Abstract
Capsomeres are considered to be an alternative to viruslike particle (VLP)-based vaccines as they can be produced in prokaryotic expression systems. So far, no detailed side-by-side comparison of VLPs and capsomeres has been performed. In the present study, we immunized mice with insect cell-derived human papillomavirus type 16 VLPs and capsomeres. VLPs induced consistently higher antibody titers than capsomeres but the two forms induced similar CD8 T-cell responses after subcutaneous, intranasal, and oral immunization, and at least 20 to 40 times more L1 in the form of capsomeres than in the form of VLPs was needed to achieve comparable antibody responses. These results were confirmed by DNA immunization. The lower immunogenicity of capsomeres was independent of the isotype switch, as it was also observed for the early immunoglobulin M responses. Although there were differences in the display of surface epitopes between the L1 particles, these did not contribute significantly to the differences in the immune responses. capsomeres were less immunogenic than VLPs in Toll-like receptor 4 (TLR4)-deficient mice, suggesting that the lower immunogenicity is not due to a failure of capsomeres to trigger TLR4. We observed better correlation between antibody results from enzyme-linked immunosorbent assays and neutralization assays for sera from VLP-immunized mice than for sera from capsomere-immunized mice, suggesting qualitative differences between VLPs and capsomeres. We also showed that the lower immunogenicity of capsomeres could be compensated by the use of an adjuvant system containing MPL. Taken together, these results suggest that, presumably because of the lower degree of complexity of the antigen organization, capsomeres are significantly less immunogenic than VLPs with respect to the humoral immune response and that this characteristic should be considered in the design of putative capsomere-based prophylactic vaccines.
The development of the prophylactic papillomavirus vaccines for the prevention of invasive cervical cancer started in the early 1990s with the discovery that L1, the major capsid protein of papillomaviruses, can self-assemble into viruslike particles (VLPs) following expression in different systems, including a vaccinia virus expression system, baculovirus-infected insect cells, and Schizosaccharomyces pombe (20, 25, 27, 40, 48). Immunization with VLPs, which are morphologically similar to native virions, leads to the induction of high titers of neutralizing antibodies and L1-specific cytotoxic T lymphocytes (12, 16, 42, 45). Furthermore, in the cottontail rabbit papillomavirus and the canine oral papillomavirus models, it was shown previously that immunized animals are protected against challenge with infectious virions (8, 26, 51). As an achievement based on these initial studies and many clinical trials in the following years, two VLP-based vaccines were recently introduced to the market (21, 31, 54). Gardasil, produced by Merck & Co, contains in addition to the VLPs of the two oncogenic human papillomavirus types 16 and 18 (HPV16 and HPV18) particles of the low-risk types 6 and 11 and was approved in the United States and in Europe in 2006. In 2007, the second vaccine, Cervarix (GlaxoSmithKline), which includes VLPs of the two high-risk types HPV16 and HPV18, became commercially available in Australia and Europe. Nevertheless, the production, storage, and intramuscular administration of VLP-based vaccines is cost-intensive, and therefore, the development of an economically advantageous vaccine is warranted for the introduction of HPV vaccines into developing countries, the areas where about 83% of cervical cancer cases occur (34). L1 capsomeres, the pentameric subcapsid particles, are considered to be a possible cost-effective alternative to VLP-based vaccines because they can be produced in Escherichia coli. Several studies have shown that immunization with capsomeres by different administration routes induces neutralizing antibodies and cytotoxic T lymphocytes (15, 33, 42, 60). However, so far only few data from comparisons of the immunogenicities of different L1 particles are available, and most of the studies have involved capsomeres derived from E. coli. As protein preparations from bacteria bear the risk of contamination with endotoxins, which have a strong enhancing effect on the immune response, they cannot be directly compared with VLP preparations from insect cells. Alternatively, capsomeres can be produced in insect cells if two conserved cysteine residues, which are essential for the capsid assembly because they form intercapsomeric disulfide bonds, are eliminated (23, 29, 47). By using both insect cell-derived VLPs and capsomeres, the immunogenicities of the two L1 particle forms can be directly compared. Data obtained previously by Fligge et al. using intraperitoneal immunization and by our group using oral immunization already suggested differences in immunogenicity between capsomeres and VLPs (17, 53).
Analyzing the immunogenicities of different L1 particles in close detail, we showed in this study that VLPs induced considerably higher antibody titers than capsomeres but that the two particle forms induced similar CD8 T-cell responses, not only after subcutaneous (s.c.), intranasal, or oral immunization with low doses of insect cell-derived L1 protein but also in a DNA immunization experiment using two constructs leading to the expression of either VLPs or capsomeres. Capsomeres induced not only lower immunoglobulin G (IgG) responses but also lower IgM responses than VLPs, indicating that the differences are not based on differences in the efficiency of isotype switching. Furthermore, the lower immunogenicity of capsomeres did not seem to be due to a lack of epitopes or a failure to trigger Toll-like receptor 4 (TLR4).
MATERIALS AND METHODS
Mice.
Six- to 8-week-old C57BL/6 (Charles River Wiga Breeding Laboratories, Sulzfeld, Germany) and C3H/HeJ and C3H/HeOuJ (The Jackson Laboratory, Bar Harbor, ME) mice were maintained under specific-pathogen-free conditions.
Purification of HPV16 L1 particles.
HPV16 L1 VLPs and capsomeres were produced using recombinant baculoviruses containing the wild-type HPV16 L1 gene or a mutated L1 gene (carrying the mutations C175A and C428A) as described previously, with minor modifications (53). In short, High Five insect cells (Invitrogen, Germany) were infected for 3 days with recombinant baculoviruses and harvested by centrifugation. Cell pellets were resuspended in 20 ml of extraction buffer (5 mM MgCl2, 5 mM CaCl2, 1 M NaCl, 0.01% Triton X-100, 20 mM HEPES [pH 7.4], and 1 mM phenylmethylsulfonyl fluoride), and proteins were extracted by sonication. The lysate was cleared, loaded onto a two-step gradient consisting of 7 ml of sucrose (30%, wt/vol) on top of 7 ml of CsCl (58%, wt/vol), and then centrifuged at 96,500 × g at 10°C for 3 h in an SW32 rotor (Beckman ultracentrifuge). The interphase between the sucrose and the CsCl and the complete CsCl layer were mixed and transferred into Quick-Seal tubes (Beckman). After another centrifugation step for 16 to 18 h at 20°C and 184,000 × g in a Sorval TFT 65.13 rotor, the tubes were punctured at the bottom with 20-gauge needles and 1-ml fractions were collected. The L1-containing fractions were evaluated by an antigen capture enzyme-linked immunosorbent assay (ELISA) and Western blot analysis, and the structures of the particles were characterized by electron microscopy (32).
Immunization of and sample collection from mice. (i) Protein immunization.
HPV16 VLPs or capsomeres were dialyzed against 20 mM HEPES (pH 7.4)-0.5 M NaCl. The L1 concentration was determined by comparing different dilutions of the L1 stock solutions with bovine serum albumin standards (2, 1, 0.5, 0.25, and 0.125 μg/μl) in the Bradford assay and Coomassie gel analyses. Final concentrations for immunization experiments were adjusted by diluting the L1 particles in 1× phosphate-buffered saline (PBS). For s.c. immunization, C57BL/6 mice were immunized either three times biweekly with 3 to 48 ng of L1 protein or once with 4, 20, or 100 μg of particles. C3H/HeJ and C3H/HeOuJ mice were immunized twice over a 2-week interval by s.c. injection with 2.5 or 15 μg of L1 particles. Intranasally immunized mice were anesthetized by intraperitoneal injection with a solution containing 100 μg of ketamine (Ketavet; Pharmacia, Germany)/g and 8 μg of xylazine (Rompun; Bayer, Germany)/g. One to 27 μg of L1 was applied dropwise in a total volume of 10 μl. For oral immunization, 50 μg of VLPs or capsomeres mixed with 10 μg of CpG DNA (CpG ODN 1826; 5′-TCCATGACGTTCCTGACGTT-3′) (25), which was synthesized with a nuclease-resistant phosphorothioate backbone, in 200 μl of PBS was applied by gavage. To analyze the influences of different adjuvants on the immunogenicities of L1 particles, 10-μg doses of VLPs or capsomeres were either administered without adjuvant or adsorbed to 500 μg of aluminum hydroxide (A8222; Sigma, Germany). Additionally, mice were immunized with 10 μg of VLPs or capsomeres in combination with a mixture of 500 μg of aluminum hydroxide and an adjuvant system containing 50 μg of monophosphoryl lipid A derived from Salmonella enterica serovar Minnesota R595 and 50 μg of synthetic trehalose dicorynomycolate in squalene and Tween 80 (catalog no. S6322; Sigma, Germany).
(ii) DNA immunization.
For DNA immunization, either the humanized HPV16 L1 (L1h) gene (28), a variant humanized L1 (L1h2xCysM) gene containing two mutations (C175A and C428A), or a humanized HPV16 L2 (L2h) gene were subcloned in the pUF3 vector under the control of the cytomegalovirus immediate-early promoter (62). The plasmids were purified using the EndoFree plasmid mega kit (Qiagen, Germany), and endotoxin levels were below the detection limit of the QCL-1000 Limulus amebocyte lysate assay (Lonza, Germany). Fifty-microgram aliquots of the different constructs were applied intradermally using a tattoo device (7).
(iii) Sample collection.
Sera were collected from anesthetized animals via retro-orbital sinus puncture at the time points indicated in the figure legends, and 8 to 14 days after the last immunization, final blood samples were collected by cardiac puncture.
ELISAs. (i) Detection of IgG or IgM in sera by VLP capture ELISAs.
