Abstract
Patients recruited in virus-based cancer clinical trials and immunocompromised individuals in need of vaccination would profit from viral strains with defined attenuation mechanisms. We generated measles virus (MV) strains defective for the expression of either the V protein, a modulator of the innate immune response, or the C protein, which has multiple functions. The virulence of these strains was compared with that of the parental wild-type MV in a natural host, Macaca mulatta. Skin rash, viremia, and the strength of the innate and adaptive immune responses were characterized in groups of six animals. Replication of V- or C-protein-defective viruses was short-lived and reached lower levels in peripheral blood mononuclear cells and lymphatic organs compared to the wild-type virus; none of the mutants reverted to the wild type. The neutralizing antibody titers and MV-specific T-cell responses were equivalent in monkeys infected with the viral strains tested, documenting strong adaptive immune responses. In contrast, the inflammatory response was better controlled by wild-type MV, as revealed by inhibition of interleukin-6 and tumor necrosis factor alpha transcription. The interferon response was also better controlled by the wild-type virus than by the defective viruses. Since V- and C-defective MVs induce strong adaptive immune responses while spreading less efficiently, they may be developed as vaccines for immunocompromised individuals. Moreover, MV unable to interact with single innate immunity proteins may be developed for preferential replication in tumors with specific contexts of vulnerability.
Certain safe and effective live attenuated vaccines were generated simply by repeated passage of virulent strains on heterologous cells, resulting in multiple mutations and sometimes gene deletions (30, 43). Nevertheless, vaccine strains with defined mechanisms and levels of attenuation would be preferable for new applications of viruses for cancer therapy (18, 22) and the vaccination of immunocompromised hosts (37). Many viruses express proteins that control the antiviral response, and viruses without these proteins are promising candidates for specific vector and vaccine applications. Measles virus (MV) host control proteins include V protein, whose mechanisms of innate immunity modulation are well understood, and C protein, whose mechanisms of action are less well defined. Both V and C proteins are dispensable for replication in cultivated cells (35, 41).
The V protein shares the amino-terminal domain of the P protein, but its 68 carboxyl-terminal highly conserved amino acids forming a zinc-binding domain (31) are translated from a different open reading frame (ORF) accessed by the cotranscriptional insertion of a pseudotemplate G residue (9). MV V functions as an interferon (IFN) antagonist through its interaction with both MDA5 and the STAT proteins (3, 7, 8, 10, 27, 29, 47). V can also interfere with signaling by interleukin-6 (IL-6) (29). Interference with the IFN response facilitates virus replication by blocking the induction of antiviral proteins, such as double-stranded RNA-dependent protein kinase R, 2,5-oligoadenylate synthetase (OAS), or MxA (37).
The C protein, produced by translation of an ORF initiated 19 nucleotides downstream of the P/V start codon, colocalizes with the MV ribonucleoprotein in infected cells (6). It reduces viral transcription in a chloramphenicol acetyltransferase reporter minigenome assay (39) and acts as an infectivity factor (12). It may (26, 42) or may not (48) interfere directly with the IFN response, and different mechanisms of its action have been considered (26, 42).
V- or C-defective MV strains were generated (35, 41), and their spread was characterized in different animal hosts (25, 32, 49, 50). However, the P gene of the original MV infectious cDNA (36) used as the parental genome for these viruses has two defects: a V-protein cysteine residue that complexes zinc is mutated (41), as well as a tyrosine necessary for STAT1 interaction (13). Thus, interpretation of the results of animal studies based on these viruses is not straightforward, because the V protein of the original infectious cDNA is not fully functional (13, 27).
Nevertheless, studies of the relevance of the V and C proteins for virulence of another morbillivirus are available. V- or C-defective canine distemper virus (CDV) strains were generated, and their spread was characterized in a natural host, ferrets. In these animals, V sustains swift invasion of mucosal tissue and lymphatic organs, whereas C is necessary only for subsequent infection phases (52). On the other hand, a small study based on a C-deficient derivative of a wild-type MV strain documented the importance of this protein for spread in the lymphatic tissue of cynomolgus monkeys (48).
Here we characterized the virulence of V- or C-protein-defective MV in groups of six rhesus monkeys. V- or C-defective MV was generated based on the virulent infectious cDNA of the wild-type strain IC-B (46). The rhesus monkey infection model was selected because it reproduces human disease faithfully (5, 11, 23, 55). The levels of mRNA induction of IFN, of IFN-induced proteins, and of other cytokines were characterized at the peak of viral replication (day 7 postinfection) and 1 week later, after the rash. The humoral and cellular immune responses were quantified at later time points. Our data indicate that the V- and C-defective viruses induce strong adaptive immune responses and that both V and C proteins are required to control the inflammatory response and innate immunity.
MATERIALS AND METHODS
Cells and viruses.
Vero/hSLAM cells (28) and the helper 293-3-46 cell line (36) were maintained as monolayers in Dulbecco's modified Eagle's medium (Mediatech Inc., Herndon, VA) supplemented with 10% fetal calf serum (FCS), 1% penicillin/streptomycin (Mediatech), and 0.5 and 1.2 mg/ml G418 (Mediatech), respectively. Raji cells were maintained in RPMI medium containing 10% FCS.
Recombinant MVs were generated by the method of Radecke et al. (36). Briefly, the helper cell line 293-3-46 stably expressing MV N, MV P, and T7 polymerase was transfected by calcium phosphate precipitation using the ProFection kit (Promega, Madison, WI) with two plasmids, one coding for the relevant MV genome and the other for the MV polymerase (pEMCLa). Three days after transfection, the helper cells were overlaid on Vero/hSLAM cells, the appearance of infectious centers was monitored, and then single syncytia were picked and propagated on Vero/hSLAM cells. To prepare virus stocks, Vero/hSLAM cells were infected at a multiplicity of infection (MOI) of 0.03 and incubated at 37°C. Cells were scraped off in Opti-MEM (Gibco/Invitrogen Corp., Grand Island, NY), and particles were released by two freeze-thaw cycles.
Virus stock titers were determined by 50% end-point dilution (50% tissue culture infective dose [TCID50]) on Vero/hSLAM cells using the Spearman-Kärber method (17). Viral loads were quantified in monkey peripheral blood mononuclear cells (PBMC) by end-point dilution coculture with Raji cells (55). Virus in lymph node mononuclear cells (LNMC) was measured by overlay of 106 LNMC on 2 × 106 Vero/hSLAM cells and assessed by the appearance of syncytia.
Plasmid construction.
