responses and their role in protection after HIV-1 infection. Proc. Natl. Acad. Sci. USA 1024512-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. A. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 686103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braaten, D., E. K. Franke, and J. Luban. 1996. Cyclophilin A is required for the replication of group M human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus SIVCPZGAB but not group O HIV-1 or other primate immunodeficiency viruses. J. Virol. 704220-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brockman, M. A., A. Schneidewind, M. Lahaie, A. Schmidt, T. Miura, I. Desouza, F. Ryvkin, C. A. Derdeyn, S. Allen, E. Hunter, J. Mulenga, P. A. Goepfert, B. D. Walker, and T. M. Allen. 2007. Escape and compensation from early HLA-B57-mediated cytotoxic T-lymphocyte pressure on human immunodeficiency virus type 1 Gag alter capsid interactions with cyclophilin A. J. Virol. 8112608-12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brockman, M. A., G. O. Tanzi, B. D. Walker, and T. M. Allen. 2006. Use of a novel GFP reporter cell line to examine replication capacity of CXCR4- and CCR5-tropic HIV-1 by flow cytometry. J. Virol. Methods 131134-142. [DOI] [PubMed] [Google Scholar]
- 13.Chang, L. J., V. Urlacher, T. Isakuma, Y. Cui, and J. Zucali. 1999. Efficacy and safety analyses of a recombinant human immunodeficiency virus type 1 derived vector system. Gene Ther. 6715-728. [DOI] [PubMed] [Google Scholar]
- 14.Cheng, Y., and W. H. Prusoff. 1973. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 223099-3108. [DOI] [PubMed] [Google Scholar]
- 15.Deforche, K., R. Camacho, K. V. Laethem, B. Shapiro, Y. Moreau, A. Rambaut, A. M. Vandamme, and P. Lemey. 2007. Estimating the relative contribution of dNTP pool imbalance and APOBEC3G/3F editing to HIV evolution in vivo. J. Comput. Biol. 141105-1114. [DOI] [PubMed] [Google Scholar]
- 16.Douglas, C. C., D. Thomas, J. Lanman, and P. E. Prevelige, Jr. 2004. Investigation of N-terminal domain charged residues on the assembly and stability of HIV-1 CA. Biochemistry 4310435-10441. [DOI] [PubMed] [Google Scholar]
- 17.Feeney, M. E., Y. Tang, K. A. Roosevelt, A. J. Leslie, K. McIntosh, N. Karthas, B. D. Walker, and P. J. Goulder. 2004. Immune escape precedes breakthrough human immunodeficiency virus type 1 viremia and broadening of the cytotoxic T-lymphocyte response in an HLA-B27-positive long-term-nonprogressing child. J. Virol. 788927-8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forshey, B. M., U. von Schwedler, W. I. Sundquist, and C. Aiken. 2002. Formation of a human immunodeficiency virus type 1 core of optimal stability is crucial for viral replication. J. Virol. 765667-5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamble, T. R., F. F. Vajdos, S. Yoo, D. K. Worthylake, M. Houseweart, W. I. Sundquist, and C. P. Hill. 1996. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell 871285-1294. [DOI] [PubMed] [Google Scholar]
- 20.Gao, X., A. Bashirova, A. K. Iversen, J. Phair, J. J. Goedert, S. Buchbinder, K. Hoots, D. Vlahov, M. Altfeld, S. J. O'Brien, and M. Carrington. 2005. AIDS restriction HLA allotypes target distinct intervals of HIV-1 pathogenesis. Nat. Med. 111290-1292. [DOI] [PubMed] [Google Scholar]
- 21.Gatanaga, H., D. Das, Y. Suzuki, D. D. Yeh, K. A. Hussain, A. K. Ghosh, and H. Mitsuya. 2006. Altered HIV-1 Gag protein interactions with cyclophilin A (CypA) on the acquisition of H219Q and H219P substitutions in the CypA binding loop. J. Biol. Chem. 2811241-1250. [DOI] [PubMed] [Google Scholar]