Assay plates (96 well; Becton Dickinson) were coated overnight at 4°C with protein G-purified polyclonal rabbit IgG (blocked against insect cell and baculovirus proteins) specific for HPV16 VLPs (85 ng/well). All following incubation steps were carried out for 1 h each at 37°C, antigens and antibodies were diluted in blocking solution (3% skim milk in PBS-0.3% Tween 20 [PBS/T]), and each step was followed by washing three times with PBS/T. After the incubation of the plates with blocking solution, 350 ng of VLPs per well was added. The mouse sera were diluted 1:50 or titrated up to a dilution of 1:1,409,600. As a secondary antibody, either IgG-specific antibody (goat anti-mouse IgG conjugated with horseradish peroxidase; Dianova, Germany) or IgM-specific antibody (goat anti-mouse IgM conjugated with horseradish peroxidase; Southern Biotechnology) in a 1:3,000 dilution was added. The plates were then washed six times with PBS/T, and freshly prepared staining solution {100 mM sodium acetate, 50 mM NaH2PO4, pH 4.2, 1 mg of ABTS [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)]/ml, 0.012% H2O2} was added. Ten to 20 min later, absorbance at 405 nm was measured using a Titertek plate reader. IgG titers were expressed as the reciprocal of the highest dilution giving an absorbance above the cutoff value (the average for the negative control mice plus three times the standard deviation).
(ii) Direct ELISAs using different monoclonal antibodies.
Plates (96 well; Becton Dickinson) were coated overnight at 4°C with 1 μg of VLPs or capsomeres per well. The plates were washed three times with PBS/T and incubated for 1 h at 37°C with blocking solution. Hybridoma supernatants containing a panel of monoclonal antibodies which were described before (10, 11, 30, 39) were kindly provided by N. D. Christensen (College of Medicine, Pennsylvania State University) Supernatants were diluted in blocking solution by using the dilution factors determined by Rizk et al. (38) and added to the plates. After being incubated for 1 h at 37°C, the plates were washed and stained as described above.
Preparation of pseudovirions and neutralization assay.
The production of pseudovirions was performed by the cotransfection of 293TT cells with a plasmid containing the HPV16 L1h and L2h genes and a simian virus 40 origin of replication (pCDNA4-HPV16L1h-IRES-L2h/SV40ori) and a second plasmid encoding the reporter protein secreted alkaline phosphatase (SEAP) under the control of the cytomegalovirus promoter (pCMVSEAP) by a procedure described earlier (36) with minor modifications (18).
As target cells for the neutralization assay, 293TT cells were seeded at a concentration of 22,500 cells per well onto 96-well plates in Dulbecco's modified Eagle's medium (Sigma, Germany) supplemented with 10% fetal calf serum (FCS; GIBCO BRL, Germany) and 1% penicillin-streptomycin (Life Technologies, Germany). The following day, the pseudovirions were diluted in Dulbecco's modified Eagle's medium (1:5,000) and mixed with the sera at different dilutions (Psvserum). After 15 min of incubation at room temperature, the medium of the 293TT cells was replaced by 200 μl of the pseudovirion solution. As a control, cells were incubated with pseudovirions alone (Psvalone) or with pseudovirions in the presence of a known neutralizing polyclonal antiserum specific for HPV16 (PsvAb). SEAP in cell culture supernatants was detected 5 days later with the chemiluminescence SEAP reporter gene assay by following the instructions of the manufacturer (Roche, Germany). The neutralization activity was calculated using the following formula: percent neutralization = (value for Psvalone − value for Psvserum)/(value for Psvalone − value for PsvAb) × 100. Sera with at least 70% neutralizing activity were regarded as neutralization positive.
ELISPOT assays and restimulation of L1-specific cytotoxic T lymphocytes.
Enzyme-linked immunospot (ELISPOT) assays were performed as described previously (53). In short, 96-well MultiScreen IP sterile plates (MAIPS; Millipore, Germany) were moistened with 70% ethanol, washed, and coated overnight at 4°C with 600 ng of anti-mouse gamma interferon (IFN-γ) capture antibody (clone R4-6A2; BD Pharmingen, Germany) per well. After four washes of the plates with PBS and a 2-h blocking step with RPMI 1640 medium (Sigma, Germany) supplemented with 10% FCS (GIBCO BRL, Germany) and 1% penicillin-streptomycin (Life Technologies, Germany), the splenocytes were seeded in triplicate in a twofold dilution series from 2 × 106 to 2.5 × 105 cells per well. As controls, cells were incubated with 200 ng of pokeweed mitogen (Sigma, Germany; positive control) or medium only (background). To detect L1-specific CD8 T-cell responses, an HPV16 L1 peptide comprising amino acids 165 to 173 [L1(165-173) peptide] was added to a final concentration of 0.2 μM. Subsequently, the plates were incubated at 37°C for 16 to 18 h and secreted IFN-γ was detected using a biotinylated anti-mouse IFN-γ antibody (clone XMG1.2; BD Pharmingen, Germany), streptavidin-alkaline phosphatase (BD Pharmingen, Germany), and a 5-bromo-4-chloro-3-indolylphosphate (BCIP)-nitroblue tetrazolium liquid substrate system (Sigma, Germany) as described before (53). The quantification of the spots was performed using an ELISPOT reader (ELISPOT reader system ELR02; AID GmbH, Germany). The number of spots in the background control wells was subtracted from the values for wells incubated with peptide. Mice were defined to be positive by the ELISPOT assay if the number of IFN-γ-positive spots per 106 cells was higher than the average number of IFN-γ-positive spots per 106 cells from negative control mice plus three times the standard deviation.
To restimulate L1-specific T lymphocytes, RMA-S cells were loaded with 0.2 μM HPV16 L1(165-173) peptide for 1.5 h at 37°C and irradiated with 100 Gy. Loaded RMA-S cells (2 × 106) were cocultured with 1.5 × 107 to 3 × 107 splenocytes at 37°C and 5% CO2. After 5 days, 250,000 to 31,250 restimulated T lymphocytes were analyzed in an ELISPOT assay as described above.
TLR activation assay.
THP1-Blue CD14 cells (InvivoGen, France) overexpress the CD14 molecule and are stably transfected with an NF-κB-inducible reporter gene encoding SEAP. Samples (100 μl) of a cell suspension containing 1.6 × 106 cells/ml were seeded onto 96-well plates in modified RPMI 1640 medium (RPMI 1640 medium [Sigma, Germany] supplemented with 10% FCS, 1% penicillin-streptomycin, 4.5 g of glucose/ml, 10 mM HEPES, 1 mM sodium pyruvate, 10 μg of blasticidin [Calbiochem, Germany]/ml, and 200 μg of zeocin [InvivoGen]/ml). As positive controls, the cells were stimulated with different TLR ligands (InvivoGen): Pam3CSK4 (TLR1/2; 200 ng/ml), a heat-killed preparation of Listeria monocytogenes (TLR2; 106 cells/ml), poly(I:C) (TLR3; 25 μg/ml), S. enterica serovar Typhimurium flagellin (TLR5; 100 ng/ml), FSL-1 (TLR6/2; 10 ng/ml), and CpG ODN 1826 (TLR9; 5 μM). Lipopolysaccharide (LPS), a potent activator of TLR4, was titrated in a 10-fold dilution series and added to the cells at final concentrations ranging from 0.0015 to 1,500 endotoxin units/ml. PBS and a VLP preparation purified from H5 insect cells infected with wild-type baculovirus served as negative controls. The ability of different L1 particles to activate TLRs was analyzed by incubating the cells with different amounts (400, 100, and 25 μg per ml) of HPV16 L1 VLPs or capsomeres in three independent preparations. All controls and L1 samples were added in a volume of 10 to 100 μl of the cell suspension and tested in duplicate. After 18 to 24 h of incubation of the cells at 37°C and 5% CO2, the activities of SEAP in the cell culture supernatants were determined using the chemiluminescence SEAP reporter gene assay according to the instructions of the manufacturer (Roche, Germany).
Statistical analyses.
For the comparison of the correlations between neutralization activities and IgG titers, Fisher's exact test was used. All other data sets were compared using the Wilcoxon rank sum test. A P value below 0.05 was considered to be statistically significant.
RESULTS
Papillomavirus VLPs and capsomeres are highly immunogenic antigens if applied s.c., intramuscularly, intranasally, or orally, and they induce high titers of neutralizing antibodies and L1-specific cytotoxic T lymphocytes (5, 15-17, 19, 25, 33, 41-43, 53, 60). Nevertheless, so far there are only limited data available concerning a direct comparison of different L1 particles with respect to their immunogenicities. Most immunization studies have been performed using E. coli-derived L1 pentamers; however, bacterially expressed L1 preparations are frequently contaminated by endotoxins, which make a direct comparison with insect cell-derived VLPs difficult due to unspecific immunostimulatory effects. We compared the induced humoral and cellular immunogenicities of VLPs and capsomeres after s.c., oral, intranasal, or DNA immunization.
s.c. immunization.
To produce HPV16 VLPs and capsomeres, the wild-type HPV16 L1 gene and the L12xCysM gene (carrying the mutations C175A and C428A) (47) were expressed in insect cells by using recombinant baculoviruses. As immunization with large amounts of L1 may mask differences in the immunogenicities of VLPs and capsomeres, limiting doses of L1 were used for the immunization experiments.