Site-directed mutagenesis was performed using the QuikChange system (Stratagene, La Jolla, CA) on a cassette vector covering the P-gene sequence. To produce p(+)MVwtIC323.Vko and p(+)MVwtIC323.Cko, site-directed mutagenesis was performed on pCGPmeI-MVwtIC323-PmeI (P. Devaux, unpublished data) using primers 5′-CAGCACTTCCGAGACACCCATTAAAGAGGGCACTGACGCGAGATTGGCCTCATTTG and 5′-GGCAGAAGAGCAGGCACGCCACGTCAAAAACGGACTAGAATGCATCCGGGCTCTCAAGG, respectively (the nucleotides exchanged are in bold type). Full-length cDNA p(+)MVwtIC323.Vko and p(+)MVwtIC323.Cko were obtained by transferring the mutated BssHII-BstEII fragment into the full-length cDNA p(+)MVwtIC323 (46). The integrity of the full-length constructs was verified by sequencing.
Western blot analysis of cell extracts.
Cells (5 × 105) were infected with a multiplicity of infection of 0.05. After 36 h, the cells were washed in phosphate-buffered saline, incubated for 10 min at 4°C in cell lysis buffer (Cell Signaling, Beverly, MA) containing protease inhibitors (protease inhibitor cocktail set I [Calbiochem, La Jolla, CA]), and phosphatase inhibitors (phosphatase inhibitor cocktail set II [Calbiochem]) and centrifuged at 5,000 × g for 15 min at 4°C. The supernatants were denatured with urea buffer (200 mM Tris HCl [pH 6.8], 8 M urea, 0.1 mM EDTA [pH 8], 5% sodium dodecyl sulfate, 0.03% bromophenol blue) containing 1.5% dithiothreitol for 10 min at 90°C. Samples were fractionated on 4 to 15% sodium dodecyl sulfate-polyacrylamide gels (Bio-Rad, Hercules, CA), blotted onto polyvinylidene difluoride membranes (Immobilon-P [Millipore, Billerica, MA]), and subjected to enhanced chemiluminescence detection using the antibodies indicated.
Rhesus monkey infections.
MV-seronegative rhesus monkeys, housed at the California National Primate Research Center in accordance with the regulations of the Association for the Assessment and Accreditation of Laboratory Animal Care, were bled under ketamine sedation. Six monkeys per group were challenged by conjunctival/intranasal inoculation of 104.5 TCID50 of either the wild-type virus MVwtIC323 (WT) or mutant virus MVwtIC323.Vko (mutant virus in which the V gene was knocked out) (Vko) or MVwtIC323.Cko (mutant virus in which the C gene was knocked out) (Cko). The animals were monitored daily for anorexia, depression, coughing, diarrhea, and skin rash. They were bled on days 0, 7, 14, and 28 postchallenge. Viremia was quantified by end-point dilution coculture with Raji cells as described previously (34). The limit of detection was 1 TCID50 in 106 PBMC. Infectious virus titers were calculated by the method of Reed and Muench (38).
Characterization of the humoral immune response in monkeys.
Neutralizing antibody to MV was measured by the method of Zhu et al. (55). Briefly, starting at 1/10, serial twofold dilutions of heat-inactivated (56°C for 30 min) serum were added to wells of a 96-well plate in duplicate, in 50 μl, and mixed with an equal volume of freshly diluted MV containing 50 PFU. After incubation for 1 h at 37°C in a 5% CO2 incubator, Vero cells were added at 8 × 103 cells per well in 100 μl. The plates were incubated for 3 days at 37°C and 5% CO2. After staining for MV nucleoprotein, the neutralizing antibody titer was calculated as the highest dilution showing 50% reduction in viral antigen of control wells that contained virus without serum.
Cell-mediated immunity.
MV-specific T cells were counted using a gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay as previously described (34). Briefly, PBMC were resuspended at 5 × 106 cells/ml in a 48-well flat-bottom plate in AIM V medium (Gibco/Invitrogen Corp.) supplemented with 10% FCS and stimulated overnight with live MV Edmonston (American Type Culture Collection) at a concentration of 103 TCID50/100 μl. Positive-control stimulation was with 10 ng/ml phorbol 12-myristate 13-acetate and 1 μg/ml ionomyocin (Sigma, St. Louis, MO). Following overnight incubation, the cells were transferred to a 96-well ELISPOT plate coated with antibody to rhesus IFN-γ (U-Cytech BV, Utrecht, The Netherlands) and developed according to the manufacturer. Spot-forming cells (SFC) were counted using a dissecting microscope, and the number of spots in duplicate wells was averaged. A positive result was at least 10 spots per well and greater to or equal to the mean plus 2 standard deviations of the medium control. The spot number in medium control wells was subtracted from the experimental spot count, and the number of SFC was adjusted to 106 PBMC.
Viral RNA extraction and reverse transcription-PCR (RT-PCR).
Lymph nodes from monkeys were collected at day 7 postinfection, and LNMC were isolated using lymphocyte separation medium (ICN Biomedicals, Aurora, OH), immediately frozen in 10% dimethyl sulfoxide (Sigma, St. Louis, MO) plus 90% newborn bovine serum (Gemini BioProducts, Calabasas, CA), and stored in liquid nitrogen until RNA isolation.
Total RNA was isolated from 2 × 106 cells using the RNeasy mini kit (Qiagen, Valencia, CA). The bicistronic N-P mRNA was then reverse transcribed using the SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer's instructions and primer 5′-CTACTTCATTATTATCTTCATC. A forward primer from the N gene, 5′-GGCAAGAGATGGTAAGG, and a reverse primer from the end of P, 5′-CATCTGGTGGAACTTGG, were then used for PCR amplification of a 2,090-base-pair fragment. After purification on 1% agarose gel, the newly synthesized cDNA was sequenced with the reverse primer 5′-GTTGTCTGATATTTCTGAC and forward primer 5′-AGAGGCAACAACTTTCC located upstream of the start coding sequence and the editing site region of the P gene, respectively.
Amplification of cytokine and interferon mRNA by real-time RT-PCR.
Total RNA was isolated from PBMC with Trizol (Invitrogen) according to the manufacturer's protocol. All samples were treated with DNA-free DNase (Ambion, Austin, TX) for 1 h at 37°C. The cDNA was prepared using random hexamer primers (Amersham-Pharmacia Biotech, Inc., Piscataway, NJ) and Moloney murine leukemia virus reverse transcriptase (Invitrogen). Real-time PCR was performed as previously described (1, 2). Briefly, samples were tested in duplicate, and the PCR for the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and the target gene from each sample were run in parallel on the same plate. The reaction was carried out in a 96-well optical plate (Applied Biosystems, Foster City, CA) in a 25-μl reaction mixture volume containing 5 μl cDNA plus 20 μl Mastermix (Applied Biosystems). All sequences were amplified using the 7900 default amplification program: 2 min at 50°C and 10 min at 95°C, followed by 45 cycles with each cycle consisting of 15 s at 95°C and 1 min at 60°C. Results were analyzed with 7900 sequence detection system software, version 2.1 (Applied Biosystems).