- 22.Geels, M. J., C. A. Jansen, E. Baan, I. M. De Cuyper, G. J. van Schijndel, H. Schuitemaker, J. Goudsmit, G. Pollakis, F. Miedema, W. A. Paxton, and D. van Baarle. 2006. CTL escape and increased viremia irrespective of HIV-specific CD4+ T-helper responses in two HIV-infected individuals. Virology 345209-219. [DOI] [PubMed] [Google Scholar]
- 23.Goodenow, M., T. Huet, W. Saurin, S. Kwok, J. Sninsky, and S. Wain-Hobson. 1989. HIV-1 isolates are rapidly evolving quasispecies: evidence for viral mixtures and preferred nucleotide substitutions. J. Acquir. Immune Defic. Syndr. 2344-352. [PubMed] [Google Scholar]
- 24.Goulder, P. J., C. Brander, Y. Tang, C. Tremblay, R. A. Colbert, M. M. Addo, E. S. Rosenberg, T. Nguyen, R. Allen, A. Trocha, M. Altfeld, S. He, M. Bunce, R. Funkhouser, S. I. Pelton, S. K. Burchett, K. McIntosh, B. T. Korber, and B. D. Walker. 2001. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature 412334-338. [DOI] [PubMed] [Google Scholar]
- 25.Goulder, P. J., M. Bunce, P. Krausa, K. McIntyre, S. Crowley, B. Morgan, A. Edwards, P. Giangrande, R. E. Phillips, and A. J. McMichael. 1996. Novel, cross-restricted, conserved, and immunodominant cytotoxic T lymphocyte epitopes in slow progressors in HIV type 1 infection. AIDS Res. Hum. Retrovir. 121691-1698. [DOI] [PubMed] [Google Scholar]
- 26.Goulder, P. J. R., R. E. Phillips, R. A. Colbert, S. McAdam, G. Ogg, M. A. Nowak, P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, A. J. McMichael, and S. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3212-217. [DOI] [PubMed] [Google Scholar]
- 27.Gulukota, K., and C. DeLisi. 2001. Neural network method for predicting peptides that bind major histocompatibility complex molecules. Methods Mol. Biol. 156201-209. [DOI] [PubMed] [Google Scholar]
- 28.Harris, R. S., K. N. Bishop, A. M. Sheehy, H. M. Craig, S. K. Petersen-Mahrt, I. N. Watt, M. S. Neuberger, and M. H. Malim. 2003. DNA deamination mediates innate immunity to retroviral infection. Cell 113803-809. [DOI] [PubMed] [Google Scholar]
- 29.Huet, S., D. F. Nixon, J. B. Rothbard, A. Townsend, S. A. Ellis, and A. J. McMichael. 1990. Structural homologies between two HLA B27-restricted peptides suggest residues important for interaction with HLA B27. Int. Immunol. 2311-316. [DOI] [PubMed] [Google Scholar]
- 30.Janini, M., M. Rogers, D. R. Birx, and F. E. McCutchan. 2001. Human immunodeficiency virus type 1 DNA sequences genetically damaged by hypermutation are often abundant in patient peripheral blood mononuclear cells and may be generated during near-simultaneous infection and activation of CD4+ T cells. J. Virol. 757973-7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Q. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaslow, R. A., M. Carrington, R. Apple, L. Park, A. Munoz, A. J. Saah, J. J. Goedert, C. Winkler, S. J. O'Brien, C. Rinaldo, R. Detels, W. Blattner, J. Phair, H. Erlich, and D. L. Mann. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2405-411. [DOI] [PubMed] [Google Scholar]
- 33.Kelleher, A. D., C. Long, E. C. Holmes, R. L. Allen, J. Wilson, C. Conlon, C. Workman, S. Shaunak, K. Olson, P. Goulder, C. Brander, G. Ogg, J. S. Sullivan, W. Dyer, I. Jones, A. J. McMichael, S. Rowland-Jones, and R. E. Phillips. 2001. Clustered mutations in HIV-1 gag are consistently required for escape from HLA-B27-restricted cytotoxic T lymphocyte responses. J. Exp. Med. 193375-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keulen, W., N. K. Back, A. van Wijk, C. A. Boucher, and B. Berkhout. 1997. Initial appearance of the 184Ile variant in lamivudine-treated patients is caused by the mutational bias of human immunodeficiency virus type 1 reverse transcriptase. J. Virol. 713346-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koenig, S., A. J. Conley, Y. A. Brewah, G. M. Jones, S. Leath, L. J. Boots, V. Davey, G. Pantaleo, J. F. Demarest, C. Carter, C. Wannebo, J. R. Yannelli, S. A. Rosenberg, and H. C. Lane. 1995. Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat. Med. 1330-336. [DOI] [PubMed] [Google Scholar]
