Abstract
Antibody-dependent cellular cytotoxicity (ADCC) is a potentially effective adaptive immune response to human immunodeficiency virus (HIV) infection. The study of ADCC responses has been hampered by the lack of simple methods to quantify these responses and map effective epitopes. We serendipitously observed that standard intracellular cytokine assays on fresh whole blood from a cohort of 26 HIV-infected subjects identified non-T lymphocytes expressing gamma interferon (IFN-γ) in response to overlapping linear peptides spanning HIV-1 proteins. The effector cells were CD3− CD4− CD8− CD14− CD2+ CD56+/− NK lymphocytes and degranulated granzyme B and perforin in response to antigen stimulation. Serum transfer assays demonstrated that the specific response was mediated by immunoglobulin G. Fresh blood samples from half of the HIV-infected cohort demonstrated robust HIV peptide-specific IFN-γ expression by NK cells, predominately to Env, Pol, and Vpu HIV-1 proteins. Responses were readily mapped to define minimal epitopes utilizing this assay. Antibody-dependent, HIV-specific NK cell recognition, involving components of both innate and adaptive immune systems, represents a potentially effective immune response to induce by vaccination.
Inducing human immunodeficiency virus (HIV)-specific T-cell immunity and neutralizing antibodies is considered a likely prerequisite for effective immune protection against HIV type 1 (HIV-1) infection (7). Other elements of the adaptive and innate immune system may, however, be required to facilitate more robust protective immunity against HIV-1.
There is increasing interest in the relationship between natural killer (NK) cells and HIV-1 infection (1, 8). NK cells comprise 15% of peripheral blood lymphocytes and form one arm of the innate immune system via their ability to kill virus-infected cells that down-regulate or modify major histocompatibility complex class I (MHC-I) molecules. NK cell function is regulated by a balance between inhibitory and activating receptors. The CD2 receptor identifies targets for killing, while a second receptor, CD94, covalently assembles with NKG2 membrane molecules to potentially block (NKG2A) or activate (NKG2C) NK-mediated cytotoxicity via recognition of HLA-E. Interactions between NK receptors and HLA-B have been linked to a delayed progression to AIDS in HIV-positive individuals (19). HIV-1 evades the host's NK cell response via (i) differential down-regulation of MHC-I molecules on the surface of infected cells, (ii) dysregulated production of cytokines that enhance NK cell function, (iii) direct inhibitory effects of certain viral proteins on NK cell functions, and (iv) changes in the expression of NK cell receptors (15-17, 20-22).
Antibody-dependent cell-mediated cytolysis (ADCC) is an adaptive immune response mediated in large part by NK cells. The Fc portions of antiviral ADCC immunoglobulin G (IgG) antibodies are bound by the CD16 Fc receptor on NK cells, triggering lysis of the target. Release of gamma interferon (IFN-γ) by NK cells has an important function in the virus-specific immune response although NK cells also secrete interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α), granulocyte-macrophage colony-stimulating factor, and degranulated perforin and granzyme B. ADCC has been associated with protection from simian immunodeficiency virus disease in macaques (4, 10), delayed progressive HIV infection in humans (2), protection of intravenous drug users from HIV-1 infection (28), and lower genital HIV viral loads (23).
Little is known about the HIV-1 antigens that stimulate ADCC activity in humans. Linear ADCC epitopes have been described for Env (3) and Nef (33) in HIV-1-infected long-term slow progressors (LTSP). LTSP subjects are typically persons who have been infected with HIV for more than 10 years and who remain healthy without anti-HIV treatment.
The utility of ADCC responses in controlling HIV-1 has not been sufficiently studied in part because of the difficulty in assaying these responses (13). Previous assays have required the preparation of radiolabeled or fluorescent target cell lines pulsed with whole proteins or viruses for functional killing assays (9). It has been relatively difficult to map epitopes with these assays. Simple whole-blood assays of cytokine expression in responses to overlapping peptides have revolutionized the study of HIV-specific T-cell immunity (18). Similar advances would be very helpful in the study of ADCC responses.
We recently studied a cohort of HIV-infected subjects with antiretroviral drug-resistant HIV-1 (29). While studying standard CD8 and CD4 T-cell immunity to HIV-1 by IFN-γ intracellular cytokine staining of whole blood stimulated by overlapping HIV-1 peptide sets, we observed substantial HIV-specific expression of IFN-γ by lymphocytes that were not CD8 or CD4 T cells. We subsequently showed that the effector cells were NK lymphocytes and that IgG in plasma mediated the response. Herein we define the breadth and characteristics of HIV-specific antibody-dependent NK cell responses detected with this intracellular cytokine staining technique.
MATERIALS AND METHODS
Subjects.
HIV-infected human subjects were recruited with institutional human ethics committee approval to donate blood samples to study HIV-specific immunity. We studied 26 HIV-1-infected subjects recruited in a study examining T-cell immunity. All subjects but one were infected with drug-resistant HIV-1 and had a mean CD4 T-cell count of 407 cells/μl and an HIV-1 viral load of 21,000 copies/ml, as previously described (29).
Peptides.
Initial responses were detected using peptide pools containing 15-mers (overlapping by 11 amino acids) spanning all HIV-1 proteins using a consensus subtype B (NIH AIDS Reagent Repository). Responses were further defined using additional purchased peptides (GL Biochem, Shanghai, China).
Analysis of cellular immunity.
Our standard intracellular cytokine staining (ICS) protocol on fresh sodium-heparinized whole blood was used for this study (29), although incubation times were varied for some experiments. Briefly, 200 μl of whole blood was incubated at 37°C with peptides at a final concentration of 1 μg/ml of peptide in the presence of costimulatory antibodies to CD28 and CD49d. Brefeldin-A (Sigma) was added after 2 h (10 mg/ml), and the incubation was terminated after 7 h. Negative (dimethyl sulfoxide alone) and positive control (combined Staphylococcus enterotoxin B and pokeweed mitogen; Sigma) wells were included. Cells were surface stained with CD4-fluorescein isothiocyanate (FITC), CD3-phycoerythrin (PE), and CD8-peridinin chlorophyll protein and lysed, permeabilized, and stained intracellularly with IFN-γ-allophycocyanin (APC). Analysis was performed on FACSort and Cell Quest software. Additional cell surface markers used to characterize the immune responses included the following: CD3-FITC (SK7 clone; catalog no. 349201), CD3-PE (SP34 clone; catalog no. 556621), CD2-PE, CD56-PE, CD14-PE, CD19-PE, CD20-PE, CD94-PE, and T-cell receptors (TCRs) αβ-PE and γδ-FITC (all BD). Staining for lytic potential used surface staining with CD107a-APC (BD) and intracellular staining with anti-granzyme B-FITC (clone GB11; Mabtech) or anti-perforin-FITC (clone Pf-344; Mabtech) as previously described (26).