The mRNA expression levels were calculated from normalized ΔCT (change in the threshold cycle) values and are reported as the increase or decrease in target gene mRNA levels in the PBMC samples at day 7 or 14 postinfection compared to the target gene mRNA levels at day 0 for each animal. CT values correspond to the cycle number at which the fluorescence due to enrichment of the PCR product reaches significant levels above the background fluorescence (threshold). In this analysis, the CT value for the GAPDH gene is subtracted from the CT value of the target gene. The ΔCT value for the test sample (day 7 or day 14) is then subtracted from the mean ΔCT value of the sample at day 0 (ΔΔCT). Assuming that the target gene and the reference gene (GAPDH) are amplified with the same efficiency (data not shown), the increase or decrease in the target gene mRNA level in a test sample compared to the mRNA level at day 0 is then calculated as the change in mRNA expression by the following equation: increase or decrease = 2−ΔΔCT (User Bulletin 2, ABI Prism 7700 Sequence Detection System, Applied Biosystems). Primer and probe sequences for rhesus IFN-α, IFN-β, tumor necrosis factor alpha (TNF-α), IL-6, IL-2, IL-4, IL-12, IFN-γ, OAS, and MxA have been published (1, 2).
Statistical analysis.
A Kruskal-Wallis one-way analysis of variance (ANOVA) was used to determine statistically significant differences of the values for neutralizing antibody titer, peak viremia, IFN-γ-positive SFC, and change in cytokine mRNA expression for the three experimental monkey groups. Post hoc testing was performed by the Dunne's multiple comparison test.
RESULTS
Generation of MV deficient in either C or V protein.
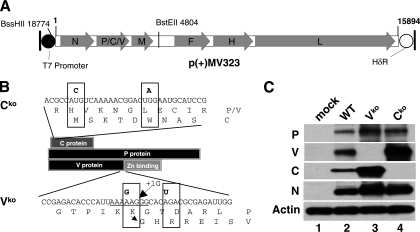
To selectively prevent C-protein expression, a mutation converting the start codon to ACG was combined with one converting codon 6 to a stop codon (Fig. 1B, top sequence, the two mutated nucleotides are shown in bold type). The combined mutations were introduced in an intermediate plasmid and then transferred to p(+)MV323 (Fig. 1A) (46). The rescued virus was named MVwtIC323.Cko, in short Cko. Viral protein expression was analyzed by immunoblotting, confirming that these mutations completely prevent C-protein production without inhibiting expression of the P or V protein (Fig. 1C, lane 4).
FIG. 1.
Generation and characterization of WT derivatives defective for expression of the V or C protein. (A) Map of the WT genome and flanking sequences in plasmid p(+)MV323 (46). The T7 promoter (black circle), coding regions (light gray arrowed boxes), and hepatitis delta ribozyme (HδR) (white circle) are indicated. The MV antigenome is represented with its 5′ end on the left, and its six genes are indicated by capital letters. Two relevant restriction sites are shown. (B) Schematic drawing illustrating the reading frames of the three proteins (C, P, and V proteins) generated from the P gene and the mutations silencing C- and V-protein expression. The two mutations selectively ablating C-protein expression (elimination of the start codon, ACG, and introduction of one stop codon [UAG]) are indicated in bold type above the nucleotide sequence (Cko). The two mutations selectively ablating V-protein expression (G mutation in the editing sequence, and introduction of the stop codon, UGA) are indicated in bold type above the nucleotide sequence (Vko). (C) Immunoblot analysis of the P-gene products expressed by WT and its two derivatives, Vko and Cko. Antisera against P protein, V protein, or C protein were used. Uninfected Vero/hSLAM cells (mock) were used as a control. N-protein and β-actin antibodies were used as controls of infection and protein load, respectively.
To prevent V-protein expression, a mutation interfering with RNA editing was cointroduced with another converting the second codon of the V ORF into a UGA stop codon (Fig. 1B, bottom sequence, the two mutated nucleotides are shown in bold type) (41). The conserved sequence UUAAAAAGGGCACAGA was mutated to UUAAAGAGGGCACUGA (the underlined polypurine tract is essential for RNA editing). We constructed a mutated infectious cDNA and rescued a virus that was named MVwtIC323.Vko, in short Vko. We analyzed viral protein expression by immunoblotting, confirming that these mutations completely prevented V-protein production (Fig. 1C, lane 3). The level of P protein is increased in the Vko than in the WT or Cko, as all P transcripts of the Vko express P, while 50% of the P transcripts of the WT or Cko express V (Fig. 1C, top blot).
Short-lived replication of Vko or Cko in rhesus monkeys.
To assess whether viruses deficient in either V- or C-protein expression retained virulence, groups of six rhesus monkeys were challenged by the conjunctival/intranasal route with 104.5 infectious units of WT, Vko, or Cko. One monkey out of the six animals injected with Cko was eliminated from the study due to a technical problem, reducing this group to five animals.
After infection with WT, rhesus monkeys often develop mild anorexia (weight loss less than 10%) and diarrhea with bacterial or parasitic dysentery, responsive to antibiotic therapy (23, 51, 55). Anorexia was observed in three of six monkeys and two of five monkeys from the WT- and Cko-infected groups, respectively, but not in the Vko-infected group. Two animals in each of the V- and C-defective groups were treated with antibiotics in the month following MV infection to control diarrhea symptoms, but none of the monkeys inoculated with WT were treated, and it is unclear whether diarrhea was caused by MV infection. Two of six rhesus monkeys infected with WT developed a measles skin rash after challenge (Table 1). This is a fairly typical result for infection with this virus in monkeys (11, 46). None of the animals infected with the V- or C-defective viruses developed a rash, suggesting that both viruses may be more attenuated than the WT (Table 1).
TABLE 1.
Virulence of WT or V- or C-deficient viruses in rhesus monkeys
| Group, monkey IDa no., and parameter | Rash | Viremia (TCID50/106 PBMC)
|
||
|---|---|---|---|---|
| Day 7 | Day 14 | Day 28 | ||
| WT-infected group | ||||
| 536 | Yes | 104.25 | 101.00 | <1b |
| 381 | No | 103.50 | <1 | <1 |
| 847 | No | 103.25 | 100.25 | <1 |
| 180 | No | 102.75 | 101.25 | <1 |
| 338 | No | 103.75 | 101.00 | <1 |
| 311 | Yes | 103.75 | 101.5 | <1 |
| Mean | 103.54 | 100.83 | <1 | |
| Vko-infected group | ||||
| 853 | No | 102.25 | <1 | <1 |
| 890 | No | 101.50 | <1 | <1 |
| 161 | No | 102.50 | 100.75 | <1 |
| 886 | No | 102.25 | <1 | <1 |
| 949 | No | 102.50 | <1 | <1 |
| 958 | No | 102.50 | <1 | <1 |
| Mean | 102.25 | 100.13 | <1 | |
| Cko-infected group | ||||
| 288 | No | 101.75 | <1 | <1 |
| 169 | No | 101.50 | <1 | <1 |
| 957 | No | 103.00 | <1 | <1 |
| 903 | No | 103.00 | <1 | <1 |
| 982 | No | 102.25 | <1 | <1 |
| Mean | 102.30 | <1 | <1 | |
ID, identification.