- 36.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 684650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, B., A. D. Gladden, M. Altfeld, J. M. Kaldor, D. A. Cooper, A. D. Kelleher, and T. M. Allen. 2007. Rapid reversion of sequence polymorphisms dominates early human immunodeficiency virus type 1 evolution. J. Virol. 81193-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lichterfeld, M., D. G. Kavanagh, K. L. Williams, B. Moza, S. K. Mui, T. Miura, R. Sivamurthy, R. Allgaier, F. Pereyra, A. Trocha, M. Feeney, R. T. Gandhi, E. S. Rosenberg, M. Altfeld, T. M. Allen, R. Allen, B. D. Walker, E. J. Sundberg, and X. G. Yu. 2007. A viral CTL escape mutation leading to immunoglobulin-like transcript 4-mediated functional inhibition of myelomonocytic cells. J. Exp. Med. 2042813-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madden, D. R., J. C. Gorga, J. L. Strominger, and D. C. Wiley. 1992. The three-dimensional structure of HLA-B27 at 2.1 A resolution suggests a general mechanism for tight peptide binding to MHC. Cell 701035-1048. [DOI] [PubMed] [Google Scholar]
- 40.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Migueles, S. A., M. S. Sabbaghian, W. L. Shupert, M. P. Bettinotti, F. M. Marincola, L. Martino, C. W. Hallahan, S. M. Selig, D. Schwartz, J. Sullivan, and M. Connors. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 972709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moriyama, E. N., Y. Ina, K. Ikeo, N. Shimizu, and T. Gojobori. 1991. Mutation pattern of human immunodeficiency virus gene. J. Mol. Evol. 32360-363. [DOI] [PubMed] [Google Scholar]
- 43.Mothé, B. R., J. Weinfurter, C. Wang, W. Rehrauer, N. Wilson, T. M. Allen, D. B. Allison, and D. I. Watkins. 2003. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 772736-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nietfeld, W., M. Bauer, M. Fevrier, R. Maier, B. Holzwarth, R. Frank, B. Maier, Y. Riviere, and A. Meyerhans. 1995. Sequence constraints and recognition by CTL of an HLA-B27-restricted HIV-1 gag epitope. J. Immunol. 1542189-2197. [PubMed] [Google Scholar]
- 45.Nixon, D. F., A. R. Townsend, J. G. Elvin, C. R. Rizza, J. Gallwey, and A. J. McMichael. 1988. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature 336484-487. [DOI] [PubMed] [Google Scholar]
- 46.O'Brien, S. J., X. Gao, and M. Carrington. 2001. HLA and AIDS: a cautionary tale. Trends Mol. Med. 7379-381. [DOI] [PubMed] [Google Scholar]
- 47.Oxenius, A., D. A. Price, A. Trkola, C. Edwards, E. Gostick, H. T. Zhang, P. J. Easterbrook, T. Tun, A. Johnson, A. Waters, E. C. Holmes, and R. E. Phillips. 2004. Loss of viral control in early HIV-1 infection is temporally associated with sequential escape from CD8+ T cell responses and decrease in HIV-1-specific CD4+ and CD8+ T cell frequencies. J. Infect. Dis. 190713-721. [DOI] [PubMed] [Google Scholar]
- 48.Pillay, T., H.-T. Zhang, J. W. Drijfhout, N. Robinson, H. Brown, M. Khan, J. Moodley, M. Adhikari, K. Pfafferott, M. E. Feeney, A. St. John, E. C. Holmes, H. M. Coovadia, P. Klenerman, P. J. R. Goulder, and R. E. Phillips. 2005. Unique acquisition of cytotoxic T-lymphocyte escape mutants in infant human immunodeficiency virus type 1 infection. J. Virol. 7912100-12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plikat, U., K. Nieselt-Struwe, and A. Meyerhans. 1997. Genetic drift can dominate short-term human immunodeficiency virus type 1 nef quasispecies evolution in vivo. J. Virol. 714233-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reimann, K. A., K. Tenner-Racz, P. Racz, D. C. Montefiori, Y. Yasutomi, W. Lin, B. J. Ransil, and N. L. Letvin. 1994. Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J. Virol. 682362-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8(+) lymphocytes. Science 283857-860. [DOI] [PubMed] [Google Scholar]