CD8 T-cell depletion.
To exclude the need for CD8 T-cell responses mediating ADCC responses, in vitro depletion of CD8 cells was undertaken using a CD8α-specific monoclonal antibody (Dynal beads M-450; Oslo, Norway). Depletion of 4 ml of whole blood was performed, as per the manufacturer's instructions, at a ratio of 50 beads to 1 CD8α+ cell. Depletion was confirmed by fluorescence-activated cell sorting using CD8α-peridinin chlorophyll protein monoclonal antibody.
Purification of Ig from human plasma.
To show that IgG in serum was mediating the ADCC response, purification of IgG from serum was performed using protein A-Sepharose Fast-Flow, as per the manufacturer's instructions (Amersham Biosciences). Briefly, protein A beads were loaded into a polyprep chromatography column (Bio-Rad), and 5 ml of subject serum was applied and allowed to drip through by gravity. The column was then washed with phosphate-buffered saline, and Ig was eluted from the column with 0.1 M glycine HCl (pH 2.8). The acidity of the eluate was then neutralized using Tris buffer. The purity of the Ig in the eluate was confirmed using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (courtesy of Adam Winterhalter, University of Melbourne). The concentration of IgG in the eluate was then measured at 5.4 g/liter, representing >96% of all Ig in the eluate (courtesy of Chemical Pathology, Royal Melbourne Hospital).
RESULTS
HIV-specific IFN-γ expression by non-T lymphocytes is commonly detected in blood from HIV-infected subjects.
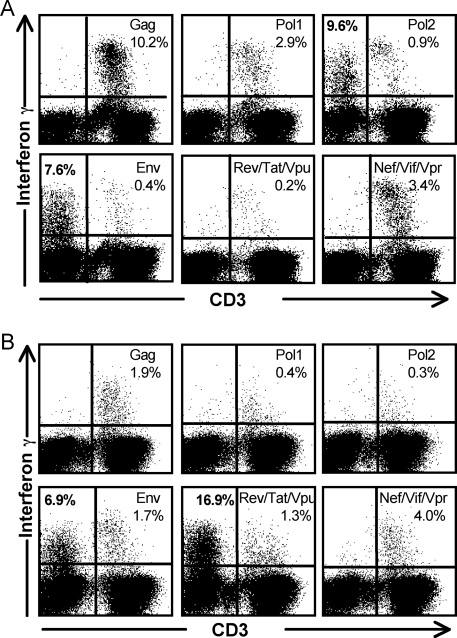
Typical ICS assays detect IFN-γ expression from CD4 or CD8+ CD3+ T lymphocytes in fresh blood stimulated with overlapping HIV-1 peptides. Other lymphocytes such as NK cells are also capable of expressing IFN-γ but, on their own, not in an antigen-specific manner. We recently studied HIV-specific T-cell immunity by ICS in fresh blood samples from a cohort of HIV-infected subjects with a median CD4 T-cell count of 407 cells/μl and an HIV viral load of 21,000 copies/ml (29). During acquisition of flow cytometric data on this cohort, we observed substantial HIV-specific expression from lymphocytes that lacked surface expression of CD3, CD4, or CD8. Examples of flow cytometric data on whole blood stimulated with various overlapping peptide sets on two of the subjects are shown in Fig. 1. In subject 23 for example, when CD8+ cells were gated first on CD3+ T lymphocytes, antigen-specific T-cell responses were detected to several HIV-1 peptide pools ranging from 0.2% to 10.2% (Table 1 and Fig. 1A). However, when cells were analyzed directly from the lymphocyte gate (based on forward and side scatter criteria only), the same data files showed non-CD3 lymphocytes expressing IFN-γ in response to Pol and Env peptides of 9.6% and 7.6%, respectively (Fig. 1A).
FIG. 1.
CD3-negative lymphocytes expressing IFN-γ in response to HIV peptides. Flow cytometric plots on gated lymphocytes from two subjects are shown. (A) Whole blood from HIV-infected subject 23 was stimulated with pools of HIV-1 consensus subtype B overlapping peptide pools in a 5-h ICS assay. Pol peptides were divided into two separate pools, and peptides for Rev, Tat, and Vpu and for Vif, Nef, and Vpr were combined. Gated lymphocytes were studied for expression of CD3 and intracellular IFN-γ. HIV-specific IFN-γ expression from non-CD3 lymphocytes in response to Pol and Env is detected. (B) Whole blood from HIV-infected subject 26 was similarly incubated with pools of overlapping 15-mer peptides from HIV-1 peptides for 5 h. Gated lymphocytes were studied for CD3 and intracellular IFN-γ expression as above. A large proportion of CD3-negative cells produced IFN-γ in response to the combined Rev, Tat, and Vpu peptide.
TABLE 1.
