Abstract
Based on previous preclinical evaluation in mice and monkeys, the chimeric TBEV/DEN4Δ30 virus, carrying the prM and E protein genes from a highly virulent Far Eastern strain of tick-borne encephalitis virus (TBEV) on the backbone of a nonneuroinvasive dengue type 4 virus (DEN4), has been identified as a promising live attenuated virus vaccine candidate against disease caused by TBEV. However, prior to use of this vaccine candidate in humans, its neurovirulence in nonhuman primates needed to be evaluated. In the present study, we compared the neuropathogeneses of the chimeric TBEV/DEN4Δ30 virus; Langat virus (LGTV), a former live TBEV vaccine; and yellow fever 17D virus vaccine (YF 17D) in rhesus monkeys inoculated intracerebrally. TBEV/DEN4Δ30 and YF 17D demonstrated remarkably similar spatiotemporal profiles of virus replication and virus-associated histopathology in the central nervous system (CNS) that were high in cerebral hemispheres but progressively decreased toward the spinal cord. In contrast, the neurovirulence of LGTV exhibited the reverse profile, progressing from the site of inoculation toward the cerebellum and spinal cord. Analysis of the spatiotemporal distribution of viral antigens in the CNS of monkeys revealed a prominent neurotropism associated with all three attenuated viruses. Nevertheless, TBEV/DEN4Δ30 virus exhibited higher neurovirulence in monkeys than either LGTV or YF 17D, suggesting insufficient attenuation. These results provide insight into the neuropathogenesis associated with attenuated flaviviruses that may guide the design of safe vaccines.
Tick-borne encephalitis (TBE) is a debilitating and often fatal neuroinfection caused by antigenically closely related RNA viruses belonging to the Flaviviridae family (9). The clinical course of TBE infection can be unapparent or manifest as a severe acute, subacute, or chronic illness. Licensed inactivated TBE virus (TBEV) vaccines are currently available in Europe and Russia; however, three doses of vaccine are required for primary immunization, and subsequent booster vaccinations every 3 years are needed to maintain protective immunity. Despite immunization of populations living in areas of endemicity using inactivated TBEV vaccines, TBE remains a pressing public health problem in Europe and Russia, where up to 14,000 human cases are reported annually (9, 42). A less expensive live TBEV vaccine that induces more durable immunity is needed.
In an effort to achieve this goal, a chimeric TBEV/DEN4 virus was created by replacing the membrane precursor (prM) and envelope glycoprotein (E) structural protein genes of a mosquito-borne dengue type 4 virus (DEN4) with the corresponding genes from the highly virulent TBEV strain Sofjin (30). Preclinical studies demonstrated that the TBEV/DEN4 chimeric virus lacked neuroinvasiveness in immunocompetent mice inoculated peripherally and that immunized mice were protected against challenge with a lethal dose of TBEV (30, 32). However, chimeric TBEV/DEN4 virus was not attenuated for neurovirulence in suckling mice inoculated intracerebrally (i.c.) (39). Recently, a TBEV/DEN4Δ30 mutant that contains a 30-nucleotide deletion (Δ30) in the 3′ noncoding region of the DEN4 part of the chimeric TBEV/DEN4 genome was generated in an effort to further attenuate this virus for mice and monkeys (39). The Δ30 mutation is genetically stable and was shown to attenuate DEN1, DEN4, and West Nile/DEN4 viruses for mice, monkeys and humans (5, 29, 45). The TBEV/DEN4Δ30 virus exhibited a high level of attenuation of neuroinvasiveness in very sensitive immunodeficient mice (39) and was less neurovirulent in adult Swiss mice following i.c. inoculation than its immediate TBEV/DEN4 parent or tick-borne Langat virus (LGTV), a former live TBEV vaccine (A. G. Pletnev, unpublished data). Thus, the development of a live attenuated vaccine against TBE by chimerization of TBEV with DEN4Δ30 virus is a promising approach.
Previous studies with rhesus monkeys inoculated subcutaneously showed that although TBEV/DEN4Δ30 virus induced a very low-level viremia compared with TBEV/DEN4 or LGTV, it was immunogenic and protected monkeys against LGTV challenge (39). However, it remains possible that this new chimeric virus, containing the structural protein genes derived from the highly neurovirulent TBEV strain, might gain access to the central nervous systems (CNS) of vaccine recipients by a hematogenous or other route. Thus, it seemed prudent to further evaluate the neurovirulence of TBEV/DEN4Δ30 virus in nonhuman primates before initiating clinical trials with humans.
The rationale for the neurovirulence testing of live attenuated viral vaccines was originally based on the need to test viruses with known neurovirulent properties, such as poliovirus and yellow fever (YF) virus (47). Monkeys in the neurovirulence test are monitored for clinical signs, viremia, and antibody responses. Neurovirulence is determined by evaluation of semiquantitative histopathological scores in the specific areas of the CNS. The viremia profile and histopathological scores must not exceed those of the reference control. Taking into consideration the fact that a monkey neurovirulence test (MNVT) for a live TBEV vaccine has never been established, reference control viruses do not exist. The specific areas of the CNS that need to be analyzed and that might potentially discriminate between flaviviruses with different neurovirulence potentials remain undefined. Therefore, we thought that a comprehensive analysis of the neuropathogenesis in nonhuman primates is needed to further our understanding of the neurovirulence potential of new live attenuated chimeric TBEV vaccine candidates.
In the present study, we performed a comparative analysis of the neurovirulence and neuropathogenesis in rhesus monkeys following intrathalamic inoculation with the chimeric TBEV/DEN4Δ30 vaccine candidate, the naturally attenuated LGTV, or the YF 17D vaccine virus. LGTV, a former TBEV vaccine candidate that retains a low but unacceptable level of residual neuroinvasiveness and neurovirulence for humans (9, 41) and that can be studied at biosafety level 2 (BSL-2), was chosen as a reference virus. The highly virulent Sofjin strain of TBEV, from which the prM and E structural protein genes in TBEV/DEN4Δ30 are derived, was not used as a comparator virus since it requires animal BSL-4 containment, which was not available to us for this study. The YF 17D vaccine virus (YF 17D) was used as another reference virus since this vaccine has been used for human vaccination for 70 years with a remarkable record of safety and efficacy (21). Our analysis of the development of CNS infection in monkeys following i.c. virus inoculation included comparisons of the clinical signs and the spatiotemporal patterns of virus replication and distributions of histopathological lesions at multiple time points. In addition, the topographical localization of viral antigens and the cellular inflammatory response were analyzed using immunohistochemistry. Our results indicate that chimeric TBEV/DEN4Δ30 virus is more neurovirulent in rhesus monkeys than either LGTV or YF 17D, suggesting that it needs to be further attenuated. These results offer insight into neuropathogenesis associated with attenuated TBEVs that may guide the design of safe vaccines against other neurotropic flavivirus infections.
MATERIALS AND METHODS
Cells and viruses.
Simian Vero cells (World Health Organization seed, passage 143 to 149) were maintained at 37°C in an atmosphere of 5% CO2 in OptiPRO SFM medium supplemented with 4 mM l-glutamine and 0.05 mg/ml of gentamicin (Invitrogen, Carlsbad, CA). The LGTV wild-type strain TP21, originally isolated from ticks in Malaysia in 1956, was received from the Rockefeller Foundation Collection, plaque purified three times in Vero cells under soft agar, and finally amplified by three passages in Vero cells. The titer of LGTV in Vero cells was 6.9 log10 PFU/ml as determined by plaque-forming assay (31). The chimeric recombinant TBEV/DEN4Δ30 virus that contained the prM and E genes of the highly virulent Sofjin strain of TBEV and a 30-nucleotide deletion in the 3′ noncoding region of the genome was originally recovered after transfection of Vero cells with RNA transcripts of its full-length chimeric cDNA genome (39). The stock virus had a titer of 6.3 log10 PFU/ml in Vero cells. The live attenuated YF virus vaccine, YF-VAX, used in this study was originally prepared by culturing the 17D-204 strain of YF virus in living avian leucosis virus-free chicken embryos (Sanofi Pasteur Inc., Swiftwater, PA). The lyophilized YF-VAX virus (∼4.74 log10 PFU) in the sealed vial (vial 5188, lot UE984AA) was reconstituted with 1 ml of the sterile diluent provided (Sodium Chloride Infection USP) and used to infect confluent Vero cell monolayers in two 225-cm2 tissue culture flasks containing 5 ml of OptiPRO SFM medium. After virus adsorption for 1 h at 37°C, the inoculum was removed and cells were rinsed once with 10 ml of OptiPRO SFM medium. Infected cells were then overlaid with 25 ml of fresh OptiPRO SFM supplemented with 4 mM l-glutamine. After incubation at 37°C for 5 days, cell culture medium was harvested, clarified by centrifugation, and frozen at −80°C. The titer of YF virus in clarified supernatant was determined by titration on Vero cells using YF virus-specific hyperimmune mouse ascetic fluid (ATCC, Manassas, VA) to immunostain plaques as previously described (31). The stock virus had a titer of 6.6 log10 PFU/ml.
Animals.
