Abstract
Influenza causes significant morbidity in tropical regions; however, unlike in temperate zones, influenza in the tropics is not strongly associated with a given season. We have recently shown that influenza virus transmission in the guinea pig model is most efficient under cold, dry conditions, which are rare in the tropics. Herein, we report the lack of aerosol transmission at 30°C and at all humidities tested. Conversely, transmission via the contact route was equally efficient at 30°C and 20°C. Our data imply that contact or short-range spread predominates in the tropics and offer an explanation for the lack of a well-defined, recurrent influenza season affecting tropical and subtropical regions of the world.
Influenza in temperate regions is characterized by well-defined outbreaks lasting 2 to 3 months and occurring once each year, in the winter (5, 11, 16). In the tropics and subtropics, by contrast, the spread of influenza viruses in the human population is detectable for a much greater proportion of the year, and the timing of outbreaks is less regular. Furthermore, the seasonality of influenza observed in the tropics varies considerably from one location to the next. Influenza A virus activity in Singapore (at a latitude of approximately 1°N) has been reported to peak twice a year, in November to January and in June to July, while influenza B virus activity showed no significant seasonal periodicity (3); a more recent analysis of influenza A virus in Singapore indicated that moderate to high activity occurred throughout the year (11). In contrast, data on laboratory-confirmed influenza virus in Thailand (13°N) shows a peak in influenza virus activity in June (14). In Brazil, an analysis at a regional level revealed a wave of pneumonia and influenza virus activity which traveled southward each year, starting in April in the equatorial north and arriving in the southernmost state, Rio Grande do Sul (at approximately 33°S), in mid-July (1). In Hong Kong (22°N), influenza A virus was isolated at moderate to high rates in all months of the year except October to December (11). Influenza virus activity in Taiwan (23°N) is high from December to March (6), coinciding with annual epidemics in temperate regions of the northern hemisphere, but it can also be moderate to high in July and August (11). While outbreaks of influenza have been associated with the rainy season in some tropical and subtropical countries (3, 4, 10, 12, 13), epidemics also occurred in these countries outside of the rainy season (3, 10, 12). Furthermore, the absence of a correlation between rainfall and influenza virus activity has been reported for other tropical and subtropical regions (6, 11, 15). Overall, it is clear that the well-defined seasonal periodicity of influenza seen in temperate regions is not observed in (sub)tropical regions. Thus, it is likely that the seasonal factors governing influenza virus transmission in temperate zones do not strongly impact influenza virus in the tropics.
Using the guinea pig as a model for influenza virus transmission, we have recently shown that host-to-host spread via respiratory droplets is acutely sensitive to both temperature and relative humidity (RH). Specifically, our results indicate that transmission is most efficient at a low temperature (5°C) and a low RH (20 to 35%), conditions prevalent during winter months in the northern and southern hemispheres. Conversely, we found that transmission via respiratory droplets failed to occur at either a high RH (80% RH and 20°C) or a high temperature (30°C and 35% RH). While these data are consistent with the lack of influenza during the summertime in temperate regions, they appear to contradict the observed incidence of influenza in the tropics. A survey of average monthly weather conditions listed on the British Broadcasting Corporation website (http://www.bbc.co.uk/weather/world/city_guides) indicated that cities located between the Tropics of Cancer and Capricorn (that is, tropical cities) experience temperature highs of about 30°C for most or all of the year and temperature lows of about 20°C or 25°C, depending on proximity to the equator. RHs showed more variation, both over time in a given location and between locations. Overall, some tropical cities could be described as hot and dry (e.g., Khartoum, where mean monthly temperatures range from 23.6°C to 37.6°C and RHs range from 19.6% to 37.9%) and some cities hot and humid (e.g., Singapore, where mean monthly temperatures range from 23.6°C to 31.0°C and RHs range from 73.3% to 78.8%). Herein, we have used the guinea pig model to further investigate the efficiency of transmission under environmental conditions which prevail throughout the year in tropical climates.
Aerosol transmission at 30°C.
First, the rate of spread by the aerosol (large or small respiratory droplet) route in an environment kept at 30°C was examined more closely. Transmission experiments were performed as previously described (8). Briefly, Hartley strain guinea pigs were inoculated intranasally with 1000 PFU of influenza A/Panama/2007/99 virus. At 24 h postinoculation, four infected animals plus four naïve guinea pigs were each transferred to a transmission cage, and the eight cages were placed in an environmental chamber (Caron model 6030) set to 30°C and the desired RH. The naïve animals were exposed to the infected animals in this way for a period of 7 days. To monitor the infection status of the exposed guinea pigs and the amount of virus shed by the inoculated guinea pigs, nasal washings were collected from all eight animals on days 2, 4, 6, and 8 postinoculation.