Robust proportions of CD3-negative lymphocytes from HIV-1 infected subjects express IFN-γ in response to pools of overlapping HIV-1 peptides
| Subject no. | Antigen-specific response to HIV-1 peptidea
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Non-T-cell response
|
% CD8 T-cell response
|
% CD4 T-cell response
|
||||||||||||||||
| Gag | Pol | Env | RTV | VNV | Total | Gag | Pol | Env | RTV | VNV | Total | Gag | Pol | Env | RTV | VNV | Total | |
| 1 | − | − | − | − | − | − | 2.6 | − | − | − | 0.5 | 3.1 | 0.2 | − | − | − | − | 0.2 |
| 2 | − | 4.7 | 3.3 | − | − | 8.0 | 0.1 | 0.5 | − | − | − | 0.6 | 0.1 | − | − | − | − | 0.1 |
| 3 | − | − | − | − | − | − | 0.8 | 0.2 | 0.2 | − | 0.1 | 1.3 | 0.1 | 0.1 | − | − | 0.1 | 0.3 |
| 4 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 5 | − | − | 0.7 | − | − | 0.7 | − | 0.1 | − | − | 0.1 | 0.2 | − | − | − | − | − | − |
| 6 | − | − | − | − | − | − | 0.1 | 0.2 | − | − | 0.2 | 0.5 | − | − | − | − | 0.1 | 0.1 |
| 7 | − | − | − | − | − | − | 0.5 | 0.3 | − | − | 0.5 | 1.3 | 0.1 | 0.2 | − | − | 0.1 | 0.4 |
| 8 | − | 1.7 | − | − | − | 1.7 | 2.4 | 1.1 | 1.5 | 0.5 | 2.2 | 7.7 | 0.1 | 0.4 | 0.1 | 0.2 | 0.2 | 1.0 |
| 9 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 10 | − | − | − | − | − | − | 0.1 | − | − | − | − | 0.1 | − | − | − | − | − | − |
| 11 | − | − | − | − | − | − | 0.2 | − | − | − | − | 0.2 | − | − | − | − | − | − |
| 12 | − | − | 0.7 | − | − | 0.7 | 0.9 | − | − | − | − | 0.9 | − | − | − | − | − | − |
| 13 | − | − | 1.1 | − | − | 1.1 | 1.1 | 0.4 | 0.1 | − | 0.4 | 1.9 | 0.1 | 0.1 | − | − | 0.1 | 0.3 |
| 14 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 15 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 16 | − | − | 1.1 | 3.4 | − | 4.5 | 2 | 1.2 | 0.3 | − | 1 | 4.5 | 0.1 | − | − | − | − | 0.1 |
| 17 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 18 | − | − | 8.2 | − | − | 8.2 | − | − | − | − | − | − | − | − | − | − | − | − |
| 19 | − | − | 1.9 | − | − | 1.9 | 3 | 0.5 | 0.4 | − | 0.8 | 4.7 | − | − | − | − | 0.3 | 0.3 |
| 20 | − | − | 2.1 | − | − | 2.1 | − | − | − | − | − | − | − | − | − | − | − | − |
| 21 | − | − | 0.6 | − | − | 0.6 | 0.4 | − | 0.4 | − | − | 0.8 | − | − | − | − | − | − |
| 22 | − | − | − | − | − | − | 0.9 | 6.4 | 1.2 | − | 1.1 | 9.6 | 0.3 | 0.6 | 0.2 | − | 0.2 | 1.3 |
| 23 | − | 10.8 | 10.6 | − | − | 21.3 | 12.7 | 3.4 | 0.6 | − | 3.6 | 20.3 | 0.6 | 0.3 | − | − | − | 0.9 |
| 24 | − | − | 2.5 | − | − | 2.5 | 3.1 | 1.8 | 1.1 | − | 0.3 | 6.3 | 0.4 | 0.1 | 0.1 | − | − | 0.6 |
| 25 | − | − | − | − | − | − | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.6 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.5 |
| 26 | − | − | 6.0 | 15.8 | − | 21.8 | 1.7 | 2.1 | 0.5 | 1.4 | 5.7 | 1.0 | 0.4 | − | − | 0.4 | 1.7 | |
| Mean ± SDb | 5.7 ± 4.6 (12) | 3.2 ± 3.3 (46) | 9.6 ± 8.8 (8) | 5.8 ± 7.4 (50) | 1.8 ± 2.9 (72) | 1.2 ± 1.8 (56) | 0.7 ± 0.6 (44) | 0.4 ± 0.2 (12) | 0.9 ± 1.0 (56) | 3.7 ± 4.9 (76) | 0.3 ± 0.3 (48) | 0.3 ± 0.2 (36) | 0.1 ± 0.1 (16) | 0.2 ± 0.1 (8) | 0.2 ± 0.1 (36) | 0.5 ± 0.5 (56) | ||
−, negative response (<0.05% or less than threefold above background response for T-cell responses and <0.5% for non-T-cell responses); RTV, pool containing peptides from HIV-1 proteins Rev, Tat, and Vpu; VNV, pool containing peptides from HIV-1 proteins Vif, Nef, and Vpr.
Values indicate the mean strength of responders; values in parentheses are the percentages of subjects responding.
We systematically reexamined fluorescence-activated cell sorting data from 26 consecutively enrolled subjects infected with HIV-1 (29). We conservatively accepted a 0.5% antigen-specific response above background as likely to be significant. Based on this, 13 (50%) of the 26 subjects studied consecutively displayed antigen-specific non-T-lymphocyte responses to at least one of the HIV-1 peptide pools studied (Table 1).
The majority of NK cell-mediated HIV-specific responses in the cohort of 26 subjects were to the Env peptide pool, ranging from 0.6% to 10.6% of all lymphocytes. Responses were also detected to Pol and to the combined pool of Rev, Tat, and Vpu in three and two subjects, respectively. Interestingly, no NK cell-mediated responses were detected to the Gag pool or the combined pool of Nef, Vif, and Vpr in the 26 subjects studied. In contrast CD8 and CD4 T-cell responses to Gag were the most common in this cohort (68% and 40% of subjects, respectively) (29).
Characterization of HIV-specific IFN-γ expression by lymphocytes not expressing CD3.
The nature of the effector lymphocytes mediating the HIV-specific IFN-γ expression was then examined. We chose one subject (subject 26) to initially characterize the HIV-specific IFN-γ expression by non-T lymphocytes since this subject had large HIV-specific non-T-cell responses (Fig. 1B). This person had been infected for more than 18 years and had slowly progressive HIV infection. A standard ICS assay with pools of overlapping linear 15-mer peptides derived from consensus subtype B HIV-1 proteins was used. HIV-specific T-lymphocyte immune responses were detected in subject 26 to a number of peptide pools spanning HIV proteins. For example, a 1.9% antigen-specific CD3 T-lymphocyte response was seen directed against a pool of Gag peptides (Fig. 1B).
A large (16.9%) antigen-specific response from non-CD3 lymphocytes was detected directed against the peptide pool containing Rev, Tat, and Vpu as well as a 6.9% response to Env (Fig. 1B). The IFN-γ-producing cells were lymphocytes (based on forward and side scatter criteria) but were CD3, CD4, and CD8 negative and, hence, not T lymphocytes. We subsequently demonstrated that these responses were maximal after a 5-h incubation, were not present without antigen stimulation, and were unaffected by the presence or absence of CD28 and CD49d antibodies used to provide costimulatory signals to T cells (data not shown). This HIV-specific response was repeatedly (n = 5) detected over a 16-month period and varied between 13.8 to 21.4% of all CD3-negative lymphocytes.
Epitope mapping of Vpu-specific IFN-γ expression by a non-T-lymphocyte immune response.