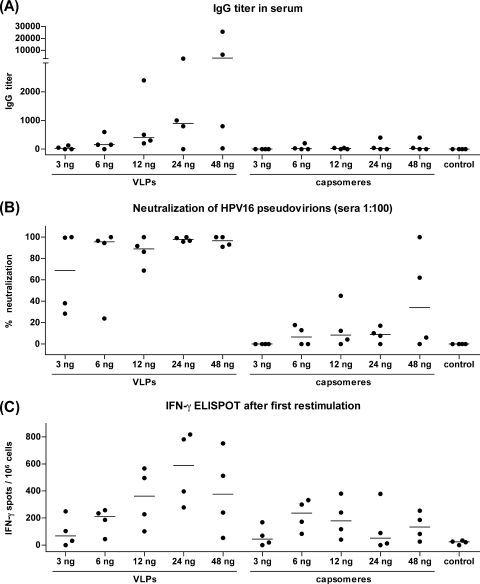
Female C57BL/6 mice (four mice per group) were immunized three times s.c. at 2-week intervals with 3 to 48 ng of L1 VLPs or capsomeres. Ten days after the last immunization, blood samples were taken and the reciprocal titers of L1-specific IgG antibodies were determined using a VLP capture ELISA (Fig. 1A; Table 1). Eighty percent of VLP-immunized mice (16 of 20) produced specific antibodies, and the application of as little as 3 ng of VLPs induced specific IgG antibodies in two of four mice; no reactivity in the sera of the control mice was found. Throughout the different groups of VLP-immunized mice, a dose dependency was observed (Table 1). In contrast, only 40% of the capsomere-immunized mice (8 of 20) developed L1-specific antibodies, and these mice clearly showed lower titers than VLP-immunized animals. Summarizing the results for the 20 VLP-immunized and the 20 capsomere-immunized mice and comparing the outcomes for these groups confirmed a statistically significant difference between the immunogenicities of VLPs and capsomeres with respect to the IgG antibody response (Wilcoxon rank sum test: P = 0.0009). Likewise, 16 of 20 mice immunized with VLPs, but only 1 of 20 mice immunized with capsomeres, were positive in the neutralization assay if the sera were diluted 1:100 (P = 0.000001) (Fig. 1B; Table 1).
FIG. 1.
s.c. immunization. C57BL/6 mice were immunized three times s.c. with 3, 6, 12, 24, or 48 ng of HPV16 L1 VLPs or capsomeres at 2-week intervals. Control mice received PBS alone. (A) The reciprocal titers of L1-specific IgG antibodies in sera collected 10 days after the third immunization were determined using a VLP capture ELISA. (B) Additionally, the sera were tested in a 1:100 dilution in a neutralization assay with HPV16 pseudovirions, and the percent neutralization is shown. (C) Splenocytes were collected 10 days after the third immunization, restimulated for 5 days with L1 peptide-loaded RMA-S cells, and analyzed in an IFN-γ ELISPOT assay. The graph shows the number of IFN-γ spots per 106 cells; the horizontal bars indicate the median values.
TABLE 1.
Immune responses after the administration of HPV16 VLPs or capsomeres using different routes of immunizationa
| Immunization route | Inoculum | Amt of L1 | IgG titer
|
% Neutralization
|
ELISPOT assay reactivity
|
|||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |||
| s.c. | VLPs | 3 ng | 25 | 0-69 | 69 | 36-100 | 68 | 24-140 |
| VLPs | 6 ng | 150 | 113-263 | 95 | 77-97 | 211 | 151-240 | |
| VLPs | 12 ng | 400 | 275-975 | 89 | 82-94 | 362 | 196-514 | |
| VLPs | 24 ng | 900 | 600-1,550 | 98 | 96-99 | 589 | 367-791 | |
| VLPs | 48 ng | 3,600 | 606-11,200 | 96 | 92-100 | 376 | 193-572 | |
| Capsomeres | 3 ng | 0 | 0-0 | 0 | 0-0 | 44 | 15-94 | |
| Capsomeres | 6 ng | 13 | 0-69 | 7 | 0-14 | 236 | 150-308 | |
| Capsomeres | 12 ng | 19 | 0-38 | 8 | 3-21 | 179 | 98-275 | |
| Capsomeres | 24 ng | 19 | 0-128 | 9 | 6-12 | 51 | 9-161 | |
| Capsomeres | 48 ng | 19 | 0-128 | 34 | 5-72 | 134 | 68-204 | |
| PBS | 0 | 0-0 | 0 | 0-0 | 24 | 16-28 | ||
| Intranasal | VLPs | 1 μg | 6,400 | 2,438-9,600 | 100 | 80-100 | 4 | 2-5 |
| VLPs | 3 μg | 8,800 | 7,600-12,000 | 100 | 100-100 | 16 | 13-20 | |
| Capsomeres | 1 μg | 0 | 0-0 | 0 | 0-0 | 0 | 0-13 | |
| Capsomeres | 3 μg | 0 | 0-300 | 31 | 19-45 | 9 | 1-19 | |
| Capsomeres | 9 μg | 51 | 38-638 | 54 | 35-79 | 17 | 13-28 | |
| Capsomeres | 27 μg | 3,250 | 2,438-4,275 | 74 | 61-84 | 49 | 40-52 | |
| PBS | 0 | 0-0 | 13 | 6-19 | 1 | 1-2 | ||
| Oral | VLPs | 50 μg (plus CpG) | 3,200 | 25-9,600 | 54 | 13-97 | NT | NT |
| Capsomeres | 50 μg (plus CpG) | 50 | 0-400 | 0 | 0-22 | NT | NT | |
| CpG only | 0 | 0-0 | 0 | 0-0 | NT | NT | ||
The table summarizes the humoral and cellular immune responses after s.c., intranasal, or oral administration of HPV16 VLPs or capsomeres. The reciprocal titers of L1-specific antibodies in the sera after the last immunization are given. Neutralizing activities of the sera in 1:100 dilutions are shown as the percent neutralization relative to that by a positive control. Reactivity in an ELISPOT assay (for s.c. immunization, an ELISPOT assay performed after restimulation, and for intranasal immunization, an ex vivo ELISPOT assay) is shown as the number of IFN-γ-positive spots per 106 cells after stimulation with an L1 H-2b-restricted peptide. IQR, interquartile range; NT, not tested.
Ten days after the third immunization, splenocytes were collected and analyzed in an IFN-γ ELISPOT assay after stimulation with the HPV16 L1(165-173) peptide, which is a major histocompatibility complex class I T-cell epitope in C57BL/6 mice of the H-2b haplotype (33). As the number of L1-specific CD8 T cells after immunization with as little as 3 to 48 ng of L1 protein was below the detection limit (data not shown), the cells were restimulated using RMA-S cells loaded with the HPV16 L1(165-173) peptide. After restimulation, 16 of 20 VLP-immunized mice and 14 of 20 capsomere-immunized mice were positive in the ELISPOT assay (Fig. 1C; Table 1). There was no clear correlation between antibody and CD8 T-cell responses, as 5 mice (3 VLP-immunized mice and 2 capsomere-immunized mice) were IgG positive but were negative by the ELISPOT assay and 11 mice (3 VLP-immunized mice and 8 capsomere-immunized mice) showed no detectable antibody responses but had CD8 T-cell responses above the cutoff level. A comparison of all mice immunized with VLPs versus all mice immunized with capsomeres resulted in a P value of 0.0284. Nevertheless, the observed difference between VLPs and capsomeres with respect to the T-cell response was less pronounced than the difference with respect to the antibody response.
Mucosal immunization.
Because VLPs were more immunogenic than capsomeres after systemic application, the effect of the immunization route on the immunogenicities of VLPs and capsomeres was investigated by evaluating the immune responses induced by the mucosal (intranasal and oral) administration of HPV16 L1 particles.
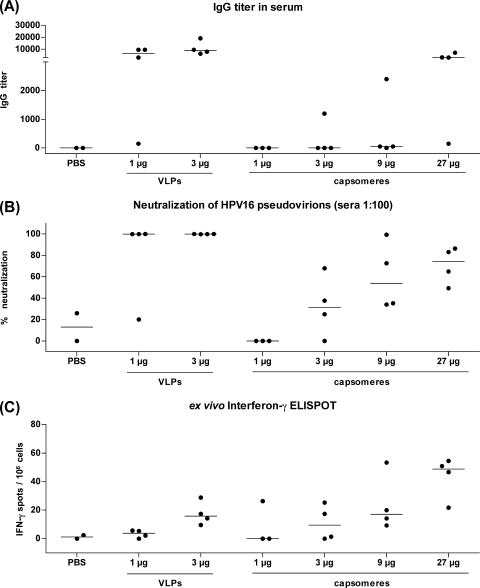
Mice were immunized intranasally with either 1 or 3 μg of VLPs (four mice per group) or with 1, 3, 9, or 27 μg of capsomeres (1 μg, three mice per group; 3, 9, and 27 μg, four mice per group). The mice were immunized three times at 2-week intervals, and 8 days after the last immunization, blood samples were taken and an ex vivo ELISPOT assay was performed. All eight mice immunized with VLPs developed L1-specific antibodies, with median titers of 6,400 (for mice immunized with 1 μg) and 8,800 (for mice immunized with 3 μg) (Fig. 2A; Table 1). In contrast, only one of seven mice receiving 1 or 3 μg of capsomeres produced L1-specific IgG antibodies (for the comparison of responses to VLPs and capsomeres in cumulated 1- and 3-μg-dose groups, P = 0.0012). Increasing the amount of L1 capsomeres used for the immunization to 9 or 27 μg of capsomeres induced humoral immune responses with higher efficiency, resulting in the development of L1 antibodies in seven of eight mice, with a median titer of 51 (9 μg) or 3,250 (27 μg), although even the 27-fold-larger amount of capsomeres still resulted in lower IgG titers than 1 μg of VLPs. In a neutralization assay with HPV16 pseudovirions, seven of eight mice immunized with 1 or 3 μg of VLPs, but none of the seven mice immunized with 1 or 3 μg of capsomeres, showed neutralization above the cutoff level (Fig. 2B). However, four of eight mice immunized with 9 or 27 μg of capsomeres were positive by the neutralization assay.
FIG. 2.
Intranasal immunization. C57BL/6 mice were immunized three times intranasally with different amounts of VLPs (1 or 3 μg) or capsomeres (1, 3, 9, or 27 μg) or with PBS alone as a negative control. Eight days after the third immunization, blood samples were taken and an ex vivo IFN-γ ELISPOT assay was performed. (A) The reciprocal titers of L1-specific IgG antibodies were determined using a VLP capture ELISA. (B) The sera were tested in a 1:100 dilution in a neutralization assay with HPV16 pseudovirions, and the graph shows the percent neutralization. (C) Numbers of IFN-γ-positive spots per 106 cells in an ex vivo ELISPOT assay are shown. The horizontal bars indicate the median values.