The lower detection limit was 1 TCID50/106 PBMC.
Viremia was assayed as a quantitative parameter of virulence in PBMC collected 7, 14, and 28 days after infection; viral titers per 106 cells are shown in Table 1. Virus was isolated from PBMC and lymph nodes of all animals tested at day 7 postinfection (Table 1). In PBMC of animals infected with V- or C-defective viruses, the viral titers (average group titers of 102.25 and 102.3 TCID50/106 PBMC, respectively) reached levels about 1 logarithm lower than those of animals infected with WT (average group titer of 103.54 TCID50/106 PBMC), confirming significant attenuation of both viruses (P = 0.007, ANOVA). At day 14, virus was detected in five of six animals infected with WT, but in only one of six monkeys infected with Vko and in none of the five monkeys infected with Cko, indicating that the propagation of the V- or C-defective viruses in the PBMC was short-lived compared to the wild-type virus (P = 0.007, ANOVA). By day 28, viremia was undetectable in all of the animals.
Mutant viruses do not revert to the wild type in rhesus monkeys.
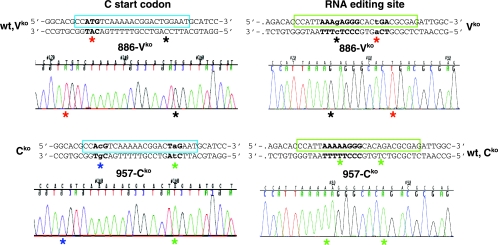
To assess whether the viral genomes of the defective viruses were stable, we analyzed the consensus sequence of viral mRNA extracted from infected LNMC isolated at day 7 postinoculation. Figure 2 presents the sequence analysis of the RT-PCR products from two animals, monkey 886 infected with Vko (886-Vko) and monkey 957 infected with Cko (957-Cko). The segments covering the initiation codon (ATG) of the C-protein-coding region and the RNA editing purine-rich sequence were sequenced.
FIG. 2.
Mutant viruses do not revert. Sequence analysis of viral RNA extracted from lymph nodes 7 days after infection with Vko (monkey 886 infected with Vko [886-Vko]) or Cko (monkey 957 infected with Cko [957-Cko]). Sequences are also shown above the chromatograms. They cover the C start codon (shown in bold type) on the left side panel, and the RNA editing site (in bold type) on the right side panel. The wild-type sequences are shown above the left top panel (wt,Vko) and the right bottom panel (wt,Cko). Mutations are indicated in lowercase type. Relevant nucleotides are highlighted with colored asterisks as follows: for 957-Cko, inactivation of the C-protein start codon (blue asterisk), mutation to a stop codon (green asterisk), and wild-type sequence covering the editing site region (two green asterisks); for 886-Vko, an inactivated editing site (right side, mutation to G, black asterisk) followed by a TGA stop codon (red asterisk) and wild-type sequence covering the C protein ATG start codon (red asterisks), and the TGG tryptophan codon (black asterisk).
Analysis of the 957-Cko sequence (Fig. 2, left side, bottom chromatogram) confirmed inactivation of the C-protein start codon (blue asterisk) and insertion of a stop codon (green asterisk). The 886-Vko chromatogram (Fig. 2, left side, top chromatogram) is the control and shows the ATG start codon sequence (red asterisk) and the TGG tryptophan codon (black asterisk).
Analysis of the 886-Vko sequence (Fig. 2, right side, top chromatogram) confirmed an inactivated editing site (mutation to G, black asterisk) followed by a TGA stop codon (red asterisk). The 957-Cko chromatogram (Fig. 2, right side, bottom chromatogram) is the control and shows the wild-type sequence covering the editing site region (two green asterisks). All viral genomes amplified from all the monkeys infected with Vko or Cko remained identical to that of the inoculated virus. In each animal, more than 1,300 nucleotides covering the N or P gene were sequenced, but no derivations from the parental sequence were found. Thus, none of the mutants reverted to the wild-type genotype.
Vko and Cko infections elicit a robust adaptive immune response.
To assess the quality of the adaptive immune responses elicited by V- or C-protein deficient MV, two parameters were measured: anti-MV neutralization titers and the number of MV-specific IFN-γ-secreting T cells.
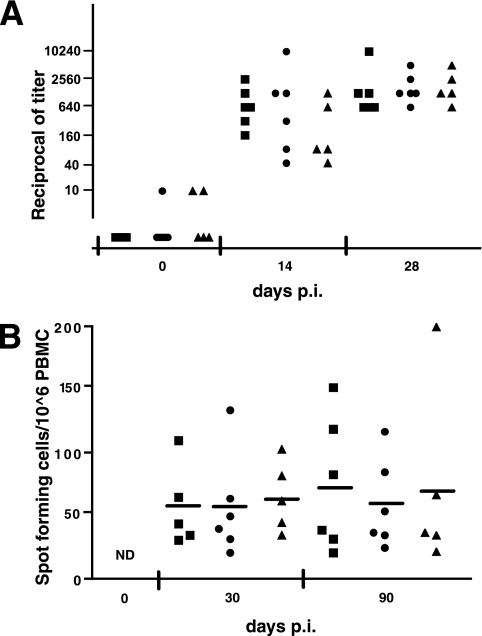
Figure 3A documents the MV neutralization titers at 0, 14, and 28 days after inoculation with WT (squares), Vko (dots), and Cko (triangles). At day 14, neutralizing antibodies were observed for all animals in response to WT infection, with titers ranging from 1:160 to 1:2,560. At day 14, the antibody response in certain monkeys infected with Vko or Cko were only slightly above background levels, but at day 28, all monkeys showed a titer of at least 1:640 in all three groups. The day 28 postinoculation mean titers of neutralizing MV antibodies were 1:2,450, 1:2,030, and 1:2,180 for groups infected with WT, Vko, and Cko, respectively. Thus, the humoral response to the two defective viruses was not significantly different compared to the response to WT inoculation (P = 0.571, ANOVA).
FIG. 3.
Ablation of the V and C proteins does not affect the adaptive immune response. (A) Neutralizing antibody response of monkeys infected with WT (squares), Vko (dots), or Cko (triangles). Sera obtained at 0, 14, and 28 days postinoculation (p.i.) were assayed for MV neutralization, and results are presented as reciprocals of the titer. Each dot represents an animal. (B) MV-specific IFN-γ-secreting T cells. At 30 and 90 days postinfection, PBMC were isolated from monkeys infected with WT (squares), Vko (dots), or Cko (triangles) and stimulated overnight with live MV Edmonston strain. The number of spot-forming cells per 106 PBMC was measured with an IFN-γ-specific ELISPOT assay. Each dot represents an animal, and a short horizontal bar indicates the mean of the group. Preinoculation samples were not tested; however, the CMI response in naïve monkeys is negative or low (40). ND, not done.