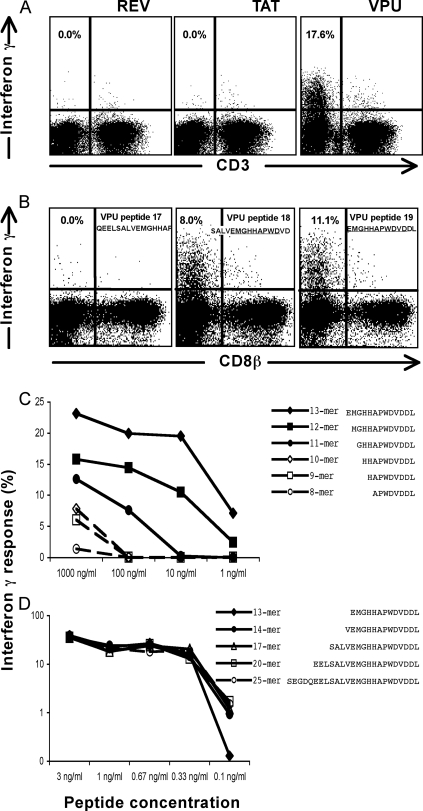
To enable a detailed study of this immune response, we mapped this Vpu-specific response to a minimal epitope. The large numbers of HIV-specific IFN-γ-expressing lymphocytes were readily mapped using smaller pools of overlapping 15-mer peptides. Within the Rev/Tat/Vpu combined pool, the response was solely directed at the Vpu pool (Fig. 2A). Vpu is an 81-amino-acid HIV-1 regulatory protein involved in late processing of the HIV-1 virion, prior to release of free virus from CD4 T cells, and prevents Env from binding to the CD4 T-cell receptor within the endoplasmic reticulum during processing (5, 11). The Vpu peptide pool is comprised of 19 overlapping peptides. Fine mapping using individual peptides showed that responses were directed at a common 11-amino-acid sequence shared between the 18th and 19th peptides of the Vpu pool, namely EMGHHAPWDVD (Fig. 2B). Truncated versions of this 11-mer were subsequently constructed to fine map this response (Table 2). The C-terminal aspartic acid residue (D) of the peptide was critical for maintaining the peptide-specific response, while sequential removal of the N-terminal amino acid residues resulted in diminishing peptide-specific responses. This was confirmed with serial dilutions of 8- to 13-mer peptides (Fig. 2C). When longer peptides were constructed by sequentially adding the appropriate Vpu peptide to the N terminus, similarly strong responses were detected with marginally higher responses detected to peptides with lengths greater than or equal to 14 amino acids at concentrations of 0.1 ng/ml (Fig. 2D).
FIG. 2.
Epitope mapping: an HIV-specific non-T-lymphocyte response recognizes Vpu peptide. Whole blood from subject 26 was incubated with HIV peptides for 5 h, and gated lymphocytes were studied for the expression of CD3 and IFN-γ. (A) The recognition of pooled Tat, Rev, and Vpu peptide (shown in Fig. 1B) was specific for a Vpu pool of 19 peptides. (B) Individual peptides within the Vpu pool of 19 peptides were studied, with the response overlapping peptides 18 and 19. (C and D) The Vpu response was mapped to minimal epitopes. A 14-mer peptide had an equally strong response to larger peptides.
TABLE 2.
Mapping of ADCC/NK responses to linear Vpu peptides
| Peptide namea | Non-T-lymphocyte responseb | Sourcec | Peptide sequenced |
|---|---|---|---|
| Peptide 17 of Vpu pool | 0.00 | NIH | Q E E L S A L V E M G H A P |
| 13-mer EK (peptide 19) | 6.82 | NIH | E M G H H A P W D V D D L |
| 10-mer EV | 0.00 | China | E M G H H A P W D V |
| 10-mer MD | 5.64 | China | M G H H A P W D V D |
| 9-mer ED | 0.00 | China | E M G H H A P W D |
| 9-mer MV | 0.00 | China | M G H H A P W D V |
| 9-mer GD | 4.70 | China | G H H A P W D V D |
| 8-mer EW | 0.00 | China | E M G H H A P W |
| 8-mer MD | 0.00 | China | M G H H A P W D |
| 8-mer GV | 0.00 | China | G H H A P W D V |
| 8-mer HD | 2.02 | China | H H A P W D V D |
The first and last residues of the peptide are indicated.
Significant responses are in boldface.
NIH, National Institutes of Health, AIDS Reagent Program; China, GL Biochem (Shanghai, China).
The common 11-mer amino acid sequence shared by Vpu peptides 18 and 19 is underlined.
Phenotypic analysis of the peptide-specific non-T-lymphocyte IFN-γ response.
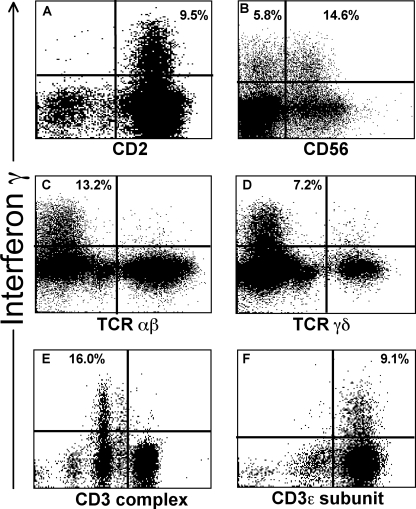
The effector cells expressing IFN-γ in response to the Vpu epitope were unlikely to be T lymphocytes since the cells did not express CD3. Further detailed phenotypic analysis showed that these IFN-γ-producing lymphocytes did not express either of the subunits of the CD8 heterodimer, namely CD8α or CD8β (Fig. 2B; also data not shown). Since the lymphocytes were expressing IFN-γ, we considered NK cells were likely candidates for the effector cells. We did, however, formally test for the expression of the monocyte marker CD14 and the B-lymphocyte markers CD19 and CD20, none of which was expressed by the IFN-γ-expressing cells. The number of monocytes that fall into the lymphocyte gate (where we detected the specific IFN-γ production) would be too small to account for the observed cell population. All effector cells expressed CD2 on their surfaces (Fig. 3A). CD2 mediates intracellular adhesion between leukocytes and other cells, targets cells for lysis, and is typically found on both T cells and most CD3-negative NK cells. The Vpu-reactive cells expressing IFN-γ were confined to the CD2+ CD3− lymphocyte population and comprised 21.6 to 38.5% of this population over multiple (n = 5) assays throughout 16 months. Interestingly, one-half of the CD2+ CD3− IFN-γ-expressing lymphocytes from subject 26 expressed the NK marker CD56 (Fig. 3B).
FIG. 3.
Cell markers on Vpu-specific IFN-γ-expressing lymphocytes. Whole blood from subject 26 was pulsed with the Vpu epitope. Gated lymphocytes are shown in all plots. The proportions of cells expressing IFN-γ are shown in the relevant quadrant. (A) IFN-γ-expressing lymphocytes all expressed CD2. CD3-negative gated lymphocytes are shown. (B) Only a proportion of the IFN-γ-expressing lymphocytes expressed CD56. CD2+ CD3− gated lymphocytes are shown. Percentages in the upper-right and upper-left quadrants show the proportion of CD56+ and CD56− cells that express IFN-γ, respectively. Overall ∼50% of IFN-γ-expressing cells are CD56+. (C and D) IFN-γ-expressing lymphocytes did not express intracellular TCRαβ or TCRγδ. Gated lymphocytes are shown. (E and F) There was no expression of the CD3 complex by IFN-γ-expressing lymphocytes, but intracellular expression of the CD3 epsilon subunit was detected. Gated lymphocytes are shown.