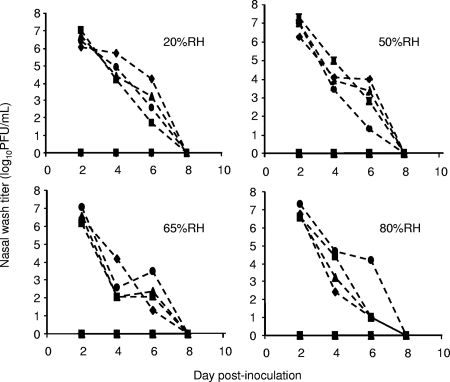
Aerosol transmission experiments were performed at 30°C and 20%, 50%, 65%, and 80% RH. In all four of these experiments, we detected no virus in the nasal washings of exposed guinea pigs (Fig. 1). Furthermore, a comparison of preinfection and 17-day postinfection sera by hemagglutination inhibition assay showed that none of the exposed animals produced antibodies against influenza A/Panama/2007/99 virus, while all intranasally inoculated guinea pigs did seroconvert (data not shown). In addition, we have previously shown that at 30°C and 35% RH, aerosol transmission does not occur between guinea pigs (8). Thus, at 30°C and all RHs tested, no aerosol transmission of influenza virus was observed.
FIG. 1.
Aerosol transmission of influenza virus from guinea pig to guinea pig is blocked at 30°C. Titers of influenza virus in nasal wash samples are plotted as a function of the day postinoculation. All experiments were performed at 30°C; the RH condition of each experiment is given in the upper right of each graph. Titers from intranasally inoculated guinea pigs are represented as dashed lines; titers from exposed guinea pigs are shown with solid lines. Squares, diamonds, triangles, and circles refer to individual animals. Exposed and inoculated guinea pigs with like symbols were placed on the same shelf.
Virus growth in guinea pigs at 30°C.
To gain insight into the reason for the observed lack of transmission at 30°C, we examined virus titers for the nasal washings of inoculated guinea pigs housed under these conditions. The titers obtained from the inoculated animals on days 2, 4, 6, and 8 postinfection in all five experiments (including that at 35% RH) were averaged and plotted in Fig. 2. To provide a reference for comparison, nasal washings were collected from eight inoculated guinea pigs housed at 20°C, averaged in the same manner, and also plotted in Fig. 2. No marked difference in the amounts of virus shed by each group of guinea pigs was seen, indicating that the lack of transmission was not due to a decrease in virus shed from the guinea pig upper respiratory tract at 30°C relative to 20°C.
FIG. 2.
Guinea pigs housed at 30°C shed influenza virus at titers similar to those for guinea pigs housed at 20°C. Average viral titers in nasal wash samples collected from animals housed at either 30°C (solid line) or 20°C (dashed line) are plotted as a function of the time postinfection. Error bars indicate standard deviations.
Contact transmission at 30°C.
The high titers of virus isolated from the inoculated animals housed at 30°C suggest that the effect of warm ambient temperature on transmission does not act at the level of the donor host. We reasoned that the block in transmission must therefore act either at the level of the recipient host or directly on the virus while it travels between hosts. To distinguish between these two possibilities, we performed a second set of transmission experiments at 30°C in which the inoculated and exposed animals were placed together in the same cage (method described in reference 9). Termed a “contact transmission experiment,” this setup allows direct and indirect contact between the donor and recipient animals (as well as short-range aerosol transmission). We predicted that, if an ambient temperature of 30°C prevents transmission through an effect on the recipient host, then contact transmission at 30°C would be less efficient than contact transmission at 20°C. Conversely, if heat acts on the virus in the environment to prevent viral spread, then based on the assumption that the virus spends much less time between hosts when the donor and recipient are housed together, transmission in a contact setting should remain efficient at 30°C. As shown in Fig. 3, the latter prediction proved to be correct: contact transmission at 30°C occurred at a rate and an overall efficiency similar to those for contact transmission at 20°C (virus was detected in 75 to 100% of exposed animals following just 24 h of exposure).
FIG. 3.
Contact transmission of influenza virus from guinea pig to guinea pig is efficient at 30°C. Titers of influenza virus in nasal wash samples are plotted as a function of the day postinoculation. The RH and temperature conditions of each experiment are indicated above each graph. Titers from intranasally inoculated guinea pigs are represented as dashed lines; titers from exposed guinea pigs are shown with solid lines. Squares, diamonds, triangles, and circles refer to individual animals. Exposed and inoculated guinea pigs with like symbols were housed in the same cage.
Implications for the epidemiology of influenza.
Our data with the guinea pig model predict that human-to-human transmission of influenza viruses by the aerosol route is very rare in tropical climates, occurring only in climate-controlled, indoor settings and on occasions when the outdoor temperature drops to 20°C. Results obtained when infected and exposed guinea pigs were housed in the same cage, however, suggest that influenza virus transmission at very close range or by direct contact would be efficient under tropical climatic conditions. We therefore propose that the predominant mode of influenza virus spread differs between temperate and tropical regions: aerosol transmission plays a major role in temperate climates, while contact or close-range spread are more important in the tropics. Furthermore, if the mode of transmission does indeed vary depending on climate, then this variation may explain the differing seasonal patterns of influenza in temperate and tropical regions. Specifically, transmission by the aerosol route is sensitive to RH and temperature, contributing to increased influenza virus activity during the winter months in temperate zones; conversely, transmission by the contact route is insensitive to RH and temperature, with the result that influenza in the tropics may occur throughout the year or in sporadic outbreaks with no clearly defined seasonality. Our data also suggest that the transmission of influenza virus by the contact route should occur during the summer months in temperate climates. Influenza outbreaks in the summer are occasionally reported (2, 7), and these instances may indeed be due to spread by direct or indirect contact. Perhaps increased surveillance for influenza virus infections during the off-peak season would reveal a higher burden of disease than is currently recognized. Alternatively, there may exist additional factors, other than warm temperature and high RH, which suppress influenza virus transmission by all routes during the summer months.