To assess the strength of the cell-mediated immune (CMI) response, MV-specific IFN-γ-secreting T cells were counted by ELISPOT assay at 1 and 3 months after challenge (Fig. 3B). We did not plan a 14-day postinfection analysis because after consideration of the weak responses detected in past experiments at this time, the blood samples were prioritized to measure viral load and cytokine responses. One month postinfection, all animals infected with recombinant MV showed a strong CMI response (Fig. 3B, group averages of 57 SFC/106 PBMC for the WT-infected group and 57 and 65 for the Vko- and Cko-infected groups, respectively). This response was maintained 3 months after infection for all monkeys (Fig. 3B, group averages of 74 SFC/106 PBMC for the WT-infected group and 59 and 72 for the Vko- and Cko-infected groups, respectively). Taken together, these results show that the humoral and cellular immune responses directed against MV were similar in monkeys infected with all three MV clones, indicating that the absence of the V or C protein did not significantly affect the adaptive immune response (P > 0.5, ANOVA).
Vko and Cko induce a stronger inflammatory response.
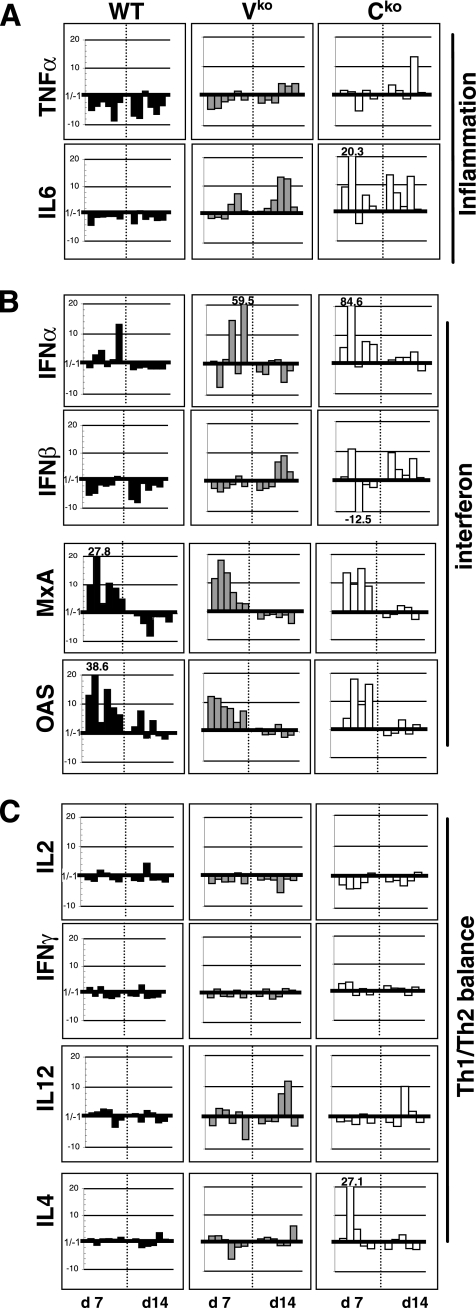
To gain insight into the mechanisms of action of the V and C proteins in vivo, we compared the transcription of different genes: type I or type II IFN, the IFN-induced MxA and OAS genes, and the genes of five cytokines. These transcripts were chosen to characterize the inflammatory response (TNF-α and IL-6), the innate immune response (IFN-α, IFN-β, OAS, and MxA), and the Th1/Th2 balance (IL-2, IFN-γ, IL-12, and IL-4). The level of each mRNA was measured in PBMC collected at day 7 or 14 postinfection, and was expressed as the change in expression compared to the level on the day of inoculation (day 0), normalized to 1 for each host (Fig. 4).
FIG. 4.
Changes in cytokine mRNA expression in infected rhesus monkeys. Monkeys were infected with WT (dark gray histograms), Vko (light gray histograms), or Cko (white histograms). The level of mRNA for each cytokine was normalized to 1 at day 0 for all monkeys. Each histogram shows the change in mRNA level at day 7 (d 7) and day 14 (d14) postinfection compared to the level at day 0. The results of individual monkeys are shown in the same order for all cytokines and time points.
Figure 4, top, shows the level of expression of the two proinflammatory cytokines, TNF-α and IL-6. In animals infected with WT, a strong down-regulation of TNF-α was observed at days 7 and 14. IL-6 expression was baseline or slightly reduced (Fig. 4A). In the absence of V or C protein, the levels of TNF-α on days 7 and 14 were elevated to the baseline level or above the baseline level for most animals. This up-regulation was significant only for the monkeys infected with Cko (P < 0.05). On day 14, IL-6 expression was up-regulated, and this difference was significant for both viruses (P < 0.01 for Cko and P < 0.05 for Vko). In the absence of C protein, IL-6 up-regulation was observed as early as day 7 postinfection in four of five monkeys (P < 0.05), suggesting that the C protein is more relevant for IL-6 expression control. These results suggest that both V and C proteins contribute to the down-regulation of inflammatory cytokines, but the C protein has a stronger effect than V does.
V and C proteins interfere with the IFN response.
To determine whether the V or C protein interferes with type I IFN production in vivo, we measured the IFN-α and IFN-β mRNA levels after infection. Strong induction of IFN-α was observed at day 7 in one, two, and four monkeys infected with WT, Vko, or Cko, respectively (Fig. 4B). At day 14, the IFN-α levels returned to normal for all animals. Transcription of IFN-β was down-regulated at both days 7 and 14 in monkeys infected by WT (Fig. 4B). In the absence of V or C protein, IFN-β transcription was close to baseline at day 7 and up-regulated at day 14, after virus was cleared from PBMC (Fig. 4B). This up-regulation was significant only for the monkeys infected with Cko (P = 0.014, ANOVA). Only three of six monkeys infected with Vko showed a strong up-regulation of IFN-β. These results suggest that even if no virus is detected in the PBMC isolated at day 14, viral infection is ongoing in the host, and the PBMC are activated. Both V and C proteins have an inhibitory effect on the expression of IFN-β in vitro; our study confirms that C protein has a similar effect in vivo, but the effect of V remains uncertain. In contrast, there was no significant effect of the V and C proteins on the strong induction of the mRNAs for the IFN-induced proteins MxA and OAS documented at the peak of infection (day 7 postinoculation).
Lack of a Th2 shift.
Altered synthesis of IL-12 and a shift toward a Th2 response have been monitored after administration of live attenuated and inactivated vaccines to rhesus monkeys (33). To assess whether the V and C proteins are implicated in the shift, we measured the transcription levels of two Th1 cytokines: IL-2, which is responsible for T-cell growth, and IFN-γ, which controls macrophage activation. We also measured transcription of the Th2 cytokine, IL-4, which activates B cells and promotes the immunoglobulin M-immunoglobulin G class switch. Surprisingly, 7 and 14 days after infection with WT, the level of expression of all the T-cell cytokines remained close to those measured before infection (Fig. 4C). IFN-γ production and Th1 cells are stimulated by IL-12, and MV can suppress IL-12 production in certain systems. However, with a few exceptions, there were only minimal changes in the level of IL-12 mRNA during infection with any MV strains. Thus, during infection with WT, cytokine mRNA induction was not biased toward a Th2 pattern and deletion of the V or C protein had no effects (Fig. 4C).