To confirm that the effector cells were not T lymphocytes, we showed that both surface and intracellular staining for the TCRαβ and TCRγδ receptors was negative (Fig. 3C and D; also data not shown). Intracellular expression of CD4 and CD8 was also negative (data not shown). We also stained for intracellular expression of CD3, since it was formally possible that there was down-regulation of surface T-cell markers during the in vitro stimulation step. The CD3 molecule is composed of several subunits. We could not detect intracellular staining of CD3 using the SK7 clone, which detects the CD3 complex (Fig. 3C, left plot). However, we did detect the CD3 epsilon subunit intracellularly using the SK34 clone (Fig. 3F). The SP34 clone targets the denatured form of CD3ɛ (27), and NK cells express the denatured form of the CD3ɛ subunit within their cytoplasm (25), while the SK7 clone (BD catalog no. 349201) is from a panel of monoclonal antibodies raised against surface membrane CD3 and targets CD3ɛ complexed with either CD3γ or CD3δ subunits (27, 30). The expression of only the CD3ɛ subunit is consistent with the effector cells’ being NK cells. To further formally exclude the possibility that CD8+ T cells were mediating this Vpu-specific IFN-γ expression, we performed ex vivo CD8 T-cell depletion using a CD8α-specific antibody. CD8-depletion did not abrogate the Vpu-specific response (data not shown).
T cells and NK cells secrete both TNF-α and IFN-γ, while IL-2 and IL-4 are typically secreted by T lymphocytes only and not NK cells. Additional analyses were therefore undertaken of the effector cells and included an analysis of the cytokine profile of peptide-specific lymphocytes. The Vpu-responding lymphocytes expressed intracellular TNF-α but did not express IL-2 or IL-4 (data not shown).
Vpu-specific responses require plasma, suggesting an ADCC-like mechanism.
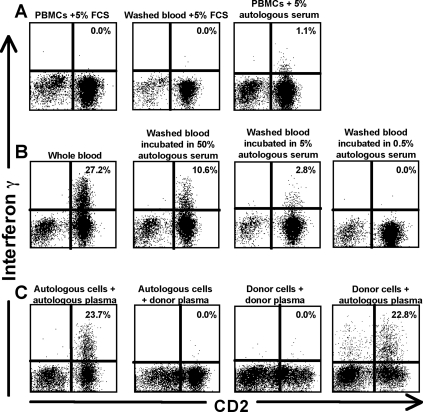
The initial studies on the Vpu-specific response were performed on fresh unfractionated whole blood, raising the possibility that antibodies were involved in mediating this response. To assess the requirement for autologous serum and cells in mediating this activity, we performed a series of serum transfer assays, incubating autologous and donor cells with mixes of autologous serum, donor serum, and fetal calf serum. Incubation of Ficoll-separated peripheral blood mononuclear cells (PBMCs) or washed whole blood (with the plasma removed) from subject 26 in fetal calf serum abrogated the response (Fig. 4A). However, when subject 26's PBMCs were incubated in 5% autologous serum, there was partial restitution of the response (Fig. 4B). Incubating washed whole blood from subject 26 with higher concentrations of autologous serum (50%) yielded higher IFN-γ responses although antigen-specific responses were lost when the concentration of autologous serum was ≤0.5%. Heat inactivation of autologous serum prior to incubation in washed whole blood from subject 26 did not affect the result (data not shown), indicating that complement was not required for the response.
FIG. 4.
Vpu-specific expression of IFN-γ in subject 26 is mediated by autologous serum. (A) Ficoll-separated PBMCs or fresh blood washed twice to remove plasma from subject 26 was incubated with either fetal calf serum or autologous serum comprising 5% of the culture together with the Vpu peptide epitope in an ICS assay. (B) Whole blood from subject 26 was washed to remove plasma and incubated with autologous serum comprising 50%, 5%, or 0.5% of the culture together with the Vpu peptide epitope. (C) Autologous PBMCs and plasma from subject 26 were mixed with PBMCs or plasma from an HIV-negative donor.
To determine the requirement for autologous effector cells in mediating the Vpu-specific response, donor cells and autologous serum were stimulated with the Vpu peptide epitope. The antigen-specific activity was wholly mediated by serum/plasma and not reliant on autologous cells from subject 26 (Fig. 4C). The Vpu-specific response of 23.7% in subject 26 (Fig. 4C, left plot) was equivalent to that detected using donor cells from an HIV-negative volunteer that were incubated with plasma from subject 26 (response, 22.8%) (Fig. 4C). Conversely, no Vpu-specific response was detected when incubations were performed using plasma from an HIV-negative volunteer. We subsequently confirmed that serum from multiple HIV-positive subjects (n = 12) with HIV-specific NK-cell mediated IFN-γ expression in whole blood could also mediate IFN-γ expression in PBMCs from HIV-negative donors but not in the absence of HIV-positive serum (not shown).
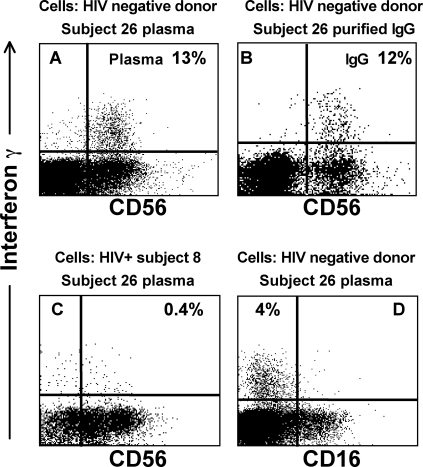
ADCC activity is dependent on IgG antibodies’ binding the target antigen and on the Fc portion of the IgG molecule binding effector cells such as NK cells. We therefore purified plasma IgG from subject 26 to assess whether IgG was mediating this Vpu-specific IFN-γ expression. Incubating whole blood from an HIV-negative volunteer in the presence of the target Vpu peptide and Ig purified from the plasma of subject 26 resulted in an equivalent response to that seen with whole plasma (Fig. 5A and B).
FIG. 5.
IgG mediates the Vpu-specific IFN-γ expression. Whole blood from additional volunteers was incubated with plasma (or plasma components) from subject 26 in the presence of the Vpu epitope. The sources of the responder cells and plasma fraction are shown above each plot. (A) HIV-negative volunteer. All IFN-γ+ cells were CD56 positive. (B) When incubation was performed in the presence of an aliquot of Ig purified from the plasma of the HIV-infected subject, the response was preserved. (C) HIV-positive donor cells from subject 8 were incubated with plasma from subject 26. Only half of the IFN-γ-expressing cells expressed CD56. (D) IFN-γ+ cells did not express CD16. HIV-negative donor cells incubated with plasma from subject 26 are shown.
CD56 down-regulation on HIV-specific NK cell responses is specific to HIV-positive donor cells.
