Abstract
Hypertension is a side effect of systemically administered glucocorticoids, but the underlying molecular mechanism remains poorly understood. Ingestion of dexamethasone by rats telemetrically instrumented increased blood pressure progressively over 7 days. Plasma concentrations of Na+ and K+ and urinary Na+ and K+ excretion remained constant, excluding a mineralocorticoid-mediated mechanism. Plasma NO2−/NO3− (the oxidation products of NO) decreased to 40%, and the expression of endothelial NO synthase (NOS III) was found down-regulated in the aorta and several other tissues of glucocorticoid-treated rats. The vasodilator response of resistance arterioles was tested by intravital microscopy in the mouse dorsal skinfold chamber model. Dexamethasone treatment significantly attenuated the relaxation to the endothelium-dependent vasodilator acetylcholine, but not to the endothelium-independent vasodilator S-nitroso-N-acetyl-d,l-penicillamine. Incubation of human umbilical vein endothelial cells, EA.hy 926 cells, or bovine aortic endothelial cells with several glucocorticoids reduced NOS III mRNA and protein expression to 60–70% of control, an effect that was prevented by the glucocorticoid receptor antagonist mifepristone. Glucocorticoids decreased NOS III mRNA stability and reduced the activity of the human NOS III promoter (3.5 kilobases) to ≈70% by decreasing the binding activity of the essential transcription factor GATA. The expressional down-regulation of endothelial NOS III may contribute to the hypertension caused by glucocorticoids.
Keywords: dexamethasone, dihydrocortisol, RNase protection assay, Western blot, Reporter gene assay
Endothelium-derived NO is a physiologically significant vasodilator. Pharmacological inhibition of the endothelial NO synthase (NOS III) increases blood pressure (1), and mice lacking the NOS III gene are hypertensive (2). Endothelial NO is also an inhibitor of platelet aggregation and adhesion (3, 4) and can prevent leukocyte adhesion to the endothelium by down-regulating the leukocyte adhesion glycoprotein complex CD11/CD18 (5). Finally, NO also has been shown to inhibit DNA synthesis, mitogenesis, and proliferation of vascular smooth muscle cells (6, 7). Therefore, endothelial NO is likely to represent a protective antiatherogenic principle (8), and up- or down-regulation of NOS III could have important consequences for vascular homeostasis (9).
Hypertension with diverse sequelae such as increased atherosclerosis, stroke, cerebral hemorrhage, and hypertensive cardiomyopathy is the major cardiovascular side effect of systemically administered glucocorticoids (10, 11). The mechanism underlying this hypertension remains poorly understood (11). Although cortisol-induced hypertension is characterized by Na+ retention and volume expansion, studies with synthetic glucocorticoids such as dexamethasone suggest that the hypertension is, to a substantial degree, independent of mineralocorticoid effects (11). There is evidence for the presence of glucocorticoid receptors on endothelial cells (12) and vascular smooth muscle cells (13). Therefore, actions of glucocorticoids on the vasculature are conceivable. Indeed, effects of glucocorticoids on vascular resistance have been demonstrated and have been explained in part by an increased response of the vasculature to catecholamines and angiotensin II (10, 11).
We now demonstrate in vitro and ex vivo that glucocorticoids down-regulate endothelial NOS III expression by decreasing the stability of NOS III mRNA and reducing NOS III gene transcription. The resulting reduction in endothelial NO production could contribute to the increase in blood pressure seen with glucocorticoid therapy.
Methods
Reagents.
Actinomycin D, dexamethasone, NG-nitro-l-arginine methylester (L-NAME) and S-nitroso-N-acetyl-d,l-penicillamine (SNAP), goat anti-rabbit antibody conjugated to alkaline phosphatase, horse anti-mouse antibody conjugated to alkaline phosphatase, and mouse monoclonal antibody to β-tubulin were obtained from Sigma. DMEM and Ham’s F-12 nutrient mixture were from Life Technologies (Paisley, Scotland). Restriction enzymes, random hexamer primers, SureClone Ligation Kit, Taq DNA polymerase, Taq polymerase reaction buffer and T7Sequencing Kit were from Amersham Pharmacia. Custom oligonucleotides were from MWG Biotech (Heidelberg, Germany). 3-Isobutyl-1-methylxanthine and superoxide dismutase were from Boehringer Ingelheim. pCR-Script and NucTrap probe purification columns were from Stratagene. DNase I, RNase A, RNase T1, proteinase K, T3/T7 RNA polymerase, and nitrate reductase were from Roche Diagnostics. Luciferase assay system with reporter lysis buffer was from Promega. [α-32P]UTP was from ICN. Mifepristone (RU 38486) was a generous gift of Roussel-UCLAF. Rabbit polyclonal antibody to NOS III was from Transduction Laboratories (Lexington, KY).