- 52.Schneidewind, A., M. A. Brockman, R. Yang, R. I. Adam, B. Li, S. Le Gall, C. R. Rinaldo, S. L. Craggs, R. L. Allgaier, K. A. Power, T. Kuntzen, C. S. Tung, M. X. LaBute, S. M. Mueller, T. Harrer, A. J. McMichael, P. J. Goulder, C. Aiken, C. Brander, A. D. Kelleher, and T. M. Allen. 2007. Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J. Virol. 8112382-12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sette, A., A. Vitiello, B. Reherman, P. Fowler, R. Nayersina, W. M. Kast, C. J. Melief, C. Oseroff, L. Yuan, J. Ruppert, J. Sidney, M. F. del Guercio, S. Southwood, R. T. Kubo, R. W. Chesnut, H. M. Grey, and F. V. Chisari. 1994. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J. Immunol. 1535586-5592. [PubMed] [Google Scholar]
- 54.Sidney, J., S. Southwood, C. Oseroff, M.-F. del Guercio, A. Sette, and H. M. Grey. 1998. Measurement of MHC/peptide interactions by gel filtration, p. 18.3.1-18.3.19. In J. E. Coligan, B. E. Bierer, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology. John Wiley & Sons, Hoboken, N.J.
- 55.Sidney, J., S. Southwood, and A. Sette. 2005. Classification of A1- and A24-supertype molecules by analysis of their MHC-peptide binding repertoires. Immunogenetics 57393-408. [DOI] [PubMed] [Google Scholar]
- 56.Streeck, H., M. Lichterfeld, G. Alter, A. Meier, N. Teigen, B. Yassine-Diab, H. K. Sidhu, S. Little, A. Kelleher, J. P. Routy, E. S. Rosenberg, R. P. Sekaly, B. D. Walker, and M. Altfeld. 2007. Recognition of a defined region within p24 Gag by CD8+ T cells during primary human immunodeficiency virus type 1 infection in individuals expressing protective HLA class I alleles. J. Virol. 817725-7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang, J., S. Tang, E. Lobashevsky, A. D. Myracle, U. Fideli, G. Aldrovandi, S. Allen, R. Musonda, and R. A. Kaslow. 2002. Favorable and unfavorable HLA class I alleles and haplotypes in Zambians predominantly infected with clade C human immunodeficiency virus type 1. J. Virol. 768276-8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Towers, G. J., T. Hatziioannou, S. Cowan, S. P. Goff, J. Luban, and P. D. Bieniasz. 2003. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat. Med. 91138-1143. [DOI] [PubMed] [Google Scholar]
- 59.Vartanian, J. P., A. Meyerhans, M. Sala, and S. Wain-Hobson. 1994. G→A hypermutation of the human immunodeficiency virus type 1 genome: evidence for dCTP pool imbalance during reverse transcription. Proc. Natl. Acad. Sci. USA 913092-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walker, B. D., C. Flexner, K. Birch-Limberger, L. Fisher, T. J. Paradis, A. Aldovini, R. Young, B. Moss, and R. T. Schooley. 1989. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 869514-9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yant, L. J., T. C. Friedrich, R. C. Johnson, G. E. May, N. J. Maness, A. M. Enz, J. D. Lifson, D. H. O'Connor, M. Carrington, and D. I. Watkins. 2006. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 805074-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu, X. G., M. Lichterfeld, K. L. Williams, J. Martinez-Picado, and B. D. Walker. 2007. Random T-cell receptor recruitment in human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells from genetically identical twins infected with the same HIV-1 strain. J. Virol. 8112666-12669. [DOI] [PMC free article] [PubMed] [Google Scholar]