The Vpu-specific IFN-γ-expressing lymphocytes in subject 26 had most of the characteristics of NK cells; however, only approximately 50% of the HIV-specific IFN-γ-expressing autologous NK cells expressed CD56. Down-regulation of CD56 on NK cells is commonly observed in HIV-infected subjects, so we therefore assessed CD56 expression on donor HIV-negative cells incubated with plasma IgG from HIV-infected subject 26.
The Vpu-specific IFN-γ-expressing cells from an HIV-negative donor were virtually all CD56 positive (Fig. 5A and B). This was confirmed in additional HIV-negative donors (data not shown). However, when plasma from subject 26 was incubated with whole blood from a second HIV-positive person (subject 8), again only approximately 50% of Vpu-specific IFN-γ-expressing cells were CD56 positive (Fig. 5C), as previously demonstrated in subject 26 (Fig. 3B). We also studied another key NK surface marker, CD16, which is the Fc receptor. Responder cells were exclusively CD16 negative (Fig. 5D). This may be a result of the receptor's being occupied by IgG and preventing antibody binding, for example, by immune complexes (31).
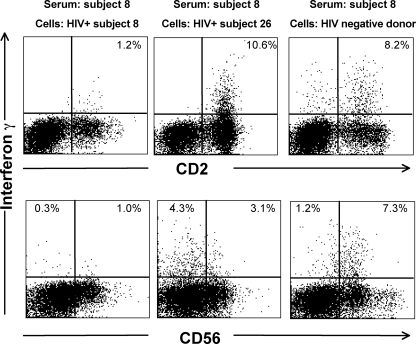
We confirmed that the CD56 downregulation was specific for HIV-positive donor cells by incubating serum of a separate HIV-positive subject with either autologous cells or cells from another HIV-positive donor and a HIV-negative donor. Serum from subject 8 (with progressive HIV infection), who had a Pol peptide-specific response of 1.2% on fresh blood using autologous cells, induced a significantly larger response in CD3-negative lymphocytes from both another HIV-infected donor (subject 26, an LTSP) and an HIV-negative donor (Fig. 6).
FIG. 6.
Serum from an additional HIV-positive subject mediates non-T-lymphocyte IFN-γ expression from multiple cell donors. Serum from HIV-positive subject 8, who responded to the Pol1 peptide pool, was incubated with either autologous PBMCs, with PBMCs from another HIV-positive subject (subject 26), or with PBMCs from an HIV-negative donor. Intracellular IFN-γ expression was examined in CD3-negative lymphocytes stained for either CD2 (top plots) or CD56 (bottom plots).
NK cell-specific response liberates granzyme B and perforin.
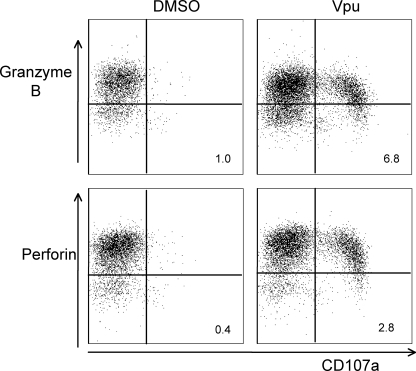
A characteristic of ADCC responses mediated by NK cells is their capacity to kill target cells expressing the relevant antigen. A surrogate marker of the lytic capacity of cells readily analyzed by flow cytometry is the expression of the degranulation marker CD107a (LAMP-1) and the loss of intracellular granzymes and perforin. We therefore stained HIV-negative donor cells stimulated with serum from subject 26 and the Vpu epitope for expression of these cytolytic markers (Fig. 7). As expected, unstimulated CD3− CD2+ CD56+ NK lymphocytes had low levels of CD107a expression and high levels of intracellular granzyme B and perforin. Vpu stimulation of NK cells resulted in high levels of CD107a staining, and the cells with the highest levels of CD107a surface expression lost significant intracellular amounts of both granzyme B and perforin.
FIG. 7.
Cytolytic markers of NK cells in response to Vpu peptide. Serum from subject 26 was incubated with either control dimethyl sulfoxide or the HIV-1 Vpu peptide 19 epitope and HIV-negative donor PBMCs for 6 h. NK cells (CD3− CD2+ CD56+ gated lymphocytes) were examined for surface expression of the degranulation marker CD107a and intracellular expression of the cytolytic effector molecules granzyme B and perforin. There was significant CD107a expression in response to Vpu, and the cells with highest expression of CD107a also lost intracellular granzyme B and perforin. Percentages of CD107a+ granzyme B-negative and CD107a+ perforin-negative cells are shown in the lower-right quadrants.
DISCUSSION
An ICS assay on whole blood detects non-T-lymphocyte peptide-specific cellular immune responses in HIV-infected subjects. The responder cells are CD3− CD4− CD8−, express IFN-γ and TNF-α in response to linear amino acid sequences in vitro, and express surface CD2 and CD56 and intracellular CD3ɛ-subunit. This HIV-specific immune response is dependent on the presence of autologous IgG. The peptide-specific IFN-γ-expressing cells have the characteristics of NK cells. The cells express the degranulation marker CD107a and release the cytolytic molecules granzyme B and perforin upon antigen stimulation. We postulate that this assay detects HIV-specific NK cell-mediated ADCC activity.
This simple ICS assay on whole blood to detect and map antibody-dependent, HIV-specific NK cell recognition responses using overlapping peptides is a quantum advance over previous methods to analyze ADCC immunity. No separate radioactive or fluorescently labeled target cells need to be prepared for functional killing assays (10, 14). Unlike assays using whole proteins, ADCC responses detected by utilizing overlapping peptides are readily mapped using individual peptides, potentially rapidly identifying useful vaccine epitopes. The fraction of NK lymphocytes expressing IFN-γ in response to HIV proteins was remarkably large, frequently exceeding the fraction (and total number) of T lymphocytes expressing IFN-γ in response to HIV proteins.
The variable expression of CD56 on NK cells from HIV-infected subjects is consistent with other reports showing that NK cells in viremic individuals frequently lose some CD56 expression (21). This was confirmed multiple times and by using cells from HIV-infected donors. The cytolytic activity of NK cells from viremic HIV-infected subjects is reduced compared with aviremic HIV-positive individuals or healthy hosts (21). Importantly, we demonstrated that in HIV-negative volunteers, the peptide-specific IFN-γ-expressing cells repeatedly all expressed CD56.