VLPs and capsomeres induced the development of L1-specific CD8 T-cell responses (here defined by IFN-γ release upon restimulation with a major histocompatibility complex class I-restricted L1 peptide) after intranasal application, as detected by an ex vivo ELISPOT assay, with a clear dose dependency observable for both particle forms (Fig. 2C; Table 1). A comparison of mice immunized with 1 to 3 μg of VLPs or capsomeres showed no statistically significant difference among the groups (P = 0.6126).
In a subsequent experiment, mice were immunized orally with VLPs and capsomeres. As oral immunization is less efficient than s.c. or intranasal immunization, 50 μg of L1 particles in combination with 10 μg of CpG DNA was administered four times at 2-week intervals via gavage (five mice per group). Fourteen days after the last immunization, blood samples were taken and analyzed by a VLP capture ELISA and a neutralization assay. Consistent with the observations after s.c. and intranasal immunization and with the data concerning the oral immunogenicities of L1 particles that we published recently (53), VLPs were significantly more efficient at inducing humoral immune responses after oral application than capsomeres (Fig. 3A; Table 1) (for comparison of IgG titers, P = 0.0295; for comparison of neutralization activities, P = 0.0145).
FIG. 3.
Oral immunization. C57BL/6 mice were immunized four times orally with 50 μg of VLPs or capsomeres in combination with 10 μg of CpG ODN 1826 at 2-week intervals. (A) Reciprocal titers of L1-specific antibodies in sera collected 14 days after the fourth immunization were determined using a VLP capture ELISA. (B) The ability to inhibit HPV16 pseudovirions in a neutralization assay is shown as the percent neutralization. The horizontal bars indicate the median values.
DNA immunization.
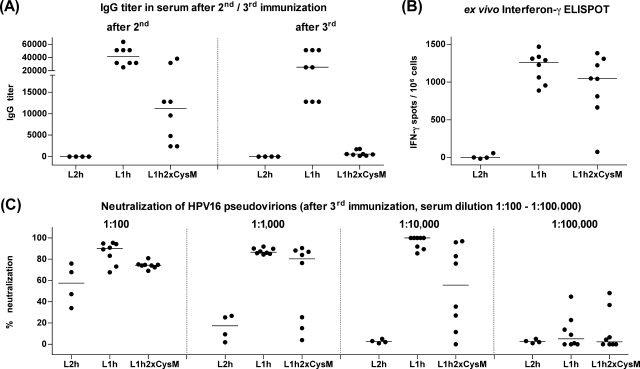
The capsomeres and VLPs used for the immunizations described above were both purified from insect cells to exclude the adjuvant effects of possible endotoxin contamination in E. coli-derived capsomere purifications. It has been reported previously that hypomethylated CpG sequences of insect cell DNA can enhance immune responses (49, 50), and it is possible that additional factors are present in insect cell lysates and preferentially copurify with VLPs. To address this issue, we immunized mice with endotoxin-free DNA preparations of expression vectors containing either an HPV16 L1h gene, an L1h2xCysM gene (carrying the mutations C175A and C428A), or an L2h gene as a negative control. We confirmed that L1h and L1h2xCysM genes were expressed at the same levels after the transient transfection of 293TT cells in vitro (data not shown). Mice (L1h and L1h2xCysM, eight mice per group; L2h, four mice per group) were immunized three times at 2-week intervals with 50 μg of DNA by using a tattoo device (7), and blood samples were taken 14 days after the second and 8 days after the third immunization. All mice immunized with L1h or L1h2xCysM developed L1-specific antibodies, although the antibody titers in mice immunized with the L1h2xCysM construct were significantly lower than those in mice immunized with L1h (Fig. 4A; Table 2), and the difference decreased with increasing numbers of immunizations (for results after the second immunization, P = 0.0007; for results after the third immunization, P = 0.007). In the neutralization assay, all sera of mice immunized three times with L1h or L1h2xCysM were positive in a 1:100 dilution (Fig. 4C). All mice immunized with L1h, but only four of eight mice immunized with L1h2xCysM, developed neutralizing-antibody titers of at least 10.000. Only one of the mice immunized with L2h developed neutralizing antibodies, presumably against L2, which confirms earlier observations that L2 is a rather poor immunogen. In an ex vivo IFN-γ ELISPOT assay, which was performed 8 days after the third immunization, all mice immunized with L1h and seven of eight mice immunized with L1h2xCysM were positive (P = 0.1949) (Fig. 4B; Table 2).
FIG. 4.
DNA immunization. C57BL/6 mice were immunized three times with pUF3 vectors containing either an L1h gene, an L1h2xCysM gene (carrying C175A and C428A mutations), or an L2h gene as a negative control. (A) The reciprocal titers of L1-specific IgG antibodies in sera collected 14 days after the second or 8 days after the third immunization were determined using a VLP capture ELISA. (B) Eight days after the third immunization, splenocytes were taken and analyzed in an ex vivo IFN-γ ELISPOT assay. Shown are the numbers of IFN-γ spots per 106 cells. (C) Sera collected after the third immunization were tested in different dilutions (1:100, 1:1,000, 1:10,000, and 1:100,000) in a neutralization assay using HPV16 pseudovirions, and the percent neutralization is shown. The horizontal bars indicate the median values.
TABLE 2.
Immune responses after DNA immunizationa
| Immunization construct | No. of immunizations | Serum dilution | IgG titer
|
% Neutralization
|
Reactivity in ELISPOT assay
|
|||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |||
| L2h | 2 | 0 | 0-0 | |||||
| 3 | 0 | 0-0 | ||||||
| 3 | 1:100 | 58 | 44-70 | |||||
| 3 | 1:1,000 | 17 | 7-26 | |||||
| 3 | 1:10,000 | 0 | 0-0 | |||||
| 3 | 1:100,000 | 0 | 0-0 | |||||
| 3 | 1 | 0-15 | ||||||
| L1h | 2 | 25,600 | 12,800-51,200 | |||||
| 3 | 41,600 | 32,000-51,200 | ||||||
| 3 | 1:100 | 90 | 81-94 | |||||
| 3 | 1:1,000 | 87 | 86-90 | |||||
| 3 | 1:10,000 | 100 | 91-100 | |||||
| 3 | 1:100,000 | 5 | 0-16 | |||||
| 3 | 1,261 | 1,036-1,319 | ||||||
| L1h2xCysM | 2 | 550 | 394-875 | |||||
| 3 | 11,200 | 4,200-17,600 | ||||||
| 3 | 1:100 | 74 | 74-75 | |||||
| 3 | 1:1,000 | 80 | 23-87 | |||||
| 3 | 1:10,000 | 56 | 23-86 | |||||
| 3 | 1:100,000 | 2 | 0-14 | |||||
| 3 | 1,046 | 775-1,246 | ||||||
The table summarizes the humoral and cellular immune responses after DNA immunization using different L1 constructs. The reciprocal titers of L1-specific antibodies after the second and third immunizations are shown. Sera collected after the third immunization were tested at different dilutions (1:100, 1:1,000, 1:10,000, and 1:100,000) in a neutralization assay with HPV16 pseudovirions, and neutralizing activities of the sera are shown as the percent neutralization relative to that by the positive control. Reactivity in an ex vivo ELISPOT assay is shown as the number of IFN-γ-positive spots per 106 cells after stimulation with an L1 H-2b-restricted peptide. IQR, interquartile range.
Balanced CD8 T-cell responses after immunization with VLPs and capsomeres are not due to anti-L1 antibodies limiting T-cell boosting.
Interestingly, though VLPs induced significantly higher humoral immune responses than capsomeres, the CD8 T-cell responses after three immunizations were much more balanced. It has been reported previously that preexisting neutralizing antibodies can prevent an effective boosting of the cellular immune response (13, 14). High titers of neutralizing antibodies produced rapidly after immunization with VLPs might therefore prevent an effective boosting of the CD8 T-cell response. In mice immunized with capsomeres, this effect might not be as strong.
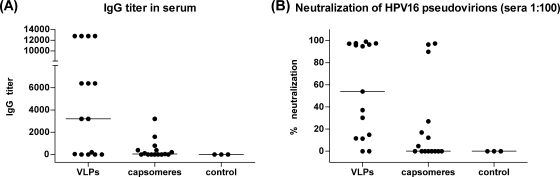
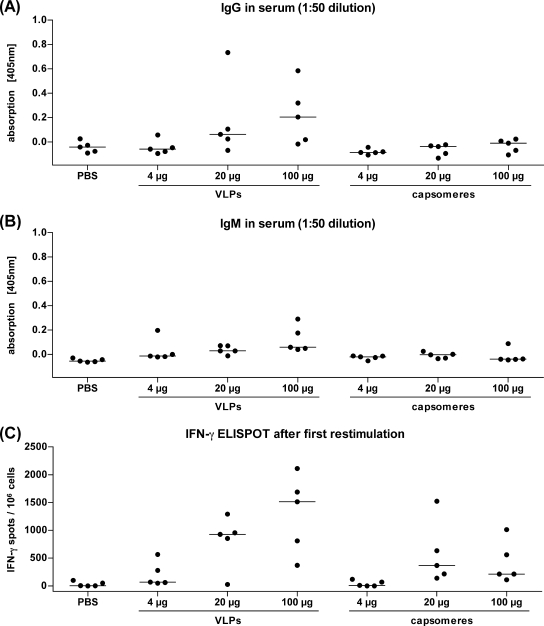
To address this question, mice (five per group) were immunized once with 4, 20, or 100 μg of VLPs or capsomeres and, 10 days later, blood samples were collected. Sera from 5 of 15 mice immunized with VLPs (2 of 5 immunized with 20 μg and 3 of 5 immunized with 100 μg) were IgG positive if tested in a 1:50 dilution by a VLP capture ELISA (Fig. 5A). In contrast, none of the mice immunized with capsomeres developed detectable levels of L1-specific IgG antibodies. As in the ex vivo ELISPOT assay only three mice showed signals above the cutoff level, another ELISPOT assay after one round of restimulation was performed. After the restimulation, 11 of 15 mice immunized with VLPs and 8 of 15 mice immunized with capsomeres (Fig. 5C) scored positive for L1-specific CD8 T cells. Although the signals in sera from VLP-immunized mice were in general higher than those in sera from capsomere-immunized mice, the difference was not statistically significant (P = 0.0814).