DISCUSSION
To characterize the relevance of the V and C proteins for viral spread in a natural host, we infected rhesus monkeys with WT or with V- or C-defective viruses. Our studies prove that both proteins contribute to viral control of the IFN response and reveal that they also modulate the inflammatory response. In WT-infected rhesus monkeys, no Th1/Th2 cytokine shift was monitored in the first 2 weeks of infection. In another study in rhesus monkeys, a moderately skewed type 2 response was documented after immunization with a live vaccine, and a stronger type 2 skewed response was shown after immunization with a formalin-inactivated virus vaccine (33). In reports based on children who were infected with circulating wild-type MV strains, altered levels of cytokines were monitored months after acute infection, but a Th2 skewed response was not consistently observed (4, 24, 56). Thus, the primate studies with wild-type viruses, and natural human infections do show some alterations of cytokine levels, but not always toward a Th2 response. Since our analysis was targeted to the first 2 weeks of infection, it might have missed subsequent changes.
Our studies revealed that only WT, but not its V- or C-defective derivatives, fully controlled the inflammatory responses, as monitored by the levels of TNF-α and IL-6 mRNA in PBMC collected 1 or 2 weeks postinfection. These two proinflammatory cytokines do not have direct antiviral activity but lead to clearance of infection by facilitating granulocyte activation or traffic to infection sites (14, 19). Indeed, reduced levels of TNF-α expression were observed after experimental MV infection of monkeys (33), and no increase in the level of expression of TNF-α or IL-6 protein from PBMC of patients with acute measles (4). An up-regulation of TNF-α mRNA was recently reported in PBMC from infants naturally infected with MV (56), but this difference is accounted by time of sampling, as our last time point (day 14) approximates the first sampling point in this report. It is also noteworthy that the V protein of parainfluenza virus 5 prevents IL-6 production; its zinc-binding carboxyl-terminal region, whose cysteine spacing is perfectly conserved in the MV V protein, is required for this function (20).
The MV V protein, and to some extent the C protein, have IFN-inhibiting activities in cultivated cells, and certain mechanisms underlying these activities are well characterized (8, 26, 27, 29, 54). Nevertheless, our data indicate that in a natural host some IFN induction occurs: rhesus monkeys developed elevated levels of IFN-α, MxA, and OAS transcripts 7 days after infection with any MV strain. One week later, the levels of these transcripts returned to preinfection levels. A significant difference between the infections with WT and the defective viruses was the level of the IFN-β transcripts 14 days postinfection, but how this relates to the defects in expression of these proteins is unclear. Differences in the kinetics of expression of IFN-α, MxA, and OAS transcripts in the very early phases of infection, that is, before day 7, may have contributed to the different outcomes of the infections with WT and defective viruses.
These studies also show that both V and C proteins are necessary to sustain high levels of viremia in rhesus monkeys. A similar conclusion was previously drawn for infection of cynomolgus monkeys with another C-protein-defective MV derived from the wild-type MV (48), which was tested in a group of three hosts. Analogously, a V-protein-defective CDV caused only mild signs of disease and reached titers 50 to 100 times lower than those of the wild-type CDV in the ferret, a natural host (52). Thus, both V and C proteins are required to maintain virulence, and their deletion has similar effects on viral pathogenesis in monkeys, even if the functions of these proteins are different.
Previous studies of the effects of the V and C proteins on MV virulence, which were based on the original infectious cDNA (36), are more difficult to interpret because of functional defects in its P gene (see below). Nevertheless, these studies do indicate that complete omission of V-protein expression attenuates MV more than combined single amino acid substitutions (25, 32, 49, 50). Moreover, V-protein overexpression appeared to favor viral replication, under certain circumstances, in human thymus/liver implants engrafted into SCID mice (50). Characterization of the mechanism of action of the C protein is less advanced, and it remains possible that the main function of this protein is simply to ensure accuracy of RNA synthesis, avoiding the formation of double-stranded RNA inducing the IFN response.
Since both V- and C-defective viruses induce high levels of neutralizing antibodies and strong cellular immune responses, they may be developed as more attenuated vaccines for immunocompromised individuals. On the other hand, this additional attenuation may be detrimental for oncolytic therapy applications. In this context, it is important to note that three current clinical trials of oncolysis (22) are based on more attenuated viruses derived from the original MV infectious cDNA (36). The V proteins of these viruses are mutated at both tyrosine 110 and cysteine 272. Tyrosine 110 supports specifically the STAT1 interaction (8, 13), and its mutation may exclusively affect this function, but mutation of cysteine 272 may disallow zinc binding, thereby precluding interactions not only with MDA5 but also with other cellular proteins (10, 29). Indeed, a virus with the Tyr110 and Cys272 mutations has reduced oncolytic efficacy in a human ovarian carcinoma preclinical model compared to another MV with the P gene of a wild-type strain (15).
Since inclusion of a wild-type P gene in a MV may raise a safety issue, viruses unable to interact with single innate immunity proteins may be a better option for preferential replication in tumors. For example, many cancer cells cannot produce IFN or respond to IFN stimulation, and such abnormalities make them highly susceptible to viral infection (21, 44). In particular, certain types of cancer have been associated with low STAT1 expression or loss of signaling (16, 45, 53). We have generated a MV mutant unable to interact selectively with STAT1 (P. Devaux, A. Hudacek, and R. Cattaneo, unpublished results). This virus may be fully replication competent in a STAT1-defective tumor, while being attenuated in healthy tissue. Analogously, viruses unable to interact with MDA5 or other cellular proteins may allow targeting types of cancer with other contexts of vulnerability. Analysis of the host response to infections of MV unable to interact with STAT1, MDA5, or other cellular proteins may not only provide insights on mechanisms of attenuation but also guide the development of vectors for cancer clinical trials.
Acknowledgments
We thank Charles Samuel, Griffith Parks, and Veronika von Messling for reading the manuscript, Sompong Vongpunsawad for excellent technical support, and Hannah Koble for secretarial assistance.
The National Institutes of Health (grant R01AI63476 to R.C. and grant RR11069 to the California National Primate Research Center) and the Mayo Foundation supported this research.
Footnotes
Published ahead of print on 2 April 2008.