Several additional studies are suggested by this work. Much of our initial data characterizing these responses focused on one finely mapped Vpu-specific response. Preliminary data suggest identical characteristics from multiple, mapped Env-specific ADCC responses (data not shown). The cytotoxic function of the IFN-γ-expressing NK cells also remains to be formally tested. The NK cells express the degranulation marker CD107a and release granzyme B and perforin in the presence of serum and the relevant epitope, strongly suggesting that the cells have cytotoxic potential. The titers of ADCC antibodies that trigger IFN-γ expression from NK cells also requires further study; our initial experiments suggest that titers of >1:20 are detected (Fig. 5). A highly variable proportion of autologous NK cells is triggered to express IFN-γ in response to IgG in serum in this assay; the reasons for this are not clear but likely reflect dysfunctional autologous NK cells (17, 20). Interestingly, when serum from subject 26 (an LTSP) with a robust Vpu-specific response was incubated with PBMCs from subject 8 (with progressive HIV-1 infection and a CD4 T-cell count of 110 cells/μl), a much smaller proportion of NK cells expressed IFN-γ (Fig. 5C). The reverse occurred when subject 8's serum (which responded to Pol peptides) was used to stimulate cells from subject 26 or an uninfected donor (Fig. 6). Our results suggest that NK cell dysfunction during progressive HIV-1 infection adversely affects the ability to perform effector functions in the presence of ADCC antibodies. Although the assay we describe is essentially measuring at least two components of the ADCC response (the presence/titer of the antibody and the function of NK cells), both are required in vivo, and this assay may have more in vivo relevance than assaying only the one component required for the response. Future studies using this assay could probe the levels of dysfunction of NK cells in HIV-infected subjects.
Importantly, our data do not define the utility of NK/ADCC activity in controlling HIV infection. NK cell-mediated ADCC responses were commonly detected using this ICS methodology, with half of the cohort of subjects with antiretroviral-resistant HIV-1 also displaying such immune responses. We are currently enrolling and longitudinally following HIV-infected cohorts to determine the role of NK cell-mediated ADCC activity in delaying progressive HIV disease. The role of ongoing viremia in maintaining antigen-specific NK cell-mediated ADCC responses also remains to be defined. Interestingly, three of the eight aviremic subjects in our cohort had peptide-specific NK-mediated ADCC responses detected, including one subject (subject 2) who had undetectable plasma HIV RNA for 11 months prior to enrolment in the study.
A striking feature of these putative ADCC/NK-cell responses is how commonly they target the Env protein (Fig. 1 and Table 1). Env has thus far been a disappointing target for HIV vaccines due to its large conformational instability, hypervariable regions, and difficult antibody access to the conserved regions (6, 32). Identifying short linear targets in the envelope protein for vigorous ADCC will facilitate the study of this potentially important immune response.
Linear CD8 T-cell epitopes are subject to mutational escape and loss of T-cell recognition (24). Current work is defining the evolution of mutations that escape ADCC responses. We are comparing ADCC recognition of native amino acid sequences in HIV-infected individuals to consensus sequence peptides. The simplicity of the assay and ready generation of mutant peptides greatly facilitate these studies.
It remains to be shown how the immune responses we have described in this report recognize the peptide antigens used in this study, i.e., whether additional antigen-presenting cells are required and what processing and cellular presentation are required. The recognition of Env, Pol, and Vpu antigens, but not Gag or Nef, by the NK cell-mediated ADCC response is curious. Surface expression of Env is clearly well described, and there is increasing evidence that the Vpu associates with the cell membrane and with MHC-II molecules, as recently reported (12). Interesting cell trafficking and surface expression of HIV proteins and vaccine targets may be revealed by further study of ADCC responses using this technology. Low levels of surface expression of whole HIV proteins or their degradation products may be sufficient to generate such antibody responses over the long course of HIV infection.
In summary, we describe a novel ICS-based method for defining NK cell-mediated HIV-specific antibody-dependent responses that are likely detecting ADCC responses. Further work will better define the phenotypes and functional capacities of the responding cells and their in vivo relevance in a larger cohort. This could lead to attempts to induce such responses and evaluate their in vivo efficacy.
Acknowledgments
We thank A. Brooks, J. McCluskey, A. Winterhalter, R. Ffrench, E. Rollman, and R. De Rose for helpful advice. We thank the subjects who generously provided blood samples and the referral clinic staff of the Melbourne Sexual Health Clinic.
Footnotes
Published ahead of print on 19 March 2008.
REFERENCES
- 1.Ahmad, A., and R. Ahmad. 2003. HIV's evasion of host's NK cell response and novel ways of its countering and boosting anti-HIV immunity. Curr. HIV Res. 1295-307. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad, R., S. T. Sindhu, E. Toma, R. Morisset, J. Vincelette, J. Menezes, and A. Ahmad. 2001. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J. Clin. Immunol. 21227-233. [DOI] [PubMed] [Google Scholar]
- 3.Alsmadi, O., R. Herz, E. Murphy, A. Pinter, and S. A. Tilley. 1997. A novel antibody-dependent cellular cytotoxicity epitope in gp120 is identified by two monoclonal antibodies isolated from a long-term survivor of human immunodeficiency virus type 1 infection. J. Virol. 71925-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks, N. D., N. Kinsey, J. Clements, and J. E. Hildreth. 2002. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res. Hum Retrovir. 181197-1205. [DOI] [PubMed] [Google Scholar]
- 5.Bour, S., and K. Strebel. 2003. The HIV-1 Vpu protein: a multifunctional enhancer of viral particle release. Microbes Infect. 51029-1039. [DOI] [PubMed] [Google Scholar]
- 6.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5233-236. [DOI] [PubMed] [Google Scholar]
- 7.Douek, D. C., P. D. Kwong, and G. J. Nabel. 2006. The rational design of an AIDS vaccine. Cell 124677-681. [DOI] [PubMed] [Google Scholar]