FIG. 5.
Similar CD8 T-cell responses are induced by single immunizations with HPV16 VLPs or capsomeres. C57BL/6 mice were immunized once with 4, 20, or 100 μg of VLPs or capsomeres or with PBS alone as a negative control. Ten days after the immunization, blood samples and splenocytes were collected. (A and B) The sera were analyzed in a VLP capture ELISA to detect IgG (A) and IgM (B). ELISA signals of sera diluted 1:50 are shown. (C) The splenocytes were restimulated with L1 peptide-loaded RMA-S cells and analyzed in an IFN-γ ELISPOT assay. Shown are the numbers of IFN-γ spots per 106 cells. The horizontal bars indicate the median values.
Observed lower humoral immune responses induced by capsomeres are not due to deficiencies in isotype switching.
It has been reported previously for the hepatitis B virus core antigen that the epitope density has an influence on the Ig isotype switching (24). We therefore wanted to analyze the IgM responses of mice immunized with VLPs or capsomeres. After one immunization with 4, 20, or 100 μg of L1 protein, 10 of 15 mice immunized with VLPs but only 2 of 15 mice immunized with capsomeres developed L1-specific IgM antibodies (Fig. 5B). Analyses of the IgM responses of the mice immunized several times s.c., intranasally, or orally or immunized with L1 DNA at different time points confirmed that capsomeres also triggered the IgM responses less efficiently than VLPs (data not shown).
Lack of epitopes is not the explanation for the lower immunogenicity of capsomeres.
A comparison of a total of 200 mice immunized with L1 particles, including animals not described in this study, demonstrated that there was better correlation between VLP-specific IgG antibodies and neutralization activities for VLP-immunized mice than for capsomere-immunized mice among animals with low IgG titers (P < 0.0001 for all mice with a titer below 4,000) (Table 3). This finding implies that the humoral immune responses induced by VLPs or capsomeres differ not only quantitatively, but also qualitatively. To evaluate the epitope distribution of capsomeres in comparison to that of VLPs, the different L1 particles were analyzed in an ELISA using a panel of different HPV16 L1-specific monoclonal antibodies recognizing conformational or linear epitopes which have been characterized before (details are in reference 38 and references therein). The monoclonal antibodies H16.B20, H16.D9, and H16.S1 reacted neither with VLPs nor with capsomeres in the ELISA (Table 4), as they bind preferentially denatured particles (11). The nonneutralizing conformation-specific antibodies H16.11B, H16.8B, and H16.13D reacted more strongly with capsomeres than with VLPs, indicating that they may bind to epitopes present on the insides of the particles. The two antibodies recognizing the linear epitope comprising amino acids 174 to 185 (H16.C2 and H16.H5) reacted with VLPs but not with capsomeres, presumably because of the change of amino acid 175 from cysteine to alanine in the capsomeres. Two additional antibodies recognizing linear epitopes (H16.15G and H16.J4) reacted only with VLPs. Several neutralizing conformation-specific antibodies (H16.7E, H16.2F, H16.3A, and H16.4A) reacted more strongly with VLPs than with capsomeres. The most prominent difference was noticeable for H16.E70, a neutralizing antibody binding an epitope on the apexes of capsomeres (9, 57), which reacted strongly with VLPs but did not bind capsomeres. However, this epitope has been shown previously to be nonessential for the induction of high titers of neutralizing antibodies (57). The remaining monoclonal antibodies, one antibody recognizing a linear epitope (CamVir1) and eight conformation-specific neutralizing antibodies, showed no clear difference in binding VLPs or capsomeres. The strong reaction of capsomeres with H16.V5 is of particular interest, as the H16.V5 epitope has been described previously as an immunodominant epitope important for the induction of high titers of neutralizing antibodies and efficient CD8 T-cell responses (46, 57).
TABLE 3.
Correlation of neutralization activities and IgG titers in sera from VLP- versus capsomere-immunized micea
| Immunogen | No. of mice | No. of neutralizing sera in four IgG titer groups (%)
|
|||
|---|---|---|---|---|---|
| Titer of <25 | Titer of 25-200 | Titer of 201-4,000 | Titer of >4,000 | ||
| VLPs | 102 | 5/15 (33.3) | 6/12 (50.0) | 26/32 (81.3) | 42/45 (93.3) |
| Capsomeres | 98 | 0/40 (0.0) | 5/22 (22.7) | 19/31 (61.3) | 6/6 (100.0) |
| Total | 200 | 5/55 (9.1) | 11/34 (32.4) | 45/63 (71.4) | 48/51 (94.1) |
The IgG titers and neutralization activities in sera of a total of 200 mice (BALB/c and C57BL/6) after s.c., intranasal, oral, or DNA immunization with HPV16 L1 VLPs or capsomeres are summarized. In addition to the results from the immunization experiments described in this study, previously published (53) and unpublished data were included. The sera were classified depending on the IgG titers (<25, 25 to 200, 201 to 4,000, or >4,000). Shown is the number of neutralization activity-positive sera in relation to the total number of sera in the respective group. For mice with low IgG titers, we observed better correlation between VLP-specific IgG antibodies and neutralization activities in sera from VLP-immunized mice than in sera from capsomere-immunized mice (P < 0.0001 for all mice with a titer below 4,000).
TABLE 4.
Reactivities of HPV16 L1 VLPs and capsomeres with different L1-specific monoclonal antibodiesa
| Antibody | Type | Essential amino acids | Reactivity of:
|
|
|---|---|---|---|---|
| VLPs | Capsomeres | |||
| H16.7E | C+ | 38-65 | 2.193 | 0.821 |
| H16.3A | C+ | 172-505 | 1.437 | 0.605 |
| H16.4A | C+ | 172-505 | 2.386 | 1.090 |
| H16.U4 | C+ | 172-505 | 1.184 | 0.736 |
| H16.14J | C+ | 172-505 | 1.343 | 0.961 |
| H16.9A | C+b | 1-173 | 0.332 | 0.605 |
| H16.2F | C+ | 266-297, 339-365 | 2.640 | 0.786 |
| H16.5A | C+ | 266-297, 339-365 | 2.241 | 2.233 |
| H16.V5 | C+ | 266-297, 339-365 | 2.871 | 2.682 |
| H263.A2 | C+ | 266-297, 339-365 | 0.850 | 0.587 |
| H16.E70 | C+ | 282 | 1.825 | 0.065 |
| Ritti01 | C+ | ? | 0.837 | 0.579 |
| H16.1A | C*b | 266-297, 339-365 | 1.641 | 1.254 |
| H16.11B | C− | ? | 0.331 | 0.957 |
| H16.13D | C− | ? | 0.112 | 0.519 |
| H16.8B | C− | ? | 0.152 | 0.664 |
| H16.H5 | L− | 174-185 | 1.368 | 0.000 |
| H16.B20 | L− | 396-415 | 0.078 | 0.185 |
| H16.D9 | L− | ? | 0.000 | 0.000 |
| H16.S1 | L−c | 111-130 | 0.056 | 0.000 |
| H16.15G | L− | 172-505 | 0.454 | 0.000 |
| H16.C2 | L− | 174-185 | 0.878 | 0.000 |
| H16.J4 | L−c | 261-280 | 0.840 | 0.000 |
| CamVir1 | L−c | 204-210 | 0.520 | 0.781 |
ELISA reactivities, expressed in absorption units, of different monoclonal antibodies to directly coated VLPs or capsomeres are shown. Values in bold indicate that the difference between the results for VLPs and capsomeres was above 0.5 absorption units, and the higher of the two values is underlined. The antibodies were classified with respect to the recognized epitope (C, conformational; L, linear) and the neutralization activity (+, neutralizing; −, nonneutralizing; *, reported in the literature to be neutralizing [38] or nonneutralizing [10]). Data about the epitopes and neutralization activities of the antibodies are summarized by Rizk et al. (38). ?, unknown.
Additional data are in reference 10.
Additional data are in reference 39.
As there were differences in the epitope patterns of the different L1 particles, we wanted to determine whether the high antibody responses of VLP-immunized mice were directed against epitopes present on VLPs but absent on capsomeres. It would also be conceivable that capsomere-immunized mice developed mainly capsomere-specific antibodies, which may not be detected in a VLP capture ELISA. Sera of mice immunized with VLPs or capsomeres were analyzed using ELISA plates directly coated with VLPs or capsomeres. Mice immunized with capsomeres showed slightly higher IgG titers when capsomeres rather than VLPs were used for detection, indicating the presence of antibodies directed against the insides of the capsomeres. However, with no exception, the IgG titers of mice immunized with VLPs were significantly higher than the IgG titers of capsomere-immunized mice, irrespective of the antigen used for ELISA coating (data not shown), indicating that the humoral immune responses in these animals were not biased toward VLP-specific epitopes.
The higher humoral immunogenicity of VLPs than capsomeres is not due to differences in TLR activation.
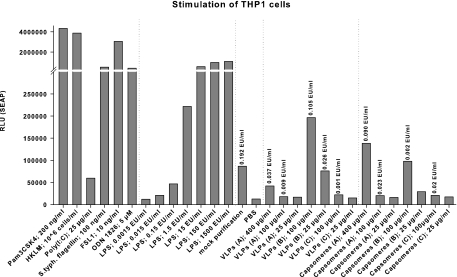
Papillomavirus VLPs can directly activate murine B lymphocytes and induce Ig class switch recombination via a TLR4- and MyD88-dependent pathway (59). To investigate if the higher humoral immunity of VLPs is based on differences between VLPs and capsomeres with respect to the activation of TLR4-MyD88 pathways, we evaluated TLR4 activation by different L1 particles in vitro in THP1-Blue CD14 cells and in vivo in a TLR4 mouse model. THP1-Blue CD14 cells are human peripheral blood monocytic cells which express TLR1 to TLR10. The activation of one of the expressed TLR types induces signaling cascades which lead to the expression of the NF-κB-dependent reporter gene (1). THP1-Blue CD14 cells were stimulated with known TLR agonists as positive controls, including different concentrations of the TLR4-activating LPS, or with different preparations of HPV16 L1 particles. Both VLPs and capsomeres induced the expression of the reporter gene, which indicates the activation of at least one TLR type, but rather large amounts of L1 were required (Fig. 6). Comparing VLPs and capsomeres with respect to the induced reporter gene expression revealed no differences between the two L1 particle forms. The results were confirmed using murine RAW 264.7 macrophages transfected with an NF-κB-inducible luciferase reporter gene (data not shown) (58).