The study was conducted using rhesus monkeys (Macaca mulatta) obtained from the NIAID Morgan Island Breeding Program, Yemassee, SC. All procedures involving monkeys were performed in BSL-2 facilities at Bioqual, Inc. (Rockville, MD) in accordance with Animal Welfare Regulations (USDA), PHS Policy on Humane Care and Use of Laboratory Animals, and the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD). Sixty-two monkeys, each weighing 2.7 to 5.0 kg, were used in this study. The monkeys were screened for neutralizing antibodies to LGTV, TBEV, DEN4, and YF virus and were found to be seronegative.
Experimental design.
Three animal experiments were performed. In the first experiment, two groups of 10 animals each were intrathalamically (i.t.) inoculated with 5.0 log10 PFU of TBEV/DEN4Δ30 or LGTV, and two animals were mock inoculated with the diluent, Leibovitz's L-15 medium (Invitrogen, Carlsbad, CA), supplemented with SPG buffer stabilizer (218 mM sucrose, 6 mM l-glutamic acid, 3.8 mM monobasic potassium phosphate, and 7.2 mM dibasic potassium phosphate, pH 7.2). Animals in this experiment were observed twice daily for 60 days postinoculation (dpi) for clinical signs of neurological illness and were euthanized only if they became moribund. In the second and third experiments (Table 1), three groups of 12 monkeys each were i.t. inoculated with 5.0 log10 PFU of TBEV/DEN4Δ30, LGTV, or YF 17D virus and euthanized at prespecified time points (3, 7, 14, 21, and 30 dpi). Four control monkeys were mock inoculated and euthanized at 7, 14, 21, and 30 dpi.
TABLE 1.
Experimental design of the comparative neuropathogenesis and neurovirulence study of TBEV/DEN4Δ30, LGTV, and YF 17D in rhesus monkeys
| Inoculated virus | Expt no.a | No. of animals euthanized on dpi:
|
|||||
|---|---|---|---|---|---|---|---|
| 3 | 7 | 14 | 21 | 30 | Total | ||
| TBEV/DEN4Δ30 | 2 and 3 | 2 | 2 | 2 + 1b,c | 2 + 1b | 2 | 12 |
| LGTV | 2 and 3 | 2 | 2 | 2 | 2 + 2b | 2 | 12 |
| YF 17D | 3 | 2 | 2 | 2 | 4 | 2 | 12 |
| Mock control | 2 and 3 | NDd | 1b | 1b | 1b | 1 | 4 |
Because a large number of animals were involved in the neuropathogenesis studies, they were conducted in separate but similarly designed and executed experiments. In experiment 1, the 22 animals used were not sacrificed and are not included in this table. Experiment 2 included 21 animals, and experiment 3 included 19 animals.
Number of animals in experiment 3.
This monkey was scheduled to be sacrificed at 21 dpi in experiment 3 but was euthanized at 13 dpi.
ND, not done.
i.t. inoculations.
The procedure for bilateral i.t. inoculation was slightly modified from that previously described (3, 20, 26). Briefly, each monkey was anesthetized by intramuscular injection of ketamine hydrochloride (10 to 15 mg/kg of body weight) before virus inoculation. The monkey's scalp was shaved, scrubbed with iodophore and swabbed with 70% alcohol. The monkey was placed on the table in ventral recumbency with its head on a chin rest. The scalp was then retracted posteriorly and firmly fixed. The parietal and temporal sutures of the skull were located by palpation and marked using a sterile surgical skin marker. The coordinates of two inoculation sites (each 5 mm posterior to the temporal sutures and 15 mm equidistant from the parietal suture) were measured using sterile surgical rulers and marked. Two incisions, each approximately 5 mm long in the coronal plane, were made through the skin and the galea aponeurotica to the skull. Two holes (each 0.8 mm in diameter) were drilled through the skull. A separate 25-gauge, 1 1/4-inch needle on a 1-ml disposable syringe containing the inoculum was inserted through each hole to a depth of approximately 1 in. at an angle approximately 20° from the vertical toward the midline and 5° anteriorly from the vertical. Inocula of 0.5 ml each were injected into the left and right thalamic regions at a low and steady rate (over 8 to 10 seconds). After both injections were performed, the scalp was released and Vetbond tissue adhesive (3 M, St. Paul, MN) was applied to close the incision sites.
Clinical evaluation.
Animals were monitored for 60 dpi, and the following clinical observations were recorded: food consumption, body weight, body temperature, coat condition, and, if present, clinical evidence of encephalitis (weakness, shaky movements, impaired coordination, tremors, and limb paralysis). Monkeys were observed twice daily and assigned numerical scores according to guidelines previously specified for YF 17D vaccine (13, 46) as follows: grade 0, no general clinical signs or signs of CNS involvement; grade 1, rough coat and decreased food consumption; grade 2, inactive and slow moving; grade 3, shaky movements, incoordination, tremors, or limb weakness; grade 4, inability to stand, limb paralysis, moribund state, or death. The higher of the two scores each day was assigned as the individual daily score.
Tissue and serum samples.
Monkeys were euthanized according to a protocol approved by the NIAID Animal Care and Use Committee. Brains and spinal cords were removed, aseptically dissected, and processed for virus titration, histopathological examination, and immunohistochemical detection of viral antigens and cellular markers. A detailed dissection scheme for monkey brains is presented in Fig. S1 in the supplemental material. Coronal-plane brain tissue blocks were selected to include major neuroanatomical compartments: cerebral cortex, hippocampus, basal ganglia, thalamus, midbrain, pons, cerebellum, and medulla oblongata. Spinal cords were divided transversally into 18 tissue samples (six from each of the cervical, thoracic, and lumbar regions). Each spinal cord tissue sample was further divided into two equal portions, one for histopathological examination and immunohistochemical detection of viral antigens and cellular markers and the other for virus titration. Serum samples were collected at 0, 1, 3, 6, 8, 10, 13 and 30 dpi to test for viremia and for measurement of neutralizing antibody titers.
Viremia and virus loads in the CNS.
Virus titers in serum and CNS tissue samples were determined by plaque-forming assay on Vero cells as previously described (31). Seventeen samples of brain tissue (1a to 17a, as shown in Fig. S1 in the supplemental material) and 18 samples of spinal cord tissues (six from each of the cervical, thoracic, and lumbar regions) were collected aseptically at necropsy and frozen at −80°C. Each frozen tissue sample was thawed, weighed, and homogenized in phosphate-buffered Hanks’ balanced salt solution (Invitrogen, Carlsbad, CA) supplemented with SPG buffer stabilizer, 5 mM sodium glutamate, 0.05 mg/ml of ciprofloxacin (Bayer HealthCare, West Haven, CT), 0.06 mg/ml of clindamycin (Pharmacia & Upjohn, Kalamazoo, MI), and 0.0025 mg/ml of amphotericin B (Quality Biologicals, Gaithersburg, MD) to yield 10% (wt/vol) tissue suspensions. Tissue suspensions were clarified by low-speed centrifugation, aliquoted, and stored at −80°C until virus titration on Vero cells.
PRNT60.
Virus-specific neutralizing antibody titers were determined by the 60% plaque reduction neutralization assay (PRNT60) in Vero cells as previously described (33, 39). The neutralizing antibody titer was defined as the dilution of serum that neutralized 60% of the virus used in the assay.
Histopathological evaluation.
Tissues fixed in 10% phosphate-buffered formalin were dissected (see Fig. S1 in the supplemental material) and processed according to standard methods for histological examination. Two sections (5 μm thick) from each paraffin-embedded tissue block were stained with hematoxylin and eosin or with gallocyanin; additional sections from selected blocks were stained with cresyl violet or luxol fast blue. Primate brain maps (18) and Macaca mulatta brain maps (NIMH, NIH; http://brainmaps.org) were used for neuroanatomical orientation and mapping of the CNS regions. Fourteen CNS, regions including cerebral cortex (frontal, temporal, parietal, and occipital), hippocampus, basal ganglia, thalamus, midbrain, pons, cerebellum, medulla oblongata, and spinal cord (cervical, thoracic, and lumbar regions) were evaluated microscopically for presence, anatomical localization, and severity of histopathological lesions. We modified the grading systems previously described (1, 28, 46) and evaluated the virus-associated histopathology by separately grading (i) cellular inflammatory infiltration (CII) and (ii) microglial activation and neuronal degeneration (MGA/ND). The grading scale incorporated five scores: 0, no lesions; 1, minimal; 2, mild; 3, moderate; 4, severe (see Table S1 in the supplemental material). The scale for grading MGA/ND was modified for the cerebellum and spinal cord in order to make a rough adjustment for the difference in the volume of gray matter in those parts of the CNS. For each of the 14 CNS regions of each monkey, a mean score and a standard deviation were calculated based on a number of readings from separate sections within each defined anatomical region, with a minimum of 2 readings (for the occipital cortex, cerebellum, and medulla oblongata) and a maximum of 12 readings (for the cervical, thoracic, and lumbar regions of spinal cord). Mean group histopathological scores (MGHS) and standard deviations for each CNS region and the entire CNS were calculated based on the total number of readings at a given time point postinfection. The differences in MGHS were analyzed using Student's t test (two tailed, heteroscedastic). Significance was assumed for P values of <0.05.