- 8.Fauci, A. S., D. Mavilio, and S. Kottilil. 2005. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat. Rev. Immunol. 5835-843. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Roman, V. R., R. H. Florese, L. J. Patterson, B. Peng, D. Venzon, K. Aldrich, and M. Robert-Guroff. 2006. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J. Immunol. Methods 30853-67. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Roman, V. R., L. J. Patterson, D. Venzon, D. Liewehr, K. Aldrich, R. Florese, and M. Robert-Guroff. 2005. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J. Immunol. 1742185-2189. [DOI] [PubMed] [Google Scholar]
- 11.Hout, D. R., E. R. Mulcahy, E. Pacyniak, L. M. Gomez, M. L. Gomez, and E. B. Stephens. 2004. Vpu: a multifunctional protein that enhances the pathogenesis of human immunodeficiency virus type 1. Curr. HIV Res. 2255-270. [DOI] [PubMed] [Google Scholar]
- 12.Hussain, A., C. Wesley, M. Khalid, A. Chaudhry, and S. Jameel. 2008. Human immunodeficiency virus type 1 Vpu protein interacts with CD74 and modulates major histocompatibility complex class II presentation. J. Virol. 82893-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karnasuta, C., R. M. Paris, J. H. Cox, S. Nitayaphan, P. Pitisuttithum, P. Thongcharoen, A. E. Brown, S. Gurunathan, J. Tartaglia, W. L. Heyward, J. G. McNeil, D. L. Birx, and M. S. de Souza. 2005. Antibody-dependent cell-mediated cytotoxic responses in participants enrolled in a phase I/II ALVAC-HIV/AIDSVAX B/E prime-boost HIV-1 vaccine trial in Thailand. Vaccine 232522-2529. [DOI] [PubMed] [Google Scholar]
- 14.Kim, J. S., P. Nag, A. L. Landay, M. Alves, M. H. Cohn, J. W. Bremer, and L. L. Baum. 2006. Saliva can mediate HIV-1-specific antibody-dependent cell-mediated cytotoxicity. FEMS Immunol. Med. Microbiol. 48267-273. [DOI] [PubMed] [Google Scholar]
- 15.Kottilil, S., T. W. Chun, S. Moir, S. Liu, M. McLaughlin, C. W. Hallahan, F. Maldarelli, L. Corey, and A. S. Fauci. 2003. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J. Infect. Dis. 1871038-1045. [DOI] [PubMed] [Google Scholar]
- 16.Kottilil, S., K. Shin, J. O. Jackson, K. N. Reitano, M. A. O'Shea, J. Yang, C. W. Hallahan, R. Lempicki, J. Arthos, and A. S. Fauci. 2006. Innate immune dysfunction in HIV infection: effect of HIV envelope-NK cell interactions. J. Immunol. 1761107-1114. [DOI] [PubMed] [Google Scholar]
- 17.Kottilil, S., K. Shin, M. Planta, M. McLaughlin, C. W. Hallahan, M. Ghany, T. W. Chun, M. C. Sneller, and A. S. Fauci. 2004. Expression of chemokine and inhibitory receptors on natural killer cells: effect of immune activation and HIV viremia. J. Infect. Dis. 1891193-1198. [DOI] [PubMed] [Google Scholar]
- 18.Maecker, H. T., H. S. Dunn, M. A. Suni, E. Khatamzas, C. J. Pitcher, T. Bunde, N. Persaud, W. Trigona, T. M. Fu, E. Sinclair, B. M. Bredt, J. M. McCune, V. C. Maino, F. Kern, and L. J. Picker. 2001. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J. Immunol. Methods 25527-40. [DOI] [PubMed] [Google Scholar]
- 19.Martin, M. P., X. Gao, J. H. Lee, G. W. Nelson, R. Detels, J. J. Goedert, S. Buchbinder, K. Hoots, D. Vlahov, J. Trowsdale, M. Wilson, S. J. O'Brien, and M. Carrington. 2002. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat. Genet. 31429-434. [DOI] [PubMed] [Google Scholar]
- 20.Mavilio, D., J. Benjamin, M. Daucher, G. Lombardo, S. Kottilil, M. A. Planta, E. Marcenaro, C. Bottino, L. Moretta, A. Moretta, and A. S. Fauci. 2003. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc. Natl. Acad. Sci. USA 10015011-15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mavilio, D., G. Lombardo, J. Benjamin, D. Kim, D. Follman, E. Marcenaro, M. A. O'Shea, A. Kinter, C. Kovacs, A. Moretta, and A. S. Fauci. 2005. Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc. Natl. Acad. Sci. USA 1022886-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mavilio, D., G. Lombardo, A. Kinter, M. Fogli, A. La Sala, S. Ortolano, A. Farschi, D. Follmann, R. Gregg, C. Kovacs, E. Marcenaro, D. Pende, A. Moretta, and A. S. Fauci. 2006. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J. Exp. Med. 2032339-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nag, P., J. Kim, V. Sapiega, A. L. Landay, J. W. Bremer, J. Mestecky, P. Reichelderfer, A. Kovacs, J. Cohn, B. Weiser, and L. L. Baum. 2004. Women with cervicovaginal antibody-dependent cell-mediated cytotoxicity have lower genital HIV-1 RNA loads. J. Infect. Dis. 1901970-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips, R. E., S. Rowland-Jones, D. F. Nixon, F. M. Gotch, J. P. Edwards, A. O. Ogunlesi, J. G. Elvin, J. A. Rothbard, C. R. Bangham, C. R. Rizza, et al. 1991. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 354453-459. [DOI] [PubMed] [Google Scholar]
- 25.Renard, V., L. Ardouin, M. Malissen, G. Milon, M. Lebastard, A. Gillet, B. Malissen, and E. Vivier. 1995. Normal development and function of natural killer cells in CD3 epsilon delta 5/delta 5 mutant mice. Proc. Natl. Acad. Sci. USA 927545-7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rollman, E., M. Z. Smith, A. G. Brooks, D. F. Purcell, B. Zuber, I. A. Ramshaw, and S. J. Kent. 2007. Killing kinetics of simian immunodeficiency virus-specific CD8+ T cells: implications for HIV vaccine strategies. J. Immunol. 1794571-4579. [DOI] [PubMed] [Google Scholar]
- 27.Salmeron, A., F. Sanchez-Madrid, M. A. Ursa, M. Fresno, and B. Alarcon. 1991. A conformational epitope expressed upon association of CD3-epsilon with either CD3-delta or CD3-gamma is the main target for recognition by anti-CD3 monoclonal antibodies. J. Immunol. 1473047-3052. [PubMed] [Google Scholar]
- 28.Scott-Algara, D., L. X. Truong, P. Versmisse, A. David, T. T. Luong, N. V. Nguyen, I. Theodorou, F. Barre-Sinoussi, and G. Pancino. 2003. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J. Immunol. 1715663-5667. [DOI] [PubMed] [Google Scholar]
- 29.Stratov, I., C. J. Dale, S. Chea, J. McCluskey, and S. J. Kent. 2005. Induction of T-cell immunity to antiretroviral drug-resistant human immunodeficiency virus type 1. J. Virol. 797728-7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Dongen, J. J., G. W. Krissansen, I. L. Wolvers-Tettero, W. M. Comans-Bitter, H. J. Adriaansen, H. Hooijkaas, E. R. van Wering, and C. Terhorst. 1988. Cytoplasmic expression of the CD3 antigen as a diagnostic marker for immature T-cell malignancies. Blood 71603-612. [PubMed] [Google Scholar]
- 31.Wei, Q., J. W. Stallworth, P. J. Vance, J. A. Hoxie, and P. N. Fultz. 2006. Simian immunodeficiency virus (SIV)/immunoglobulin G immune complexes in SIV-infected macaques block detection of CD16 but not cytolytic activity of natural killer cells. Clin. Vaccine Immunol. 13768-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422307-312. [DOI] [PubMed] [Google Scholar]
- 33.Yamada, T., N. Watanabe, T. Nakamura, and A. Iwamoto. 2004. Antibody-dependent cellular cytotoxicity via humoral immune epitope of Nef protein expressed on cell surface. J. Immunol. 1722401-2406. [DOI] [PubMed] [Google Scholar]