FIG. 6.
In vitro TLR activation assay. THP1-Blue CD14 cells were incubated with known TLR ligands [Pam3CSK4 (TLR1/2), 200 ng/ml; a heat-killed preparation of L. monocytogenes (HKLM; TLR2), 106 cells/ml; poly(I:C) (TLR3), 25 μg/ml; S. enterica serovar Typhimurium flagellin (S. typh. flagellin; TLR5), 100 ng/ml; FSL-1 (TLR6/2), 10 ng/ml; CpG ODN 1826 (TLR9), 5 μM; or LPS (TLR4), 0.0015 to 1,500 endotoxin units (EU)/ml] as positive controls, PBS, or a preparation of mock-infected H5 cells as a negative control or with different amounts (25, 100, and 400 μg/ml) of three independent preparations (A, B, and C) each of VLPs and capsomeres. In this system, the binding of TLR agonists activates signaling pathways and results in the expression of the NF-κB-inducible SEAP reporter gene. After 24 h, the SEAP content of the cell culture supernatant was determined. Numbers above columns reflect the LPS contents of the corresponding samples. Signals are shown as relative luminescence units (RLU).
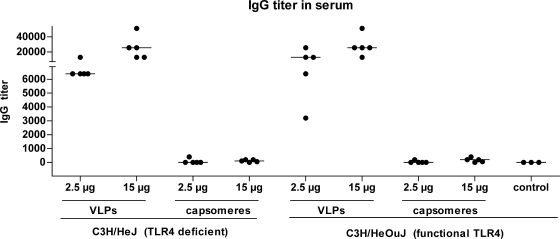
To analyze the influence of TLR4 on the humoral immunogenicities of VLPs and capsomeres, C3H/HeJ and C3H/HeOuJ mice were used as an in vivo model. C3H/HeJ mice are TLR4 deficient due to a dominant-negative point mutation within the cytoplasmatic region of the receptor (37). C3H/HeOuJ mice, which have the same genetic background as C3H/HeJ mice but express functional TLR4, were used as the control. The phenotypes of the two mouse strains were confirmed by an in vitro proliferation assay using LPS as the stimulant (data not shown). C3H/HeJ and C3H/HeOuJ mice (five mice per group) were immunized s.c. with 2.5 or 15 μg of VLPs or capsomeres over a 2-week interval, and 10 days after the second immunization, blood samples were collected. Again, VLPs induced significantly higher IgG titers in the control mice than capsomeres (for mice immunized with 2.5 μg of protein, P = 0.0079; for the 15-μg-dose group, P = 0.0084) (Fig. 7). Furthermore, similar differences between the humoral immunogenicities of VLPs and capsomeres in the TLR4-deficient C3H/HeJ mice were observed (for mice immunized with 2.5 μg of protein, P = 0.0079; for the 15-μg-dose group, P = 0.0080). Overall, the presence of functional TLR4 does not seem to have an influence on the immunogenicity of L1 particles, as no significant differences between the humoral immune responses in C3H/HeJ and C3H/HeOuJ mice were observed.
FIG. 7.
Influence of TLR4 on antibody responses after s.c. application of L1 particles. C3H/HeJ or C3H/HeOuJ mice (five per group) were immunized s.c. twice over a 2-week interval with 2.5 or 15 μg of HPV16 L1 VLPs or capsomeres. Mice immunized with PBS alone served as a negative control. Ten days after the second immunization, blood samples were collected and the titers of L1-specific IgG antibodies were determined using a VLP capture ELISA. The horizontal bars indicate the median values.
The humoral immunogenicity of L1 capsomeres can be strongly improved by using a suitable adjuvant system.
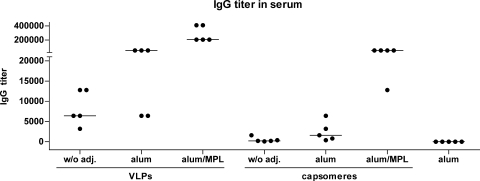
Next, we wanted to determine whether the low immunogenicity of capsomeres can be enhanced by the use of adjuvants. For this investigation, we selected aluminum hydroxide and a combination of aluminum hydroxide and the Sigma adjuvant system, as these are components of the two commercial HPV vaccines. It has to be noted, however, that the monophosphoryl lipid A adjuvant (the Sigma adjuvant system) we used is similar but not identical to the ASO4 adjuvants used in the Cervarix vaccine, which was not available for our study. In addition to monophosphoryl lipid A, the Sigma adjuvant system contains synthetic trehalose dicorynomycolate, squalene oil, and Tween 80.
Mice were immunized s.c. with 10 μg of VLPs or capsomeres. The protein was administered without adjuvant, adsorbed to 500 μg of aluminum hydroxide, or adsorbed to 500 μg of aluminum hydroxide combined with the Sigma adjuvant system, containing 50 μg of monophosphoryl lipid A and 50 μg of synthetic trehalose dicorynomycolate. The immunogenicities of both VLPs and capsomeres were increased only slightly by aluminum hydroxide alone but significantly by aluminum hydroxide combined with the Sigma adjuvant system (VLPs, P = 0.0079; capsomeres, P = 0.0079) (Fig. 8). In a direct comparison, capsomeres adsorbed to aluminum hydroxide still induced weaker antibody responses than VLPs without adjuvant. Together with aluminum hydroxide combined with the Sigma adjuvant system, capsomeres were about as immunogenic as VLPs adsorbed to aluminum hydroxide only but were less effective than VLPs adsorbed to a combination of aluminum hydroxide and the Sigma adjuvant system (P = 0.0079).
FIG. 8.
Adjuvants can in part compensate for the low immunogenicity of L1 capsomeres. C57BL/6 mice (five per group) were immunized s.c. twice over a 2-week interval with 10 μg of HPV16 L1 VLPs or capsomeres. The protein was administered without adjuvant (w/o adj.), adsorbed to 500 μg of aluminum hydroxide (alum), or adsorbed to 500 μg of aluminum hydroxide in combination with the Sigma adjuvant system containing 50 μg of monophosphoryl lipid A and 50 μg of synthetic trehalose dicorynomycolate in squalene and Tween 80 (alum/MPL). Eight days after the second immunization, blood samples were collected and the titers of L1-specific IgG antibodies were determined using a VLP capture ELISA. The horizontal bars indicate the median values.
These results lead to the conclusion that the humoral immunogenicity of capsomeres can be strongly enhanced by using a suitable adjuvant system.
DISCUSSION
The high production costs of VLP-based prophylactic HPV vaccines derived from eukaryotic cells will likely prevent rapid distribution in developing countries, the area where over 80% of the cervical cancer cases occur (35). Capsomeres are considered to be a cost-effective alternative to VLP-based vaccines because they can be produced in bacterial expression systems and because of their high degree of stability. However, despite promising results of immunization studies using E. coli-derived capsomeres (33, 43, 60), no detailed side-by-side comparison of the immunogenicities of the different L1 particles has yet been performed. The possible contamination of bacterially expressed proteins with immunostimulatory endotoxins complicates the direct comparison of capsomeres and insect cell-derived VLPs. We therefore produced both particle forms in insect cells and evaluated the immune responses induced after s.c., intranasal, or oral immunization. Consistent with the observations of Fligge et al. after the intraperitoneal application of insect cell-derived HPV33 particles (17), HPV16 VLPs induced, independent of the route of immunization, significantly higher titers of neutralizing antibodies than capsomeres. To exclude an influence of contaminating immunostimulatory molecules, which may very well differ between the particle preparations, the higher humoral immunogenicity of VLPs was confirmed using DNA immunization. Moreover, the differences between mice immunized with VLPs and those immunized with capsomeres were not a consequence of the detection system, as they were observed both in a VLP capture ELISA and in an ELISA using capsomeres as the antigen. Besides the differences with respect to the quantities of the induced humoral immune responses, the qualities of the antibodies induced by VLPs and capsomeres also seemed to differ, as there was better correlation between L1-specific IgG antibodies and neutralization activities in VLP-immunized mice than in capsomere-immunized mice among animals with low IgG titers. Using a panel of well-characterized HPV16 L1 antibodies revealed differences in the epitope distributions of VLPs and capsomeres, indicating that some neutralizing epitopes are missing on capsomeres. However, several antibodies binding important neutralizing epitopes, including HPV16.V5, which recognizes a major immunodominant epitope of HPV16 L1 (46, 57), reacted with both particle forms, which implies that the differences with respect to the epitope distribution account to only a minor degree for the lower humoral immunogenicity of capsomeres. Nevertheless, the capsomeres used in this study deviated by two point mutations (C175A and C428A) from VLPs and are different from the E. coli-derived capsomeres used in other studies, which were based on L1 constructs without mutations of cysteine residues. Therefore, future comparisons using E. coli-derived capsomeres which are free of endotoxins and other immunomodulatory contaminations should be pursued to further confirm the differences in humoral immunogenicity.
The differences with respect to the CD8 T-cell responses were less evident than those with respect to the antibody responses. In most immunization experiments, no significant differences between mice immunized with the different particle forms were observed, even in the absence of preexisting neutralizing antibodies which might diminish efficient boosting of CD8 T-cell responses (13, 14). Dendritic cells play a crucial role in the activation of antigen-specific T lymphocytes (44). As the CD8 T-cell responses induced by VLPs and capsomeres were relatively similar, we think it is unlikely that the higher humoral immunogenicity of VLPs is based on differences in the interactions of VLPs and capsomeres with dendritic cells or other antigen-presenting cells. In fact, if splenocytes of L1-immunized mice were stimulated with VLPs or capsomeres in an ex vivo ELISPOT assay, the two particle forms activated T lymphocytes equally well (unpublished data). However, it remains to be determined whether the two forms, VLPs and capsomeres, enter and activate dendritic cells equally well.