Immunohistochemistry.
For detection of TBEV and LGTV antigens, a mixture of two mouse monoclonal antibodies, 2H3 and 13D6 (1:1,000), against E protein of TBEV strain Sofjin (19, 44) was used. For detection of YF virus antigen, YF virus-specific hyperimmune mouse ascetic fluid (1:4,000) (ATCC, Manassas, VA) was used. For characterization of the cellular inflammatory response, the following antibodies were used: polyclonal rabbit anti-human CD3 (1:300; Dakocytomation, Carpinteria, CA), monoclonal mouse anti-CD20 (1:400; Biocare Medical, Concord, CA), and monoclonal mouse anti-human CD68, clone KP1 (1:1,000; Dakocytomation, Carpinteria, CA). Antigen retrieval was performed by proteinase K digestion or by boiling sections in Diva Decloaker (Biocare Medical, Concord, CA). Further processing for colorimetric detection was according to the instructions for the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) or MACH 4 universal polymer detection kit (Biocare Medical, Concord, CA), and diaminobenzidine was used as a chromogen. Negative controls included omission of the primary antibody step. Additionally, sections prepared from pelleted Vero cells, uninfected or infected with TBEV/DEN4Δ30, LGTV, or YF 17D, were used as negative or positive controls for the viral antigen detection.
RESULTS
Study design.
Three separate experiments were performed in this study. The first experiment was designed to evaluate the clinical signs in monkeys following i.t. inoculation with the chimeric live attenuated TBEV vaccine candidate (TBEV/DEN4Δ30) in comparison with another member of the TBEV complex, an antigenically closely related, naturally attenuated LGTV. The YF 17D vaccine virus, another attenuated flavivirus that we chose to use as a reference in this study, was not included in our first experiment since the clinical data for rhesus monkeys i.c. inoculated with this virus were reported elsewhere (13, 17, 21, 22). The second and third experiments (Table 1) were designed to study the neuropathogenesis in rhesus monkeys and to evaluate neurovirulence of the chimeric TBEV/DEN4Δ30 vaccine candidate in comparison with LGTV (experiment 2) or YF 17D (experiment 3). Collectively, in these two experiments, three groups of 12 animals each were inoculated i.t. with TBEV/DEN4Δ30, LGTV, or YF 17D, and the numbers of animals euthanized at five time points (3, 7, 14, 21, and 30 dpi) are indicated in Table 1. We analyzed the spatiotemporal patterns of virus replication, topographical localization of viral antigens, temporal and anatomical distributions of histopathological lesions, and immunophenotype of the inflammatory cells in the CNS of monkeys. Viremia and serum neutralizing-antibody responses were also evaluated.
Clinical evaluation.
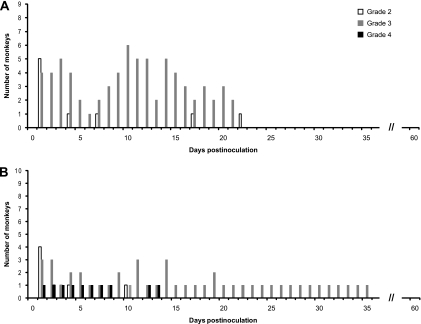
The clinical signs in 10 monkeys inoculated with TBEV/DEN4Δ30 or LGTV were evaluated as described in Materials and Methods according to the WHO grading scale (46). The distribution of daily clinical scores relevant to CNS involvement (grades 2, 3, and 4) during 60 dpi is shown in Fig. 1. One monkey from the TBEV/DEN4Δ30 group had a head tilt and lethargy and was unable to stand on the day after inoculation and was euthanized at 7 dpi because of declining health. The TBEV/DEN4Δ30 virus was not detected in the CNS of this monkey, and histopathological analysis confirmed traumatic brain injury resulting from the procedure of i.t. inoculation but did not reveal any virus-associated histopathological lesions. Thus, this monkey was excluded from the clinical evaluation. The remaining nine monkeys demonstrated a distinct biphasic neurological illness with development of grade 3 clinical signs such as shaky movements and incoordination lasting up to day 21 after inoculation (Fig. 1A). None of TBEV/DEN4Δ30-inoculated monkeys showed any clinical signs of neurological illness after 22 dpi. The clinical manifestations were different in monkeys inoculated with LGTV (Fig. 1B). One monkey from the LGTV group had a head tilt and lethargy and was unable to stand on the day after inoculation (grade 4). At 9 to 11 dpi, this monkey improved slightly, ate normally, and tried to move again but remained uncoordinated. At 12 dpi, the neurological status deteriorated, and this monkey was euthanized at 14 dpi. This monkey was not excluded from the clinical evaluation due to the fact that a moderate level of virus replication was detected in the cervical, thoracic, and lumbar regions of the spinal cord (3.3, 1.7, and 2.6 log10 PFU/g of tissue, respectively), and histopathological analysis revealed severe virus-associated MGA/ND in the spinal cord (score 4; data not shown). The biphasic neurological illnesses in the remaining nine monkeys were less marked, but grade 3 clinical signs, including shaky movements, incoordination, tremors, and limb weakness, were observed in one monkey until 35 dpi. Two mock-inoculated control monkeys were lethargic (grade 2) only during 2 dpi (data not shown).
FIG. 1.
Distribution of daily clinical scores in monkeys following i.t. inoculation with TBEV/DEN4Δ30 (A) or LGTV (B) in experiment 1. There were 10 animals inoculated with each virus. Since 1 of the 10 animals was excluded from the TBEV/DEN4Δ30 group (see Results), the daily clinical scores are shown in panel A for only 9 monkeys.
Importantly, the clinical data collected during a 60-day observation period indicated that the TBEV/DEN4Δ30 virus retained a moderate level of neurovirulence. Monkeys inoculated with this virus developed shaky movements and incoordination. However, we did not observe any other grade 3 clinical signs, such as tremors or limb weakness, and none of TBEV/DEN4Δ30-inoculated monkeys developed severe grade 4 clinical signs.
Viremia and neutralizing antibody response.
In experiments 2 and 3 (Table 1), viremia was frequently detected in monkeys inoculated with LGTV or YF 17D, but none of the monkeys inoculated with TBEV/DEN4Δ30 developed detectable viremia (<0.7 log10 PFU/ml) (Fig. 2). Of the 12 monkeys inoculated with LGTV in both experiments, 6 monkeys (50%) developed viremia at 1 and/or 3 dpi that attained a mean peak virus titer of 2.1 log10 PFU/ml (ranging from 0.7 to 3.0 log10 PFU/ml). Of the 12 monkeys inoculated with the YF17D vaccine virus, 10 monkeys (83%) became viremic at 1 and/or 3 dpi. Viremia reached a mean peak virus titer of 2.3 log10 PFU/ml (ranging from 0.7 to 2.9 log10 PFU/ml). All three viruses (TBEV/DEN4Δ30, LGTV, and YF 17D) were immunogenic and induced high levels of serum neutralizing antibodies against the homologous virus (Fig. 2). However, monkeys inoculated with LGTV or YF 17D developed neutralizing antibodies more rapidly than monkeys inoculated with TBEV/DEN4Δ30 virus.
FIG. 2.
Viremia (bars) and serum neutralizing antibody response to homologous virus (lines) in monkeys following i.t. inoculation with TBEV/DEN4Δ30, LGTV, or YF 17D. The dashed line shows the lowest serum dilution tested (1:2) by PRNT60. The mean virus titers in sera of monkeys inoculated with LGTV or YF 17D are shown by black or open bars, respectively. Values below the limit of virus detection in serum (0.7 log10 PFU/ml) are not shown.
Spatiotemporal patterns of virus replication in the CNS.
The spatiotemporal distributions of infectious virus in the CNS of monkeys inoculated with TBEV/DEN4Δ30, LGTV, or YF 17D were analyzed at five time points (Fig. 3).
FIG. 3.
Viral loads in the CNS of monkeys following i.t. inoculation with TBEV/DEN4Δ30 (A), LGTV (B), or YF 17D (C) on the indicated dpi (experiments 2 and 3). The virus titers in CNS regions of each monkey were measured by plaque-forming assay as described in Materials and Methods and are expressed as means and standard deviations. The lower limit of virus detection was 1.7 log10 PFU/g of brain or spinal cord tissue (values below the detection limit are not shown). Gray bars show viral loads in CNS regions of monkeys inoculated with TBEV/DEN4Δ30 or LGTV in experiment 3 (monkeys are indicated with asterisks).
At 3 dpi, the infectious TBEV/DEN4Δ30 virus was first detected in the CNS of monkeys within the areas adjacent (basal ganglia: caudate nucleus, globus pallidus, and putamen) and proximal (frontal cortex) to the site of inoculation (thalamus). Mean virus titers in these regions ranged from 1.8 to 2.7 log10 PFU/g of tissue (Fig. 3A). Similarly, monkeys inoculated with YF 17D had infectious virus within the range of 2.0 to 3.3 log10 PFU/g in the frontal cortex and basal ganglia, but virus was also detected in the thalamus (3.3 log10 PFU/g) and within the areas more distal to the inoculation site, i.e., the occipital cortex (1.7 log10 PFU/g) and midbrain (3.3 log10 PFU/g) (Fig. 3C). In contrast, at this early time point, infectious virus was not detected in any of the CNS regions of LGTV-inoculated monkeys.