REFERENCES
- 1.Abel, K., M. J. Alegria-Hartman, K. Zanotto, M. B. McChesney, M. L. Marthas, and C. J. Miller. 2001. Anatomic site and immune function correlate with relative cytokine mRNA expression levels in lymphoid tissues of normal rhesus macaques. Cytokine 16191-204. [DOI] [PubMed] [Google Scholar]
- 2.Abel, K., L. La Franco-Scheuch, T. Rourke, Z. M. Ma, V. De Silva, B. Fallert, L. Beckett, T. A. Reinhart, and C. J. Miller. 2004. Gamma interferon-mediated inflammation is associated with lack of protection from intravaginal simian immunodeficiency virus SIVmac239 challenge in simian-human immunodeficiency virus 89.6-immunized rhesus macaques. J. Virol. 78841-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. USA 10117264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atabani, S. F., A. A. Byrnes, A. Jaye, I. M. Kidd, A. F. Magnusen, H. Whittle, and C. L. Karp. 2001. Natural measles causes prolonged suppression of interleukin-12 production. J. Infect. Dis. 1841-9. [DOI] [PubMed] [Google Scholar]
- 5.Auwaerter, P. G., P. A. Rota, W. R. Elkins, R. J. Adams, T. DeLozier, Y. Shi, W. J. Bellini, B. R. Murphy, and D. E. Griffin. 1999. Measles virus infection in rhesus macaques: altered immune responses and comparison of the virulence of six different virus strains. J. Infect. Dis. 180950-958. [DOI] [PubMed] [Google Scholar]
- 6.Bellini, W. J., G. Englund, S. Rozenblatt, H. Arnheiter, and C. D. Richardson. 1985. Measles virus P gene codes for two proteins. J. Virol. 53908-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berghall, H., J. Siren, D. Sarkar, I. Julkunen, P. B. Fisher, R. Vainionpaa, and S. Matikainen. 2006. The interferon-inducible RNA helicase, mda-5, is involved in measles virus-induced expression of antiviral cytokines. Microbes Infect. 82138-2144. [DOI] [PubMed] [Google Scholar]
- 8.Caignard, G., M. Guerbois, J. L. Labernardiere, Y. Jacob, L. M. Jones, F. Wild, F. Tangy, and P. O. Vidalain. 2007. Measles virus V protein blocks Jak1-mediated phosphorylation of STAT1 to escape IFN-alpha/beta signaling. Virology 368351-362. [DOI] [PubMed] [Google Scholar]
- 9.Cattaneo, R., K. Kaelin, K. Baczko, and M. A. Billeter. 1989. Measles virus editing provides an additional cysteine-rich protein. Cell 56759-764. [DOI] [PubMed] [Google Scholar]
- 10.Childs, K., N. Stock, C. Ross, J. Andrejeva, L. Hilton, M. Skinner, R. Randall, and S. Goodbourn. 2007. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology 359190-200. [DOI] [PubMed] [Google Scholar]
- 11.de Swart, R. L., M. Ludlow, L. de Witte, Y. Yanagi, G. van Amerongen, S. McQuaid, S. Yuksel, T. B. Geijtenbeek, W. P. Duprex, and A. D. Osterhaus. 2007. Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 3e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devaux, P., and R. Cattaneo. 2004. Measles virus phosphoprotein gene products: conformational flexibility of the P/V protein amino-terminal domain and C protein infectivity factor function. J. Virol. 7811632-11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devaux, P., V. von Messling, W. Songsungthong, C. Springfeld, and R. Cattaneo. 2007. Tyrosine 110 in the measles virus phosphoprotein is required to block STAT1 protein phosphorylation. Virology 36072-83. [DOI] [PubMed] [Google Scholar]
- 14.Goetz, F. W., J. V. Planas, and S. MacKenzie. 2004. Tumor necrosis factors. Dev. Comp. Immunol. 28487-497. [DOI] [PubMed] [Google Scholar]
- 15.Haralambieva, I., I. Iankov, K. Hasegawa, M. Harvey, S. J. Russell, and K. W. Peng. 2007. Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Mol. Ther. 15588-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haura, E. B., J. Turkson, and R. Jove. 2005. Mechanisms of disease: insights into the emerging role of signal transducers and activators of transcription in cancer. Nat. Clin. Pract. Oncol. 2315-324. [DOI] [PubMed] [Google Scholar]
- 17.Kärber, G. 1931. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Pathol. Pharmakol. 162480-483. [Google Scholar]
- 18.Kelly, E., and S. J. Russell. 2007. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 15651-659. [DOI] [PubMed] [Google Scholar]
- 19.Kishimoto, T. 2005. IL-6: from laboratory to bedside. Clin. Rev. Allergy Immunol. 28177-186. [DOI] [PubMed] [Google Scholar]
- 20.Lin, Y., M. Sun, S. M. Fuentes, C. D. Keim, T. Rothermel, and B. He. 2007. Inhibition of interleukin-6 expression by the V protein of parainfluenza virus 5. Virology 368262-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linge, C., D. Gewert, C. Rossmann, J. A. Bishop, and J. S. Crowe. 1995. Interferon system defects in human malignant melanoma. Cancer Res. 554099-4104. [PubMed] [Google Scholar]
- 22.Liu, T. C., E. Galanis, and D. Kirn. 2007. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 4101-117. [DOI] [PubMed] [Google Scholar]
- 23.McChesney, M. B., C. J. Miller, P. A. Rota, Y. D. Zhu, L. Antipa, N. W. Lerche, R. Ahmed, and W. J. Bellini. 1997. Experimental measles. I. Pathogenesis in the normal and the immunized host. Virology 23374-84. [DOI] [PubMed] [Google Scholar]
- 24.Moss, W. J., J. J. Ryon, M. Monze, and D. E. Griffin. 2002. Differential regulation of interleukin (IL)-4, IL-5, and IL-10 during measles in Zambian children. J. Infect. Dis. 186879-887. [DOI] [PubMed] [Google Scholar]
- 25.Mrkic, B., B. Odermatt, M. A. Klein, M. A. Billeter, J. Pavlovic, and R. Cattaneo. 2000. Lymphatic dissemination and comparative pathology of recombinant measles virus in genetically modified mice. J. Virol. 741364-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakatsu, Y., M. Takeda, S. Ohno, R. Koga, and Y. Yanagi. 2006. Translational inhibition and increased interferon induction in cells infected with C protein-deficient measles virus. J. Virol. 8011861-11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohno, S., N. Ono, M. Takeda, K. Takeuchi, and Y. Yanagi. 2004. Dissection of measles virus V protein in relation to its ability to block alpha/beta interferon signal transduction. J. Gen. Virol. 852991-2999. [DOI] [PubMed] [Google Scholar]
- 28.Ono, N., H. Tatsuo, Y. Hidaka, T. Aoki, H. Minagawa, and Y. Yanagi. 2001. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 754399-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palosaari, H., J. P. Parisien, J. J. Rodriguez, C. M. Ulane, and C. M. Horvath. 2003. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J. Virol. 777635-7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parks, C. L., R. A. Lerch, P. Walpita, H. P. Wang, M. S. Sidhu, and S. A. Udem. 2001. Comparison of predicted amino acid sequences of measles virus strains in the Edmonston vaccine lineage. J. Virol. 75910-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paterson, R. G., G. P. Leser, M. A. Shaughnessy, and R. A. Lamb. 1995. The paramyxovirus SV5 V protein binds two atoms of zinc and is a structural component of virions. Virology 208121-131. [DOI] [PubMed] [Google Scholar]