It has been reported earlier that the activation of B cells by HPV16 VLPs is dependent on MyD88 and TLR4 (59). We evaluated the activation of TLRs by different L1 particles in vitro and the dependence of the induced immune responses on functional TLR4 in a mouse model. The data provided no evidence that VLPs and capsomeres trigger TLRs differently. In fact, our in vitro and in vivo data suggest that TLR4, as well as the other TLR molecules analyzed, was rather inefficiently triggered by either VLPs or capsomeres. In C3H/HeJ mice, which express a TLR4 gene with a dominant-negative mutation, the difference between VLPs and capsomeres with respect to the induced L1-specific IgG titers was very similar to the difference observed in the C3H/HeOuJ wild-type mice. Thus, the higher humoral immunogenicity of VLPs is not due to a higher efficiency of TLR4 activation.
IgM antibodies represent the early humoral immune response, as high titers of protective IgM antibodies can, without the need for isotype switching and largely without CD4 T-cell help, be induced days before IgG responses develop (3, 6). It has been reported previously that IgG, but not IgM, responses are regulated by epitope density and B-cell costimulatory thresholds (24). However, we observed that capsomeres induced not only lower IgG but also lower IgM responses than VLPs. Thus, it is unlikely that the difference in the IgG responses can be explained by different efficiencies of isotype switching. The degree of antigen organization correlates with the efficiency of specific B-cell responses and their dependence on CD4 T-cell help. Viruses or VLPs, which contain epitopes in an organized, highly repetitive form, can induce IgM and in some cases also IgG responses in T-cell-deficient mice (2, 52). The induction of high antibody titers in T-cell-deficient mice by polyomavirus (PyV)-like particles and HPV16 VLPs has also been reported previously (22, 52, 59). However, pentamers of the PyV capsid protein VP1 induce lower humoral immune responses than VLPs and do not protect T-cell-deficient mice against challenge with PyV (55). These observations are consistent with our data and results from studies performed previously demonstrating that HPV capsomeres induce lower humoral immune responses than VLPs (17, 53). It has been postulated previously that the extensive cross-linking of B-cell receptors is responsible for the activation of B cells in the absence of additional T-cell signals (3, 56). In contrast, the pattern recognition by T cells occurs via antigen-presenting cells, and T cells do not react directly to highly organized antigen patterns (4). Thus, our data indicate that the higher humoral immunogenicity of VLPs than capsomeres is based on higher levels of cross-linking of B-cell receptors due to higher-level antigen organization. The similar CD8 T-cell responses induced by different L1 assembly forms can presumably be explained by the fact that T cells are not directly dependent on repetitive antigen patterns.
Humoral immune responses are dependent on antigen organization but also on absolute and local antigen doses (61). Indeed, using larger protein amounts partly compensated for the lower humoral immunogenicity of capsomeres in our study. We estimate that at least 20 to 40 times more capsomeres than VLPs are needed to achieve comparable antibody responses. Furthermore, we have shown that immunization with capsomeres requires the use of a potent adjuvant. In conclusion, in designing a putative capsomere-based prophylactic vaccine, the lower humoral immunogenicity should be considered and overcome by increasing the antigen dose and the number of booster immunizations and using an appropriate adjuvant.
Acknowledgments
Hybridoma supernatants were a kind gift from N. D. Christensen.
The study was in part supported by a grant from the Wilhelm Sander-Stiftung (2006.099.1).
Footnotes
Published ahead of print on 2 April 2008.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4499-511. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann, M. F., and R. M. Zinkernagel. 1996. The influence of virus structure on antibody responses and virus serotype formation. Immunol. Today 17553-558. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann, M. F., and R. M. Zinkernagel. 1997. Neutralizing antiviral B cell responses. Annu. Rev. Immunol. 15235-270. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann, M. F., R. M. Zinkernagel, and A. Oxenius. 1998. Immune responses in the absence of costimulation: viruses know the trick. J. Immunol. 1615791-5794. [PubMed] [Google Scholar]
- 5.Balmelli, C., R. Roden, A. Potts, J. Schiller, P. De Grandi, and D. Nardelli-Haefliger. 1998. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J. Virol. 728220-8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgarth, N. 2000. A two-phase model of B-cell activation. Immunol. Rev. 176171-180. [DOI] [PubMed] [Google Scholar]
- 7.Bins, A. D., A. Jorritsma, M. C. Wolkers, C. F. Hung, T. C. Wu, T. N. Schumacher, and J. B. Haanen. 2005. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat. Med. 11899-904. [DOI] [PubMed] [Google Scholar]
- 8.Breitburd, F., R. Kirnbauer, N. L. Hubbert, B. Nonnenmacher, C. Trin-Dinh-Desmarquet, G. Orth, J. T. Schiller, and D. R. Lowy. 1995. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 693959-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter, J. J., G. C. Wipf, S. F. Benki, N. D. Christensen, and D. A. Galloway. 2003. Identification of a human papillomavirus type 16-specific epitope on the C-terminal arm of the major capsid protein L1. J. Virol. 7711625-11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen, N. D., N. M. Cladel, C. A. Reed, L. R. Budgeon, M. E. Embers, D. M. Skulsky, W. L. McClements, S. W. Ludmerer, and K. U. Jansen. 2001. Hybrid papillomavirus L1 molecules assemble into virus-like particles that reconstitute conformational epitopes and induce neutralizing antibodies to distinct HPV types. Virology 291324-334. [DOI] [PubMed] [Google Scholar]
- 11.Christensen, N. D., J. Dillner, C. Eklund, J. J. Carter, G. C. Wipf, C. A. Reed, N. M. Cladel, and D. A. Galloway. 1996. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 223174-184. [DOI] [PubMed] [Google Scholar]
- 12.Christensen, N. D., R. Hopfl, S. L. DiAngelo, N. M. Cladel, S. D. Patrick, P. A. Welsh, L. R. Budgeon, C. A. Reed, and J. W. Kreider. 1994. Assembled baculovirus-expressed human papillomavirus type 11 L1 capsid protein virus-like particles are recognized by neutralizing monoclonal antibodies and induce high titres of neutralizing antibodies. J. Gen. Virol. 752271-2276. [DOI] [PubMed] [Google Scholar]
- 13.Da Silva, D. M., D. V. Pastrana, J. T. Schiller, and W. M. Kast. 2001. Effect of preexisting neutralizing antibodies on the anti-tumor immune response induced by chimeric human papillomavirus virus-like particle vaccines. Virology 290350-360. [DOI] [PubMed] [Google Scholar]
- 14.Da Silva, D. M., J. T. Schiller, and W. M. Kast. 2003. Heterologous boosting increases immunogenicity of chimeric papillomavirus virus-like particle vaccines. Vaccine 213219-3227. [DOI] [PubMed] [Google Scholar]
- 15.Dell, K., R. Koesters, M. Linnebacher, C. Klein, and L. Gissmann. 2006. Intranasal immunization with human papillomavirus type 16 capsomeres in the presence of non-toxic cholera toxin-based adjuvants elicits increased vaginal immunoglobulin levels. Vaccine 242238-2247. [DOI] [PubMed] [Google Scholar]
- 16.Dupuy, C., D. Buzoni-Gatel, A. Touze, D. Bout, and P. Coursaget. 1999. Nasal immunization of mice with human papillomavirus type 16 (HPV-16) virus-like particles or with the HPV-16 L1 gene elicits specific cytotoxic T lymphocytes in vaginal draining lymph nodes. J. Virol. 739063-9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fligge, C., T. Giroglou, R. E. Streeck, and M. Sapp. 2001. Induction of type-specific neutralizing antibodies by capsomeres of human papillomavirus type 33. Virology 283353-357. [DOI] [PubMed] [Google Scholar]
- 18.Gasparic, M., I. Rubio, N. Thönes, L. Gissmann, and M. Müller. 2007. Prophylactic DNA immunization against multiple papillomavirus types. Vaccine 254540-4553. [DOI] [PubMed] [Google Scholar]
- 19.Hagensee, M. E., J. J. Carter, G. C. Wipf, and D. A. Galloway. 1995. Immunization of mice with HPV vaccinia virus recombinants generates serum IgG, IgM, and mucosal IgA antibodies. Virology 206174-182. [DOI] [PubMed] [Google Scholar]
- 20.Hagensee, M. E., N. Yaegashi, and D. A. Galloway. 1993. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J. Virol. 67315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harper, D. M., E. L. Franco, C. Wheeler, D. G. Ferris, D. Jenkins, A. Schuind, T. Zahaf, B. Innis, P. Naud, N. S. De Carvalho, C. M. Roteli-Martins, J. Teixeira, M. M. Blatter, A. P. Korn, W. Quint, and G. Dubin. 2004. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 3641757-1765. [DOI] [PubMed] [Google Scholar]
- 22.Heidari, S., A. Vlastos, T. Ramqvist, B. Clark, B. E. Griffin, M. I. Garcia, M. Perez, P. Amati, and T. Dalianis. 2002. Immunization of T-cell deficient mice against polyomavirus infection using viral pseudocapsids or temperature sensitive mutants. Vaccine 201571-1578. [DOI] [PubMed] [Google Scholar]
- 23.Ishii, Y., K. Tanaka, and T. Kanda. 2003. Mutational analysis of human papillomavirus type 16 major capsid protein L1: the cysteines affecting the intermolecular bonding and structure of L1-capsids. Virology 308128-136. [DOI] [PubMed] [Google Scholar]