By 7 dpi, similar levels of infectious virus, ranging from 1.7 to 2.9 log10 PFU/g, were detected in multiple brain regions of monkeys inoculated with TBEV/DEN4Δ30 or YF 17D (Fig. 3A and C, respectively). However, the distribution of infectious virus in the CNS of YF 17D-inoculated monkeys was more widespread, and virus disseminated in a caudal direction involving the pons of two monkeys (2.3 log10 PFU/g) and the entire spinal cord of one monkey (1.7 to 2.9 log10 PFU/g). In contrast, only one of two monkeys inoculated with LGTV had low virus titers (1.7 to 2.4 log10 PFU/g) in the cerebral cortex (frontal and occipital) and in basal ganglia.
At 14 dpi, none of YF 17D-inoculated monkeys had infectious virus in any region of the CNS. Remarkably, replication of LGTV completely shifted in a caudal direction involving the cerebellum, medulla oblongata, and entire spinal cord (Fig. 3B). On the other hand, two monkeys inoculated with TBEV/DEN4Δ30 in the second experiment (DB3K and DB7W) did not have detectable virus in the CNS, but one of two TBEV/DEN4Δ30-inoculated monkeys in the third experiment, which became moribund and was euthanized on 13 dpi (DBEK), demonstrated high virus loads (up to 5.0 log10 PFU/g) throughout entire CNS (Fig. 3A). The virus recovered from the brain and spinal cord of this monkey was sequenced across its entire genome and had the sequence of the input TBEV/DEN4Δ30 virus.
At 21 and 30 dpi, viral replication was not detected in the CNS of any monkey inoculated with TBEV/DEN4Δ30 or YF 17D. In contrast, the pattern of LGTV replication seen at 14 dpi was also observed at 21 dpi, with a subsequent decrease to below the detectable level by 30 dpi.
Spectrum of virus-associated histopathology.
Histopathological analysis of the CNS of rhesus monkeys inoculated with the attenuated flaviviruses used in the present study (TBEV/DEN4Δ30, LGTV, or YF 17D) revealed evidence of typical pathological features of an encephalitic reaction in response to viral replication (Fig. 4). The spectrum of virus-associated lesions in the CNS was comprised of features characteristic of two pathological processes: CII and response of the resident cells of the CNS (MGA/ND). i.t. inoculation resulted in tissue destruction and hemorrhage at the site of inoculation and proliferation of microglia at the needle track edges, with marked infiltration by macrophages and focal loss of myelin (data not shown). The CII in the CNS of monkeys was characterized by mononuclear inflammatory cell infiltration in the Virchow-Robin spaces (perivascular cuffing) (Fig. 4A to C), diffuse infiltration in the neuropil (Fig. 4A), and focal infiltration in the leptomeninges (Fig. 4D to F). Occasionally, focal CII was also found in the stroma of the choroid plexus. The MGA/ND in the CNS of monkeys were seen as diffuse gliosis, microglial nodules (MGNs), ND, neuronophagia, and neuronal death and loss (Fig. 4). The ND was characterized by cell shrinkage, chromatolysis, and margination of Nissl substance with eccentric or pyknotic nuclei. In the cerebellum, characteristic histopathological lesions included focal gliosis in the molecular layer combined with degeneration and loss of Purkinje cells (Fig. 4D to F). In the spinal cord, MGNs, neuronophagia, and neuronal loss were predominantly found in the ventral horns (Fig. 4G to I). These findings were used to develop a grading scale for evaluation of the severity of CII and MGA/ND in the CNS of monkeys in this study (see Table S1 in the supplemental material).
FIG. 4.
Spectrum of virus-associated histopathological lesions in the CNS of monkeys inoculated with TBEV/DEN4Δ30 (A, D, and G), LGTV (B, E, and H), or YF 17D (C, F, and I) at the peak time points (14 to 21 dpi). (A to C) Frontal cortex (21 dpi), showing perivascular cuffing by mononuclear inflammatory cells (black arrows) and (A) diffuse mononuclear inflammatory cell infiltration in the neuropil (white arrow), focal gliosis, and ND (arrowhead); (B) MGN (arrowhead); and (C) focal gliosis and neuronophagia (arrowhead). (D to F) Cerebellum (14 dpi), showing CII in the leptomeninges (black arrows), focal gliosis in the molecular layer (white arrows), and degeneration and loss of Purkinje cells (arrowheads). (G to I) Ventral horns of the cervical (G) and lumbar (H and I) spinal cord (14 to 21 dpi), showing (G) neuronophagia and acidophilic neuronal necrosis (arrowhead, 21 dpi), (H) neuronophagia and degenerating eosinophilic neuron with diffuse chromatolysis and eccentric nucleus (arrowhead, 14 dpi), and (I) foci of neuronophagia (arrowheads, 14 dpi). Hematoxylin and eosin staining was used. Original magnifications, ×200 (A to F, H, and I) and ×400 (G).
Temporal and anatomical distributions of histopathological lesions.
The severity of the virus-associated histopathology in the CNS of monkeys inoculated with TBEV/DEN4Δ30, LGTV, or YF 17D was evaluated as described in Materials and Methods. The temporal distribution of MGHS for CII and MGA/ND in the entire CNS is shown in Fig. 5A. In monkeys inoculated with TBEV/DEN4Δ30 virus, the severity of histopathological lesions (both CII and MGA/ND) in the entire CNS gradually increased after inoculation and reached a peak on 14 dpi; it remained at the same level during the following week (21 dpi) and decreased by 30 dpi (Fig. 5A). In the CNS of LGTV-inoculated monkeys, the magnitude of MGA/ND reached a maximum at 14 dpi, while the CII developed at a lower rate and reached its peak 1 week later (at 21 dpi). Consequently, the magnitude of both CII and MGA/ND significantly decreased by 30 dpi (Fig. 5A). Monkeys inoculated with YF 17D vaccine virus, similar to TBEV/DEN4Δ30-inoculated monkeys, demonstrated rapid development of histopathological lesions, which peaked at 14 dpi but sharply decreased thereafter (Fig. 5A). The control monkeys euthanized at 7, 14, 21, and 30 dpi had no virus-associated histopathology in the CNS (data not shown). Importantly, beginning at 7 dpi and until the end of the experiment, monkeys inoculated with TBEV/DEN4Δ30 virus had higher histopathological scores for the entire CNS than monkeys inoculated with LGTV or YF 17D (Fig. 5A).
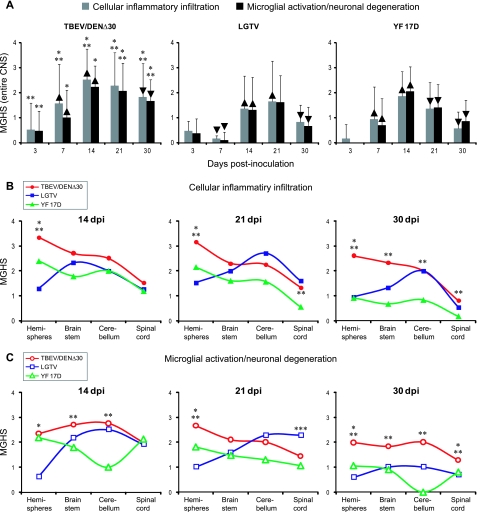
FIG. 5.
Temporal and anatomical distributions of histopathological lesions in the CNS of monkeys following i.t. inoculation with TBEV/DEN4Δ30, LGTV, or YF 17D. (A) Temporal distributions of MGHS for the CII and MGA/ND in the entire CNS. The grading scale is described in Table S1 in the supplemental material. (B and C) Anatomical profiles of the CII (B) and MGA/ND (C) at 14, 21, and 30 dpi. MGHS did not differ statistically unless specifically noted. MGHS were significantly higher (▴) or lower (▾) compared to the previous time point (P < 0.05). MGHS for the CNS regions of the TBEV/DEN4Δ30 group of monkeys were significantly higher (P < 0.05) than those for the LGTV group (indicated with one asterisk) or the YF 17D group (indicated with two asterisks) at the same time point. MGHS for the spinal cords of LGTV-inoculated monkeys were significantly higher (P < 0.05) than those for the TBEV/DEN4Δ30 or YF 17D group on 21 dpi (indicated with three asterisks). Hemispheres: cortex, hippocampus, basal ganglia (caudate nucleus, globus pallidus, and putamen), and thalamus. Brain stem: midbrain, pons, and medulla oblongata. Spinal cord: cervical, thoracic, and lumbar regions.