- 32.Patterson, J. B., D. Thomas, H. Lewicki, M. A. Billeter, and M. B. Oldstone. 2000. V and C proteins of measles virus function as virulence factors in vivo. Virology 26780-89. [DOI] [PubMed] [Google Scholar]
- 33.Polack, F. P., S. J. Hoffman, W. J. Moss, and D. E. Griffin. 2002. Altered synthesis of interleukin-12 and type 1 and type 2 cytokines in rhesus macaques during measles and atypical measles. J. Infect. Dis. 18513-19. [DOI] [PubMed] [Google Scholar]
- 34.Premenko-Lanier, M., P. A. Rota, G. H. Rhodes, W. J. Bellini, and M. B. McChesney. 2004. Protection against challenge with measles virus (MV) in infant macaques by an MV DNA vaccine administered in the presence of neutralizing antibody. J. Infect. Dis. 1892064-2071. [DOI] [PubMed] [Google Scholar]
- 35.Radecke, F., and M. A. Billeter. 1996. The nonstructural C protein is not essential for multiplication of Edmonston B strain measles virus in cultured cells. Virology 217418-421. [DOI] [PubMed] [Google Scholar]
- 36.Radecke, F., P. Spielhofer, H. Schneider, K. Kaelin, M. Huber, C. Dotsch, G. Christiansen, and M. A. Billeter. 1995. Rescue of measles viruses from cloned DNA. EMBO J. 145773-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Randall, R. E., and S. Goodbourn. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 891-47. [DOI] [PubMed] [Google Scholar]
- 38.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 27493-497. [Google Scholar]
- 39.Reutter, G. L., C. Cortese-Grogan, J. Wilson, and S. A. Moyer. 2001. Mutations in the measles virus C protein that up regulate viral RNA synthesis. Virology 285100-109. [DOI] [PubMed] [Google Scholar]
- 40.Reyes del Valle, J. R., P. Devaux, G. Hodge, N. J. Wegner, M. B. McChesney, and R. Cattaneo. 2007. A vectored measles virus induces hepatitis B surface antigen antibodies while protecting macaques against measles virus challenge. J. Virol. 8110597-10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider, H., K. Kaelin, and M. A. Billeter. 1997. Recombinant measles viruses defective for RNA editing and V protein synthesis are viable in cultured cells. Virology 227314-322. [DOI] [PubMed] [Google Scholar]
- 42.Shaffer, J. A., W. J. Bellini, and P. A. Rota. 2003. The C protein of measles virus inhibits the type I interferon response. Virology 315389-397. [DOI] [PubMed] [Google Scholar]
- 43.Staib, C., and G. Sutter. 2003. Live viral vectors: vaccinia virus. Methods Mol. Med. 8751-68. [DOI] [PubMed] [Google Scholar]
- 44.Stojdl, D. F., B. D. Lichty, B. R. tenOever, J. M. Paterson, A. T. Power, S. Knowles, R. Marius, J. Reynard, L. Poliquin, H. Atkins, E. G. Brown, R. K. Durbin, J. E. Durbin, J. Hiscott, and J. C. Bell. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4263-275. [DOI] [PubMed] [Google Scholar]
- 45.Sun, W. H., C. Pabon, Y. Alsayed, P. P. Huang, S. Jandeska, S. Uddin, L. C. Platanias, and S. T. Rosen. 1998. Interferon-alpha resistance in a cutaneous T-cell lymphoma cell line is associated with lack of STAT1 expression. Blood 91570-576. [PubMed] [Google Scholar]
- 46.Takeda, M., K. Takeuchi, N. Miyajima, F. Kobune, Y. Ami, N. Nagata, Y. Suzaki, Y. Nagai, and M. Tashiro. 2000. Recovery of pathogenic measles virus from cloned cDNA. J. Virol. 746643-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeuchi, K., S. I. Kadota, M. Takeda, N. Miyajima, and K. Nagata. 2003. Measles virus V protein blocks interferon (IFN)-alpha/beta but not IFN-gamma signaling by inhibiting STAT1 and STAT2 phosphorylation. FEBS Lett. 545177-182. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi, K., M. Takeda, N. Miyajima, Y. Ami, N. Nagata, Y. Suzaki, J. Shahnewaz, S. Kadota, and K. Nagata. 2005. Stringent requirement for the C protein of wild-type measles virus for growth both in vitro and in macaques. J. Virol. 797838-7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tober, C., M. Seufert, H. Schneider, M. A. Billeter, I. C. Johnston, S. Niewiesk, V. ter Meulen, and S. Schneider-Schaulies. 1998. Expression of measles virus V protein is associated with pathogenicity and control of viral RNA synthesis. J. Virol. 728124-8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valsamakis, A., H. Schneider, P. G. Auwaerter, H. Kaneshima, M. A. Billeter, and D. E. Griffin. 1998. Recombinant measles viruses with mutations in the C, V, or F gene have altered growth phenotypes in vivo. J. Virol. 727754-7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Binnendijk, R. S., R. W. van der Heijden, G. van Amerongen, F. G. UytdeHaag, and A. D. Osterhaus. 1994. Viral replication and development of specific immunity in macaques after infection with different measles virus strains. J. Infect. Dis. 170443-448. [DOI] [PubMed] [Google Scholar]
- 52.von Messling, V., N. Svitek, and R. Cattaneo. 2006. Receptor (SLAM [CD150]) recognition and the V protein sustain swift lymphocyte-based invasion of mucosal tissue and lymphatic organs by a morbillivirus. J. Virol. 806084-6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong, L. H., K. G. Krauer, I. Hatzinisiriou, M. J. Estcourt, P. Hersey, N. D. Tam, S. Edmondson, R. J. Devenish, and S. J. Ralph. 1997. Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3gamma. J. Biol. Chem. 27228779-28785. [DOI] [PubMed] [Google Scholar]
- 54.Yokota, S., H. Saito, T. Kubota, N. Yokosawa, K. Amano, and N. Fujii. 2003. Measles virus suppresses interferon-alpha signaling pathway: suppression of Jak1 phosphorylation and association of viral accessory proteins, C and V, with interferon-alpha receptor complex. Virology 306135-146. [DOI] [PubMed] [Google Scholar]
- 55.Zhu, Y. D., J. Heath, J. Collins, T. Greene, L. Antipa, P. Rota, W. Bellini, and M. McChesney. 1997. Experimental measles. II. Infection and immunity in the rhesus macaque. Virology 23385-92. [DOI] [PubMed] [Google Scholar]
- 56.Zilliox, M. J., W. J. Moss, and D. E. Griffin. 2007. Gene expression changes in peripheral blood mononuclear cells during measles virus infection. Clin. Vaccine Immunol. 14918-923. [DOI] [PMC free article] [PubMed] [Google Scholar]