- 24.Jegerlehner, A., T. Storni, G. Lipowsky, M. Schmid, P. Pumpens, and M. F. Bachmann. 2002. Regulation of IgG antibody responses by epitope density and CD21-mediated costimulation. Eur. J. Immunol. 323305-3314. [DOI] [PubMed] [Google Scholar]
- 25.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 8912180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirnbauer, R., L. M. Chandrachud, B. W. O'Neil, E. R. Wagner, G. J. Grindlay, A. Armstrong, G. M. McGarvie, J. T. Schiller, D. R. Lowy, and M. S. Campo. 1996. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology 21937-44. [DOI] [PubMed] [Google Scholar]
- 27.Kirnbauer, R., J. Taub, H. Greenstone, R. Roden, M. Dürst, L. Gissmann, D. R. Lowy, and J. T. Schiller. 1993. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 676929-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leder, C., J. A. Kleinschmidt, C. Wiethe, and M. Müller. 2001. Enhancement of capsid gene expression: preparing the human papillomavirus type 16 major structural gene L1 for DNA vaccination purposes. J. Virol. 759201-9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, M., P. Beard, P. A. Estes, M. K. Lyon, and R. L. Garcea. 1998. Intercapsomeric disulfide bonds in papillomavirus assembly and disassembly. J. Virol. 722160-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLean, C. S., M. J. Churcher, J. Meinke, G. L. Smith, G. Higgins, M. Stanley, and A. C. Minson. 1990. Production and characterisation of a monoclonal antibody to human papillomavirus type 16 using recombinant vaccinia virus. J. Clin. Pathol. 43488-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller, M., and L. Gissmann. 2007. A long way: history of the prophylactic papillomavirus vaccine. Dis. Markers 23331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller, M., J. Zhou, T. D. Reed, C. Rittmüller, A. Burger, J. Gabelsberger, J. Braspenning, and L. Gissmann. 1997. Chimeric papillomavirus-like particles. Virology 23493-111. [DOI] [PubMed] [Google Scholar]
- 33.Öhlschläger, P., W. Osen, K. Dell, S. Faath, R. L. Garcea, I. Jochmus, M. Müller, M. Pawlita, K. Schäfer, P. Sehr, C. Staib, G. Sutter, and L. Gissmann. 2003. Human papillomavirus type 16 L1 capsomeres induce L1-specific cytotoxic T lymphocytes and tumor regression in C57BL/6 mice. J. Virol. 774635-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parkin, D. M., and F. Bray. 2006. Chapter 2: the burden of HPV-related cancers. Vaccine 24(Suppl. 3)S11-S25. [DOI] [PubMed] [Google Scholar]
- 35.Parkin, D. M., F. Bray, J. Ferlay, and P. Pisani. 2005. Global cancer statistics, 2002. CA Cancer J. Clin. 5574-108. [DOI] [PubMed] [Google Scholar]
- 36.Pastrana, D. V., C. B. Buck, Y. Y. Pang, C. D. Thompson, P. E. Castle, P. C. FitzGerald, S. Kruger Kjaer, D. R. Lowy, and J. T. Schiller. 2004. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 321205-216. [DOI] [PubMed] [Google Scholar]
- 37.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 2822085-2088. [DOI] [PubMed] [Google Scholar]
- 38.Rizk, R. Z., R. D. Christensen, K. M. Michael, M. Müller, P. Sehr, T. Waterboer, and M. Pawlita. 2008. Reactivity pattern of 92 monoclonal antibodies with 15 human papillomavirus types. J. Gen. Virol. 89117-129. [DOI] [PubMed] [Google Scholar]
- 39.Roden, R. B., A. Armstrong, P. Haderer, N. D. Christensen, N. L. Hubbert, D. R. Lowy, J. T. Schiller, and R. Kirnbauer. 1997. Characterization of a human papillomavirus type 16 variant-dependent neutralizing epitope. J. Virol. 716247-6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose, R. C., W. Bonnez, R. C. Reichman, and R. L. Garcea. 1993. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J. Virol. 671936-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rose, R. C., C. Lane, S. Wilson, J. A. Suzich, E. Rybicki, and A. L. Williamson. 1999. Oral vaccination of mice with human papillomavirus virus-like particles induces systemic virus-neutralizing antibodies. Vaccine 172129-2135. [DOI] [PubMed] [Google Scholar]
- 42.Rose, R. C., R. C. Reichman, and W. Bonnez. 1994. Human papillomavirus (HPV) type 11 recombinant virus-like particles induce the formation of neutralizing antibodies and detect HPV-specific antibodies in human sera. J. Gen. Virol. 752075-2079. [DOI] [PubMed] [Google Scholar]
- 43.Rose, R. C., W. I. White, M. Li, J. A. Suzich, C. Lane, and R. L. Garcea. 1998. Human papillomavirus type 11 recombinant L1 capsomeres induce virus-neutralizing antibodies. J. Virol. 726151-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi, M., and J. W. Young. 2005. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J. Immunol. 1751373-1381. [DOI] [PubMed] [Google Scholar]
- 45.Rudolf, M. P., J. D. Nieland, D. M. DaSilva, M. P. Velders, M. Müller, H. L. Greenstone, J. T. Schiller, and W. M. Kast. 1999. Induction of HPV16 capsid protein-specific human T cell responses by virus-like particles. Biol. Chem. 380335-340. [DOI] [PubMed] [Google Scholar]
- 46.Ryding, J., L. Dahlberg, M. Wallen-Ohman, and J. Dillner. 2007. Deletion of a major neutralizing epitope of human papillomavirus type 16 virus-like particles. J. Gen. Virol. 88792-802. [DOI] [PubMed] [Google Scholar]
- 47.Sapp, M., C. Fligge, I. Petzak, J. R. Harris, and R. E. Streeck. 1998. Papillomavirus assembly requires trimerization of the major capsid protein by disulfides between two highly conserved cysteines. J. Virol. 726186-6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasagawa, T., P. Pushko, G. Steers, S. E. Gschmeissner, M. A. Hajibagheri, J. Finch, L. Crawford, and M. Tommasino. 1995. Synthesis and assembly of virus-like particles of human papillomaviruses type 6 and type 16 in fission yeast Schizosaccharomyces pombe. Virology 206126-135. [DOI] [PubMed] [Google Scholar]
- 49.Sun, S., C. Beard, R. Jaenisch, P. Jones, and J. Sprent. 1997. Mitogenicity of DNA from different organisms for murine B cells. J. Immunol. 1593119-3125. [PubMed] [Google Scholar]
- 50.Sun, S., H. Kishimoto, and J. Sprent. 1998. DNA as an adjuvant: capacity of insect DNA and synthetic oligodeoxynucleotides to augment T cell responses to specific antigen. J. Exp. Med. 1871145-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzich, J. A., S. J. Ghim, F. J. Palmer-Hill, W. I. White, J. K. Tamura, J. A. Bell, J. A. Newsome, A. B. Jenson, and R. Schlegel. 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. USA 9211553-11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szomolanyi-Tsuda, E., and R. M. Welsh. 1998. T-cell-independent antiviral antibody responses. Curr. Opin. Immunol. 10431-435. [DOI] [PubMed] [Google Scholar]
- 53.Thönes, N., and M. Müller. 2007. Oral immunization with different assembly forms of the HPV 16 major capsid protein L1 induces neutralizing antibodies and cytotoxic T-lymphocytes. Virology 369375-388. [DOI] [PubMed] [Google Scholar]
- 54.Villa, L. L., R. L. Costa, C. A. Petta, R. P. Andrade, J. Paavonen, O. E. Iversen, S. E. Olsson, J. Hoye, M. Steinwall, G. Riis-Johannessen, A. Andersson-Ellstrom, K. Elfgren, G. Krogh, M. Lehtinen, C. Malm, G. M. Tamms, K. Giacoletti, L. Lupinacci, R. Railkar, F. J. Taddeo, J. Bryan, M. T. Esser, H. L. Sings, A. J. Saah, and E. Barr. 2006. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br. J. Cancer 951459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vlastos, A., K. Andreasson, K. Tegerstedt, D. Hollanderova, S. Heidari, J. Forstova, T. Ramqvist, and T. Dalianis. 2003. VP1 pseudocapsids, but not a glutathione-S-transferase VP1 fusion protein, prevent polyomavirus infection in a T-cell immune deficient experimental mouse model. J. Med. Virol. 70293-300. [DOI] [PubMed] [Google Scholar]
- 56.Vos, Q., A. Lees, Z. Q. Wu, C. M. Snapper, and J. J. Mond. 2000. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol. Rev. 176154-170. [DOI] [PubMed] [Google Scholar]
- 57.White, W. I., S. D. Wilson, F. J. Palmer-Hill, R. M. Woods, S. J. Ghim, L. A. Hewitt, D. M. Goldman, S. J. Burke, A. B. Jenson, S. Koenig, and J. A. Suzich. 1999. Characterization of a major neutralizing epitope on human papillomavirus type 16 L1. J. Virol. 734882-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang, R., F. M. Murillo, H. Cui, R. Blosser, S. Uematsu, K. Takeda, S. Akira, R. P. Viscidi, and R. B. Roden. 2004. Papillomavirus-like particles stimulate murine bone marrow-derived dendritic cells to produce alpha interferon and Th1 immune responses via MyD88. J. Virol. 7811152-11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang, R., F. M. Murillo, M. J. Delannoy, R. L. Blosser, W. H. t. Yutzy, S. Uematsu, K. Takeda, S. Akira, R. P. Viscidi, and R. B. Roden. 2005. B lymphocyte activation by human papillomavirus-like particles directly induces Ig class switch recombination via TLR4-MyD88. J. Immunol. 1747912-7919. [DOI] [PubMed] [Google Scholar]
- 60.Yuan, H., P. A. Estes, Y. Chen, J. Newsome, V. A. Olcese, R. L. Garcea, and R. Schlegel. 2001. Immunization with a pentameric L1 fusion protein protects against papillomavirus infection. J. Virol. 757848-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zinkernagel, R. M., and H. Hengartner. 2001. Regulation of the immune response by antigen. Science 293251-253. [DOI] [PubMed] [Google Scholar]
- 62.Zolotukhin, S., M. Potter, W. W. Hauswirth, J. Guy, and N. Muzyczka. 1996. A “humanized” green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J. Virol. 704646-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]