Although the MGHS for the entire CNS were informative for the temporal analysis, they did not permit an analysis of the differences in anatomical distribution of the histopathological lesions observed. Therefore, for the three attenuated flaviviruses studied, we evaluated the histopathological profiles within major anatomical compartments along the rostro-caudal axis of the CNS at the potentially discriminative time points (14 to 30 dpi) (Fig. 5B and C; detailed data can be found in Fig. S2 in the supplemental material). Interestingly, the TBEV/DEN4Δ30 and YF 17D viruses demonstrated remarkably similar spatiotemporal profile of the CII: high in the hemispheres with a progressive decrease toward the spinal cord. In contrast, CII in the CNS of LGTV-inoculated monkeys was low in hemispheres but high in the brain stem, cerebellum, and spinal cord (Fig. 5B).
Furthermore, analysis of the spatiotemporal profile of MGA/ND revealed remarkable differences between these three attenuated flaviviruses, which, however, were more prominent at 14 and 21 dpi (Fig. 5C). At 14 dpi, the magnitude of MGA/ND in hemispheres induced by TBEV/DEN4Δ30 was significantly higher than that for LGTV but similar to that for YF 17D. In the brain stem and cerebellum, differences were seen between TBEV/DEN4Δ30 and YF 17D but not between TBEV/DEN4Δ30 and LGTV. The spinal cords were similarly affected in all groups of monkeys.
At 21 dpi, differences in MGA/ND profiles between TBEV/DEN4Δ30 and YF 17D could be seen only in hemispheres, whereas the LGTV MGA/ND profile was remarkably different (low in hemispheres and very high in cerebellum and spinal cord). Importantly, MGA/ND scores for the spinal cord in LGTV-inoculated monkeys at 21 dpi were significantly higher than those in both TBEV/DEN4Δ30 and YF 17D-inoculated monkeys (Fig. 5C).
At 30 dpi, fewer differences were seen in MGA/ND profiles between TBEV/DEN4Δ30, LGTV, and YF 17D, but chimeric TBEV/DEN4Δ30 virus demonstrated significantly higher MGA/ND scores throughout the CNS.
Topographical localization of viral antigens.
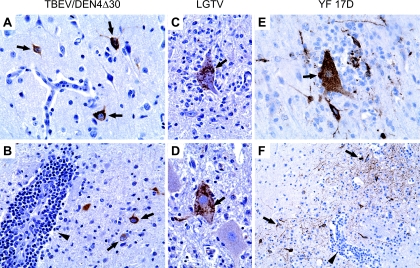
Analysis of the spatiotemporal distribution of viral antigens in the CNS of monkeys inoculated with TBEV/DEN4Δ30, LGTV, or YF 17D corroborated the data on distribution of infectious virus (Fig. 3) and revealed a prominent neurotropism associated with each attenuated virus tested. Topographically, the viral antigen-positive neurons were mostly associated with the histopathological lesions but occasionally were also seen within the intact areas. Viral antigens were localized in the neuronal perikarya and processes (Fig. 6).
FIG. 6.
Localization of viral antigens in the CNS of monkeys inoculated with TBEV/DEN4Δ30 at 7 dpi (A and B), with LGTV at 14 dpi (C and D), or with YF 17D at 7 dpi (E and F). (A) Frontal cortex, showing strongly immunolabeled neurons (arrows) within intact gray matter. (B) Thalamus, showing immunolabeled neurons (arrows) surrounding a perivascular inflammatory infiltrate (arrowhead). (C and D) Ventral horns of the lumbar spinal cord, showing neuronophagia (D) and prominent immunolabeling in the cytoplasm of degenerating neurons (C and D, arrows). (E) Parietal cortex, showing neuronophagia (arrow) and strongly immunolabeled neurons with decorated processes. (F) Putamen, showing CII (arrowhead) and prominent immunolabeling of neuronal network (arrows). Original magnifications, ×400 (A to E) and ×200 (F).
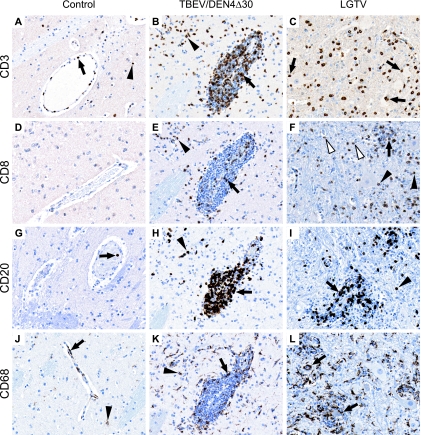
Immunohistochemical analysis of cellular inflammatory response.
The phenotype of cells involved in the inflammatory response within the CNS was analyzed using immunohistochemistry. At the peak time points of CII and MGA/ND, the inflammatory foci were composed of CD3+ T lymphocytes, CD8+ cytotoxic lymphocytes (CTLs), CD20+ B cells, and CD68+ macrophages/microglia (Fig. 7). The CII included numerous CD3+ T lymphocytes in perivascular and parenchymal compartments of the brains and spinal cords of infected monkeys (Fig. 7B and C). Within the perivascular compartments, only a small fraction of CD3+ T lymphocytes was represented by CD8+ T lymphocytes (Fig. 7, E), whereas the parenchymal compartments were mostly infiltrated by CD8+ T lymphocytes (Fig. 7E and F). CD8+ T lymphocytes were often found in close contact with morphologically intact neurons, in the immediate vicinity of the remnants of degenerated neurons, or within the MGNs (Fig. 7F). Numerous CD20+ B cells were also found within the perivascular inflammatory infiltrates and surrounding neuropil (Fig. 7H and I). CD68+ macrophages were present in the sites of inoculation (not shown) and within the perivascular infiltrates (Fig. 7K). MGA was frequently observed in the areas of neuronal damage with foci of neuronophagia (Fig. 7L). The degenerating neurons on the periphery of the MGNs were often found engulfed by the CD68+ activated microglia/macrophages.
FIG. 7.
Immunohistochemical analysis of cellular inflammatory response in the CNS. Results of immunostaining for CD3 (A to C), CD8 (D to F), CD20 (G to I), and CD68 (J to L) of mock-inoculated control monkeys (A, D, G, and J; representative sections of caudate nucleus) and monkeys inoculated with TBEV/DEN4Δ30 (B, E, H, and K; adjacent sections of the caudate nucleus) or LGTV (C, F, I, and L; representative sections showing the ventral horn of the lumbar spinal cord) at 21 dpi are shown. (A) A few CD3+ T lymphocytes adjacent to endothelial cells of the blood vessel (arrow) or in the surrounding parenchyma (arrowhead). (B) CD3+ T lymphocytes represent a fraction of mononuclear cells within the perivascular inflammatory infiltrate (arrow) and diffusely infiltrate the surrounding parenchyma (arrowhead). (C) Diffuse infiltration by CD3+ T lymphocytes. Note that many CD3+ T lymphocytes are located in close contact with neurons (arrows). (D) No CD8 immunostaining. (E) CD8+ CTLs represent only a small fraction of CD3+ T lymphocytes within the perivascular compartment (arrow) but make up most of the CD3+ T lymphocytes present in the parenchymal compartment (arrowhead). (F) Many CTLs are located in a close contact with neurons (black arrowheads) or in the immediate vicinity of the remnants of neurons (white arrowheads), as well as within the MGN (arrow). (G) Single CD20+ B cell within the lumen of the blood vessel (arrow). (H and I) CD20+ B cells represent a large fraction of lymphocytes within the perivascular infiltrates (arrows) and also invade the surrounding parenchyma (arrowheads). (J) A few CD68+ perivascular macrophages (arrow) and microglial cells (arrowhead). (K) CD68+ macrophages within the perivascular inflammatory infiltrates (arrow) and CD68+ immunoreactive activated microglia (arrowhead). (L) CD68+ activated microglia within the foci of neuronophagia (arrows). Original magnification, ×200.
DISCUSSION
Previous studies have demonstrated that the chimeric TBEV/DEN4Δ30 virus was highly attenuated for neuroinvasiveness in immunodeficient mice (39) and was less neurovirulent in adult Swiss mice than its immediate TBEV/DEN4 parent or LGTV (Pletnev, unpublished data). In addition, TBEV/DEN4Δ30 virus was shown to induce high levels of serum neutralizing antibody against TBEV in mice and rhesus monkeys and provided complete protection against challenge with TBEV or LGTV (30, 39). Nevertheless, TBEV/DEN4Δ30 retained a high level of neurovirulence in suckling mice inoculated i.c. (39). The relevance of flavivirus-induced death in suckling mice to the potential to cause CNS disease in humans is unclear since death in suckling mice is common even after i.c. inoculation of highly attenuated flaviviruses, including live attenuated YF 17D vaccine (23), which has a remarkable record of safety and efficacy in humans (21). Nonetheless, we decided to evaluate the neurovirulence of TBEV/DEN4Δ30 in nonhuman primates, especially since the chimeric virus carries the structural prM and E protein genes derived from the highly neurovirulent Sofjin strain of Far Eastern TBEV. In contrast to TBEV/DEN4Δ30, chimeric vaccine candidates for West Nile virus (WNV/DEN4Δ30) and LGTV (LGT/DEN4) have entered clinical trials in humans without monkey neurovirulence testing, since both vaccine candidates were highly attenuated for the CNS of suckling mice (35, 36, 39). Results of the phase I clinical trials in humans indicate that both vaccines are immunogenic and safe in adult volunteers without local or systemic reactogenicity. Unfortunately, seroconversion against heterologous TBEV in the LGT/DEN4 vaccine recipients was infrequent, and the level of TBEV-neutralizing antibodies induced was significantly lower than that observed against homologous LGTV, suggesting that the vaccine candidate will need to be based on the TBEV structural protein genes (48).