Acknowledgments
We thank Scott Dowell and Adolfo García-Sastre for helpful discussions and Christopher Narbus for excellent technical assistance.
This work was supported by grants from the W.M. Keck Foundation, the Centers for Disease Control (R21) (U01CI000354-01), and the Center for Investigating Viral Immunity and Antagonism (1 UC19 AI062623-023) (to P.P.). A.C.L. is a Parker B. Francis Fellow in Pulmonary Research. S.M. was supported by Sunnybrook Health Sciences Centre, Toronto, Canada, and a Ruth L. Kirschstein Physician Scientist Research Training in Pathogenesis of Viral Diseases award (Mary Klotman, principal investigator).
Footnotes
Published ahead of print on 26 March 2008.
REFERENCES
- 1.Alonso, W. J., C. Viboud, L. Simonsen, E. W. Hirano, L. Z. Daufenbach, and M. A. Miller. 2007. Seasonality of influenza in Brazil: a traveling wave from the Amazon to the subtropics. Am. J. Epidemiol. 1651434-1442. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1998. Update: outbreak of influenza A infection—Alaska and the Yukon Territory, July-August 1998. MMWR Morb. Mortal. Wkly. Rep. 47685-688. [PubMed] [Google Scholar]
- 3.Chew, F. T., S. Doraisingham, A. E. Ling, G. Kumarasinghe, and B. W. Lee. 1998. Seasonal trends of viral respiratory tract infections in the tropics. Epidemiol. Infect. 121121-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dosseh, A., K. Ndiaye, A. Spiegel, M. Sagna, and C. Mathiot. 2000. Epidemiological and virological influenza survey in Dakar, Senegal: 1996-1998. Am. J. Trop. Med. Hyg. 62639-643. [DOI] [PubMed] [Google Scholar]
- 5.Dowell, S. F. 2001. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg. Infect. Dis. 7369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh, Y. C., H. Y. Chen, J. J. Yen, D. P. Liu, L. Y. Chang, C. Y. Lu, P. L. Shao, C. Y. Lee, and L. M. Huang. 2005. Influenza in Taiwan: seasonality and vaccine strain match. J. Microbiol. Immunol. Infect. 38238-243. [PubMed] [Google Scholar]
- 7.Laurel, V. L., C. C. De Witt, Y. A. Geddie, M. C. Yip, D. M. Dolan, L. C. Canas, M. J. Dolan, and E. A. Walter. 2001. An outbreak of influenza A caused by imported virus in the United States, July 1999. Clin. Infect. Dis. 321639-1642. [DOI] [PubMed] [Google Scholar]
- 8.Lowen, A. C., S. Mubareka, J. Steel, and P. Palese. 2007. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 3e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowen, A. C., S. Mubareka, T. M. Tumpey, A. García-Sastre, and P. Palese. 2006. The guinea pig as a transmission model for human influenza viruses. Proc. Natl. Acad. Sci. USA 1039988-9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen, H. L., R. Saito, H. K. Ngiem, M. Nishikawa, Y. Shobugawa, D. C. Nguyen, L. T. Hoang, L. P. Huynh, and H. Suzuki. 2007. Epidemiology of influenza in Hanoi, Vietnam, from 2001 to 2003. J. Infect. 5558-63. [DOI] [PubMed] [Google Scholar]
- 11.Park, A. W., and K. Glass. 2007. Dynamic patterns of avian and human influenza in east and southeast Asia. Lancet Infect. Dis. 7543-548. [DOI] [PubMed] [Google Scholar]
- 12.Rao, B. L., and K. Banerjee. 1993. Influenza surveillance in Pune, India, 1978-90. Bull. W. H. O. 71177-181. [PMC free article] [PubMed] [Google Scholar]
- 13.Shek, L. P., and B. W. Lee. 2003. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr. Respir. Rev. 4105-111. [DOI] [PubMed] [Google Scholar]
- 14.Simmerman, J. M., M. Chittaganpitch, D. Erdman, P. Sawatwong, T. M. Uyeki, and S. F. Dowell. 2007. Field performance and new uses of rapid influenza testing in Thailand. Int. J. Infect. Dis. 11166-171. [DOI] [PubMed] [Google Scholar]
- 15.Sung, R. Y., R. C. Chan, J. S. Tam, A. F. Cheng, and H. G. Murray. 1992. Epidemiology and aetiology of acute bronchiolitis in Hong Kong infants. Epidemiol. Infect. 108147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viboud, C., W. J. Alonso, and L. Simonsen. 2006. Influenza in tropical regions. PLoS Med. 3e89. [DOI] [PMC free article] [PubMed] [Google Scholar]