Several factors need to be taken into account during the evaluation of the level of neurovirulence of TBEV/DEN4Δ30 in nonhuman primates. First, although the MNVT has been developed for a variety of neurotropic viruses, including poliovirus (25), measles virus (40), mumps virus (14, 20, 38), and vesicular stomatitis virus (10), the MNVT has been established for only one flavivirus, the YF 17D vaccine virus (13, 46). Second, a decision regarding an acceptable level of neurovirulence for a test virus should be made based on a comparison to one or more reference control viruses, which usually include the neurovirulent parent virus and a related vaccine virus that exhibits an acceptable level of attenuation for the CNS of humans. The YF 17D vaccine virus can be used as a surrogate, and the naturally attenuated LGTV that retains an unacceptable level of residual neurovirulence for humans (41) acts as the surrogate for the TBEV parent, which is a BSL-4 agent. Third, the histopathological scoring methodology for the evaluation of YF 17D vaccine might not be appropriate for the assessment of neurovirulence of the antigenically distant TBEV/DEN4Δ30. Finally, the standard MNVT provides only a snapshot of the pathogenesis of CNS infection, usually around 30 days after i.c. inoculation of monkeys with a given virus, and does not include evaluation of the time course of either viral replication or the development of histopathological lesions in the CNS. Thus, our major objective was to study neuropathogenesis in rhesus monkeys following i.c. inoculation with TBEV/DEN4Δ30 virus and to compare the level of neurovirulence of the chimeric vaccine candidate with that of LGTV or YF 17D vaccine virus in order to gather comprehensive safety data prior to advancing to clinical trials.
TBEV can cause acute, subacute, or chronic forms of encephalitis in humans and experimental monkeys (2, 6, 7, 11, 12, 37). In this study, the clinical data indicate that TBEV/DEN4Δ30 virus retains significant neurovirulence. Although the majority of animals inoculated with TBEV/DEN4Δ30 showed only moderate signs of neurological illness, there was one monkey that developed severe encephalitis. However, the TBEV/DEN4Δ30 virus is significantly attenuated compared to the highly virulent parental TBEV, since it has been previously shown that i.c. inoculation of monkeys with this strain resulted in the development of seizures, tremors, pareses, paralysis, and death in 100% of animals (37). Among LGTV-inoculated monkeys, one animal developed severe encephalitis, but the remaining monkeys had tremors and limb weakness consistent with those previously described (24, 43). It is noteworthy that YF 17D, a safe vaccine in humans (21), can cause signs of CNS dysfunction such as tremors in 40% of i.c. inoculated monkeys (22). The clinical data for monkeys therefore suggested that the TBEV/DEN4Δ30 virus was insufficiently attenuated for the CNS of nonhuman primates.
Although the neurovirulence of selected flaviviruses in nonhuman primates has been examined previously (11, 13, 17, 22, 24, 26-28, 37, 43), the present study is the first to analyze the kinetics of replication of attenuated flaviviruses in multiple anatomic sites of the CNS of monkeys. The neurons, as in many flaviviral infections of the CNS (4), were the primary cells expressing virus antigen. The anatomical distribution of infected neurons in the CNS of monkeys inoculated with each of the three attenuated flaviviruses correlated with the sites of virus recovery. The viral loads in the CNS of the majority of monkeys inoculated with attenuated flaviviruses in this study were relatively low (≤3.3 log10 PFU/g) compared to high titers (>6 log10 PFU/g) produced by virulent strains of TBEV (11, 15). These virological data, like the clinical observations summarized above, indicate that the TBEV/DEN4Δ30 vaccine candidate is highly attenuated compared to its parental TBEV. Another indication of the attenuation of TBEV/DEN4Δ30 was its failure to cause viremia in i.c. inoculated monkeys whereas either LGTV or YF 17D was detected in the blood. Detectable replication of infectious virus in the CNS of the majority of monkeys inoculated with TBEV/DEN4Δ30 or YF 17D ceased after day 7, coinciding with the appearance of serum neutralizing antibody. In contrast, the humoral immune response did not appear to have a significant role in the clearance of LGTV from the CNS, since its replication and caudal spread continued until day 21 despite the high titers of serum neutralizing antibodies developed at earlier time points (days 6 to 8). Although the levels of replication of TBEV/DEN4Δ30 and YF 17D virus in the CNS were generally similar, one TBEV/DEN4Δ30-inoculated monkey with severe encephalitis demonstrated high virus loads throughout the entire CNS. The virus recovered from the brain or spinal cord of this monkey was genetically identical to the inoculated TBEV/DEN4Δ30 virus, indicating that host factors, rather than mutations, might be responsible for the increased viral burden in the CNS and clinical manifestations of encephalitis.
Histopathological analysis of the CNS of rhesus monkeys inoculated with TBEV/DEN4Δ30, LGTV, or YF 17D revealed typical, although not disease-specific, histopathological lesions consistent with those previously described for flaviviral encephalitides in humans (7, 8) and in experimentally infected monkeys (2, 11, 12, 15, 24, 26-28, 37). The spectrum of virus-associated histopathology was comprised of features characteristic of two pathological processes: infiltration by inflammatory cells and reaction of the resident cells of the CNS (MGA/ND). In this study, we developed a new histopathological scoring methodology that included a separate evaluation of these two processes, and we evaluated all major neuroanatomical compartments (cerebral cortex, hippocampus, basal ganglia, thalamus, midbrain, pons, cerebellum, medulla oblongata, and spinal cord). The histopathological analysis revealed that the mean group scores for the entire CNS were significantly higher for monkeys inoculated with TBEV/DEN4Δ30 than for monkeys inoculated with either LGTV or YF 17D at all time points starting from day 7. Thus, the results of histopathological analysis of the CNS together with clinical observations and data on virus replication strongly indicate that chimeric TBEV/DEN4Δ30 virus is not sufficiently attenuated for the CNS of nonhuman primates. It is interesting that extensive histopathology in the CNS was observed with a relatively low level of TBEV/DEN4Δ30 replication, indicating that the virus evokes a strong cellular inflammatory response. In addition, using a new histopathological scoring methodology, we were able to identify distinct differences in the neuropathogenesis induced by TBEV/DEN4Δ30, LGTV, or YF 17D. For example, LGTV induced more severe lesions in the brain stem, cerebellum, and spinal cord, which became striking on day 21. These findings are consistent with previously described neurovirulence scores for LGTV, which were low in the hemispheres, intermediate in the brain stem, and high in the spinal cord (24). In contrast, the TBEV/DEN4Δ30 and YF 17D viruses demonstrated remarkably similar spatiotemporal histopathological profiles, which were high in the hemispheres with a progressive decrease toward the spinal cord. The anatomical compartments affected in monkeys inoculated with YF 17D in our study were similar to the previously described “indicator centers” (24, 28) or “target and discriminator areas” (13, 46) except that our finding of extensive involvement of the cerebral cortex is in disagreement with previous conclusions that this major CNS compartment can be designated a “spared area” (13). It should be noted here that the differences in anatomical distribution of histopathological lesions induced by each of these three viruses became less evident at 30 dpi, suggesting that histopathological analysis of the CNS in the MNVT should not be limited to only this time point.
The fact that YF 17D, LGTV and TBEV/DEN4Δ30 are antigenically distant flaviviruses might partially explain the observed differences in spatiotemporal profiles of viral replication and virus-associated histopathology in the CNS. The structural E protein of flaviviruses is involved in virus entry into cells and virion assembly and maturation, and it is believed to be a major determinant of virulence (21). The E protein of chimeric TBEV/DEN4Δ30 virus is derived from a highly virulent Far Eastern TBEV strain and shares amino acid homology of only 42% with that of YF 17D (34) and 88% with that of LGTV (16). Since TBEV/DEN4Δ30 contains the capsid and nonstructural protein genes of the nonneuroinvasive and low neurovirulent DEN4 virus, it is tempting to speculate that the prM and E proteins of TBEV, rather than the DEN4 genes, are the genetic determinants that are primarily responsible for the extensive histopathology in the CNS, but this will require additional studies in the future. Clearly, the differences in the biology of viruses and in their tropism to various neuronal populations within the CNS make direct comparisons of the levels of neurovirulence of different attenuated flaviviruses very difficult.
The pathogenesis of neurotropic flavivirus infections involves complex virus-host interactions, with a number of factors that have an effect on virus replication and its clearance by the innate and adaptive immune responses induced both in the periphery and within the CNS. Pathogenesis studies with nonhuman primates have been limited to quantitating the peripheral immune responses or the disease burden caused by replication of virus in the CNS (4). To the best of our knowledge, this is the first study with nonhuman primates to analyze the phenotype of cells involved in the inflammatory response within the CNS following i.c. inoculation with attenuated flaviviruses. We observed vigorous MGA in the areas of neuronal damage. The degenerating neurons were often found engulfed by the CD68+ activated microglia/macrophages. We show that inflammatory foci were composed of CD3+ T lymphocytes, CD8+ CTLs, CD20+ B cells, and CD68+ macrophages/microglia. Numerous CD3+ T lymphocytes were present in perivascular and parenchymal compartments of the brains and spinal cords of infected monkeys. However, only a small fraction of CD3+ T lymphocytes was represented by CD8+ CTLs within the perivascular compartments, whereas the parenchyma was infiltrated mostly by CD8+ CTLs. Importantly, CD8+ CTLs were often found in the immediate vicinity of the remnants of degenerated neurons and within the MGNs but also in close contact with morphologically intact neurons, suggesting that, while exhibiting their effector function in the clearance of neuroinfection, these cells might play a role in collateral neuronal damage. CD20+ B cells were also found within the perivascular inflammatory infiltrates, but numerous B cells were recruited into the surrounding neuropil, suggesting their involvement in virus clearance by local production of neutralizing antibodies. Further studies using quantitative immunohistochemical analysis are needed to determine whether the magnitude and dynamics of the cellular inflammatory response within the CNS are influenced by the level of neurovirulence of the virus and how these responses contribute to viral clearance and potential bystander cell injury.
Overall, the results of this study indicate that chimerization of a highly neurovirulent strain of TBEV with nonneuroinvasive DEN4 in the presence of the Δ30 mutation had an attenuating effect on neurovirulence in nonhuman primates compared to parental TBEV. However, this effect was insufficient based on comparison with two other attenuated flaviviruses, LGTV and YF 17D. Further reduction in the level of neurovirulence of the chimeric TBEV/DEN4Δ30 virus is needed. Nevertheless, the facts that the histopathological profile within the CNS of TBEV/DEN4Δ30-inoculated monkeys was remarkably similar to that observed in monkeys inoculated with YF 17D vaccine virus and that TBEV/DEN4Δ30 seems to have a reduced ability to affect the motor neurons of the spinal cord compared to LGTV or highly virulent TBEV strains (6, 11, 12, 15, 37) suggest that chimerization of TBEV with DEN4Δ30 is a useful mechanism for attenuation. An interesting aspect of this study is the comparative virological and histopathological analysis of the evolution of CNS infection in nonhuman primates following i.c. inoculation of attenuated flaviviruses. Our results provide insight into the neuropathogenesis beyond the most often analyzed time point at 30 dpi and demonstrate that attenuated flaviviruses can be more reliably discriminated by their histopathological profile at earlier time points. Taken together, these findings and our modified methodology of histopathological evaluation of neurovirulence may guide the design of safe live vaccines against neurotropic flaviviruses. Additional quantitative analysis is needed to evaluate the dynamics of CNS infiltration by different populations of inflammatory cells and responses of resident cells of the CNS induced by attenuated flaviviruses. We are currently performing a computerized morphometric analysis of the cellular inflammatory responses within the CNS of monkeys to investigate their role in neuropathogenesis and contribution to the outcome of neuroinfection.
Supplementary Material
Acknowledgments
We acknowledge the staff of Bioqual, Inc. (Rockville, MD), and the Pathology Associates Division of Charles River Laboratories (Frederick, MD) for their excellent assistance in conducting the monkey studies. We thank Richard Montali (Department of Molecular and Comparative Pathobiology, John Hopkins University) for help during experiments, Lawrence J. Faucette (Comparative Medicine Branch, NIAID, NIH) for superb assistance with immunohistochemistry, and Amber Engel (Laboratory of Infectious Diseases, NIAID, NIH) for the preparation of YF 17D virus. We also thank Stephen Whitehead and Joseph Blaney (Laboratory of Infectious Diseases, NIAID, NIH) and Pedro Piccardo (CBER, U.S. Food and Drug Administration) for helpful discussions and comments.
The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy.
This work was supported by funds provided by the NIAID Intramural Research Program.
There is not a conflict of financial or other interest.
Footnotes
Published ahead of print on 19 March 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Angsubhakorn, S., J. B. Moe, J. R. Latendresse, G. S. Ward, M. Ngamprochana, S. Sahaphong, and N. Bhamarapravati. 1986. The neurovirulence of flaviviruses in crab-eating monkeys (Macaca fascicularis). Southeast Asian J. Trop. Med. Public Health 17604-612. [PubMed] [Google Scholar]
- 2.Asher, D. M. 1975. Movement disorders in rhesus monkeys after infection with tick-borne encephalitis virus, vol. 10, p. 277-283. In B. S. Meldrum and C. D. Marsden (ed.), Advances in neurology. Raven Press, New York, NY. [PubMed] [Google Scholar]
- 3.Bodian, D., I. M. Morgan, and C. E. Schwerdt. 1950. Virus and host factors influencing the titer of Lansing poliomyelitis virus in monkeys, cotton rats and mice. Am. J. Hyg. 51126-133. [DOI] [PubMed] [Google Scholar]
- 4.Chambers, T. J., and M. S. Diamond. 2003. Pathogenesis of flavivirus encephalitis. Adv. Virus Res. 60273-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durbin, A. P., R. A. Karron, W. Sun, D. W. Vaughn, M. J. Reynolds, J. R. Perreault, B. Thumar, R. Men, C. J. Lai, W. R. Elkins, R. M. Chanock, B. R. Murphy, and S. S. Whitehead. 2001. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in the 3′ untranslated region. Am. J. Trop. Med. Hyg. 65405-413. [DOI] [PubMed] [Google Scholar]
- 6.Frolova, M. P., and V. V. Pogodina. 1984. Persistence of tick-borne encephalitis virus in monkeys. VI. Pathomorphology of chronic infection in central nervous system. Acta Virol. 28232-239. [PubMed] [Google Scholar]
- 7.Gelpi, E., M. Preusser, F. Garzuly, H. Holzmann, F. X. Heinz, and H. Budka. 2005. Visualization of Central European tick-borne encephalitis infection in fatal human cases. J. Neuropathol. Exp. Neurol. 64506-512. [DOI] [PubMed] [Google Scholar]
- 8.Gelpi, E., M. Preusser, U. Laggner, F. Garzuly, H. Holzmann, F. X. Heinz, and H. Budka. 2006. Inflammatory response in human tick-borne encephalitis: analysis of postmortem brain tissue. J. Neurovirol. 12322-327. [DOI] [PubMed] [Google Scholar]
- 9.Gritsun, T. S., V. A. Lashkevich, and E. A. Gould. 2003. Tick-borne encephalitis. Antiviral Res. 57129-146. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, J. E., F. Nasar, J. W. Coleman, R. E. Price, A. Javadian, K. Draper, M. Lee, P. A. Reilly, D. K. Clarke, R. M. Hendry, and S. A. Udem. 2007. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology 36036-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karganova, G. G., N. S. Pripuzova, N. V. Tereshkina, L. V. Gmyl', T. I. Dzhivanian, A. A. Rumyantsev, and V. A. Lashkevich. 2005. Residual neurovirulence of the chimera of Langat and Dengue-4 flaviviruses in intracerebral infection of monkeys. Vopr. Virusol. 5027-31. [PubMed] [Google Scholar]
- 12.Komandenko, N. I., V. I. Il'enko, V. G. Platonov, and A. G. Panov. 1972. Clinical picture and pathogenesis of progressive forms of experimental tick-borne encephalitis. Zh. Nevropatol. Psikhiatr. Im. S. S. Korsakova. 721000-1007. [PubMed] [Google Scholar]
- 13.Levenbook, I. S., L. J. Pelleu, and B. L. Elisberg. 1987. The monkey safety test for neurovirulence of yellow fever vaccines: the utility of quantitative clinical evaluation and histological examination. J. Biol. Stand. 15305-313. [DOI] [PubMed] [Google Scholar]
- 14.Maksimova, O. A., V. F. Popov, T. A. Bektimirov, L. V. Grigor'eva, T. N. Iunasova, O. P. Kaplunova, and O. K. Sharova. 2001. Comparative evaluation of neurovirulence of domestic and foreign live mumps vaccine. Vopr. Virusol. 4631-35. [PubMed] [Google Scholar]
- 15.Malenko, G. V., G. I. Fokina, L. S. Levina, L. L. Mamonenko, O. E. Rzhakhova, V. V. Pogodina, and M. P. Frolova. 1982. Persistence of tick-borne encephalitis virus. IV. Virus localization after intracerebral inoculation. Acta Virol. 26362-368. [PubMed] [Google Scholar]
- 16.Mandl, C. W., L. Iacono-Connors, G. Wallner, H. Holzmann, C. Kunz, and F. X. Heinz. 1991. Sequence of the genes encoding the structural proteins of the low-virulence tick-borne flaviviruses Langat TP21 and Yelantsev. Virology 185891-895. [DOI] [PubMed] [Google Scholar]
- 17.Marchevsky, R. S., M. S. Freire, E. S. Coutinho, and R. Galler. 2003. Neurovirulence of yellow fever 17DD vaccine virus to rhesus monkeys. Virology 31655-63. [DOI] [PubMed] [Google Scholar]
- 18.Martin, R. F., and D. M. Bowden. 2000. Primate brain maps: structure of the macaque brain. Elsevier, Amsterdam, The Netherlands.
- 19.Matveev, L. E., A. A. Godovikov, A. S. Karavanov, A. G. Pletnev, S. G. Rubin, I. V. Semashko, N. A. Tsekhanovskaya, M. P. Chumakov, and E. K. Pressman. 1989. The monoclonal antibodies against tick-borne encephalitis virus structural glycoprotein: preliminary characteristics. Vopr. Virusol. 34694-697. [PubMed] [Google Scholar]
- 20.Maximova, O., E. Dragunsky, R. Taffs, P. Snoy, J. Cogan, S. Marsden, and I. Levenbook. 1996. Monkey neurovirulence test for live mumps vaccine. Biologicals 24223-224. [DOI] [PubMed] [Google Scholar]
- 21.Monath, T. P. 2005. Yellow fever vaccine. Expert Rev. Vaccines 4553-574. [DOI] [PubMed] [Google Scholar]
- 22.Monath, T. P., I. Levenbook, K. Soike, Z. X. Zhang, M. Ratterree, K. Draper, A. D. Barrett, R. Nichols, R. Weltzin, J. Arroyo, and F. Guirakhoo. 2000. Chimeric yellow fever virus 17D-Japanese encephalitis virus vaccine: dose-response effectiveness and extended safety testing in rhesus monkeys. J. Virol. 741742-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monath, T. P., J. Arroyo, I. Levenbook, Z. X. Zhang, J. Catalan, K. Draper, and F. Guirakhoo. 2002. Single mutation in the flavivirus envelope protein hinge region increases neurovirulence for mice and monkeys but decreases viscerotropism for monkeys: relevance to development and safety testing of live, attenuated vaccines. J. Virol. 761932-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathanson, N., A. M. Gittelsohn, I. S. Thind, and W. H. Price. 1967. Histological studies of the monkey neurovirulence of group B arboviruses. 3. Relative virulence of selected viruses. Am. J. Epidemiol. 85503-517. [DOI] [PubMed] [Google Scholar]
- 25.Nathanson, N., and S. D. Horn. 1992. Neurovirulence tests of type 3 oral poliovirus vaccine manufactured by Lederle Laboratories, 1964-1988. Vaccine 10469-474. [DOI] [PubMed] [Google Scholar]
- 26.Nathanson, N., D. Goldblatt, I. S. Thind, M. Davis, and W. H. Price. 1965. Histological studies of the monkey neurovirulence of group B arboviruses. I. A semiquantitative grading scale. Am. J. Epidemiol. 82359-381. [DOI] [PubMed] [Google Scholar]
- 27.Nathanson, N., I. S. Thind, W. O'Leary, and W. H. Price. 1968. Histological studies of the monkey neurovirulence of group B arboviruses. IV. Evaluation of an attenuated strain (E5) of Langat virus. Am. J. Epidemiol. 88103-111. [DOI] [PubMed] [Google Scholar]
- 28.Nathanson, N., M. Davis, I. S. Thind, and W. H. Price. 1966. Histological studies of the monkey neurovirulence of group B arboviruses. II. Selection of indicator centers. Am. J. Epidemiol. 84524-540. [DOI] [PubMed] [Google Scholar]
- 29.Pletnev, A. G., D. E. Swayne, J. Speicher, A. A. Rumyantsev, and B. R. Murphy. 2006. Chimeric West Nile/dengue virus vaccine candidate: preclinical evaluation in mice, geese and monkeys for safety and immunogenicity. Vaccine 246392-6404. [DOI] [PubMed] [Google Scholar]
- 30.Pletnev, A. G., M. Bray, J. Huggins, and C. J. Lai. 1992. Construction and characterization of tick-borne encephalitis/dengue type 4 viruses. Proc. Natl. Acad. Sci. USA 8910532-10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pletnev, A. G. 2001. Infectious cDNA clone of attenuated Langat tick-borne flavivirus (strain E5) and a 3′ deletion mutant constructed from it exhibit decreased neuroinvasiveness in immunodeficient mice. Virology 28288-300. [DOI] [PubMed] [Google Scholar]
- 32.Pletnev, A. G., M. Bray, and C. J. Lai. 1993. Chimeric tick-borne encephalitis and dengue type 4 viruses: effects of mutations on neurovirulence in mice. J. Virol. 674956-4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pletnev, A. G., M. Bray, K. A. Hanley, J. Speicher, and R. Elkins. 2001. Tick-borne Langat/mosquito-borne dengue flavivirus chimera, a candidate live attenuated vaccine for protection against disease caused by members of tick-borne encephalitis virus complex: evaluation in rhesus monkeys and in mosquitoes. J. Virol. 758259-8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pletnev, A. G., V. F. Yamshchikov, and V. M. Blinov. 1990. Nucleotide sequence of the genome and complete amino acid sequence of the polyprotein of tick-borne encephalitis virus. Virology 174250-263. [DOI] [PubMed] [Google Scholar]
- 35.Pletnev, A. G., and R. Men. 1998. Attenuation of the Langat tick-borne flavivirus by chimerization with mosquito-borne flavivirus dengue type 4. Proc. Natl. Acad. Sci. USA 951746-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pletnev, A. G., R. Putnak, J. Speicher, E. J. Wagar, and D. W. Vaughn. 2002. West Nile virus/dengue type 4 virus chimeras that are reduced in neurovirulence and peripheral virulence without loss of immunogenicity or protective efficacy. Proc. Natl. Acad. Sci. USA 993036-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pogodina, V. V., N. G. Bochkova, L. S. Karan', M. P. Frolova, A. G. Trukhina, G. V. Malenko, L. S. Levina, and A. E. Platonov. 2004. Comparative analysis of virulence of the Siberian and Far-East subtypes of the tick-borne encephalitis virus. Vopr. Virusol. 4924-30. [PubMed] [Google Scholar]
- 38.Rozina, E. E., and M. Hilgenfeldt. 1985. Comparative study on the neurovirulence of different vaccine strains of parotitis virus in monkeys. Acta Virol. 29225-230. [PubMed] [Google Scholar]
- 39.Rumyantsev, A. A., R. M. Chanock, B. R. Murphy, and A. G. Pletnev. 2006. Comparison of live and inactivated tick-borne encephalitis virus vaccines for safety, immunogenicity and efficacy in rhesus monkeys. Vaccine 24133-143. [DOI] [PubMed] [Google Scholar]
- 40.Sharova, O. K., E. E. Rozina, L. S. Shteinberg, N. M. Gordienko, and I. S. Kolyanova. 1979. Morphological characteristics of the pathological process in the central nervous system of monkeys infected with variants of measles virus strain L-16. Acta Virol. 23393-397. [PubMed] [Google Scholar]
- 41.Smorodincev, A. A., and A. V. Dubov. 1986. Live vaccines against tick-borne encephalitis, p. 190-211. In A. A. Smorodincev (ed.), Tick-borne encephalitis and its vaccine prophylaxis. Meditsina, Leningrad, Russia.
- 42.Suss, J. 2003. Epidemiology and ecology of TBE relevant to the production of effective vaccines. Vaccine 21S19-35. [DOI] [PubMed] [Google Scholar]
- 43.Thind, I. S., and W. H. Price. 1966. A chick embryo attenuated strain (TP21 E5) of Langat virus. I. Virulence of the virus for mice and monkeys. Am. J. Epidemiol. 84193-213. [DOI] [PubMed] [Google Scholar]
- 44.Tsekhanovskaya, N. A., L. E. Matveev, S. G. Rubin, A. S. Karavanov, and E. K. Pressman. 1993. Epitope analysis of tick-borne encephalitis (TBE) complex viruses using monoclonal antibodies to envelope glycoprotein of TBE virus (persulcatus subtype). Virus Res. 301-16. [DOI] [PubMed] [Google Scholar]
- 45.Whitehead, S. S., B. Falgout, K. A. Hanley, J. E. Blaney, L. Markoff, and B. R. Murphy. 2003. A live attenuated dengue virus type 1 vaccine candidate with a 30-nucleotide deletion in the 3′ untranslated region is highly attenuated and immunogenic in monkeys. J. Virol. 771653-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WHO. 1998. Requirements for yellow fever vaccine. WHO Tech. Rep. Ser. 87238-40. [Google Scholar]
- 47.WHO. 2005. Final report. IABS scientific workshop on neurovirulence tests for live virus vaccines. WHO, Geneva, Switzerland. http://www.who.int/biologicals/publications/meetings/Final%20Report%20WHO%20IABS%20Scientific%20Workshop%20on%20Neurovirulence%20Tests%20Jan _Feb%202005.pdf. [DOI] [PubMed]
- 48.Wright, P. F., S. Ankrah, S. E. Henderson, A. P. Durbin, J. Speicher, S. S. Whitehead, B. R. Murphy, and A. G. Pletnev. 2008. Evaluation of the Langat/dengue 4 chimeric virus as a live attenuated tick-borne encephalitis vaccine for safety and immunogenicity in healthy adult volunteers. Vaccine 26882-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.