Telemetric Measurement of Blood Pressure and Heart Rate in Rats.
Male Wistar-Kyoto rats (from Charles River Breeding Laboratories, Sulzfeld, Germany) were housed singly under controlled environmental conditions (23 ± 1°C, light:dark, 12:12 h, light on at 7:00 a.m.). Food (regular rat chow) and water were always available. Food and water intake were measured continuously with a PC-controlled monitoring system (Feeding/Drinking Monitor, Technical & Scientific Equipment, Bad Homburg, Germany). This device records hydrostatic pressure in the water bottle and the weight of the food container. Systolic and diastolic blood pressures were measured telemetrically in freely moving animals by using implanted transmitters as described (14). Monitoring of cardiovascular parameters started 1 week after implantation of the pressure transmitters. Measurements were taken every 5 min by using the DataQuest System (Datasciences, St. Paul) and were averaged over 24 h by using dq-fit software (15). Based on the average water intake of the rats (≈100 ml/kg of body weight/day; determined before the experiment), dexamethasone was added to the drinking water at a concentration of 3 mg/liter to reach a target dose of 0.3 mg/kg/day, and L-NAME was added at 100 mg/liter to reach a target dose of 10 mg/kg/day.
Determination of NO2−/NO3− in Rat Serum.
Serum NO2−/NO3− was determined as NO2− after enzymatic reduction with NO3− reductase (16). NO2− was determined by chemiluminescence after chemical reduction to NO by using a NOA 270B Nitric Oxide Analyzer (Sievers, Boulder, CO). Rats were fasted 24 h before blood and urine collection to minimize the influence of dietary NO2−/NO3−.
Intravital Microscopy of Resistance Arterioles.
Titanium observation chambers were implanted into the dorsal skinfold of xylazin/ketamine-anesthetized C57/BL 6J mice (10 weeks old) to allow for intravital microscopy of a fine striated skin muscle. Five days after implantation, awake animals were placed under the microscope, the coverslip of the chamber was removed, and the tissue was superfused with Krebs solution (composition in mM: NaCl 120.0, KCl 4.7, KH2PO4 1.2, CaCl2·2H2O 2.5, MgSO4·7H2O 1.2, NaHCO3 25.0, glucose 11.0; pH 7.4, 34°C) for an equilibration period of 30 min. The diameters of 1–2 resistance arterioles (diameter 30–60 μm) were measured in each chamber by using an adobe photoshop (Adobe Systems, Mountain View, CA)-based image analysis at baseline and after continuous superfusion with acetylcholine (10 μM) or SNAP (10 μM) according to previously published protocols (17). Experiments were performed in untreated control mice (n = 7) and mice receiving 0.3 mg/kg/day dexamethasone in their drinking water for 1 week before the experiment.
Cell Culture.
Human umbilical vein endothelial cells, the human umbilical vein endothelial cell-derived permanent cell line EA.hy 926 (18), and bovine aortic endothelial cells (19) were grown in DMEM with 10% fetal bovine serum, 2 mM l-glutamine, penicillin, and streptomycin. The three cell types were incubated for 18–36 h with dexamethasone (10 nM to 1 μM), dihydrocortisol (100 nM to 10 μM), or beclomethasone (10 nM to 1 μM), or were left untreated (control). Then, RNA or protein was extracted or the NOS activity of the intact cells determined (see below). In experiments with the glucocorticoid receptor antagonist mifepristone (1 to 10 μM), the antagonist was added 30 min before dexamethasone.
RNase Protection Assay of NOS III mRNA.
Total RNA was isolated from the human or bovine endothelial cells and from several rat tissues by guanidinium thiocyanate/phenol/chloroform extraction (20). Radiolabeled antisense RNA probes for human NOS III and human β-actin were prepared by in vitro transcription of the cDNA clones pCR-NOS III-Hu (21) and pCR-β-actin-Hu (22) as described (21, 23). Bovine and rat cDNAs were generated by reverse transcription of mRNA from bovine aortic endothelial cells and rat aorta, respectively. Bovine or rat NOS III cDNA fragments were amplified by PCR using the same oligonucleotide primers: 5′-GACATTGAGAGCAAAGGGCTGC-3′ (sense) and 5′-CGGCTTGTCACCTCCTGG-3′ (antisense). Bovine β-actin and rat γ-actin cDNA fragments were amplified by PCR using the following oligonucleotide primers: 5′-ACCAACTGGGACGACATGGAG-3′ (sense) and 5′-CGTGAGGATCTTCATGAGGTAGTC-3′ (antisense) (24). The cDNA fragments (NOS III, 425 bp; γ-actin, 353 bp) were cloned into the EcoRV site of vector pCR-Script (generating the cDNA clones pCR-NOS III-rat and pCR-γ-actin-rat) and sequenced (T7Sequencing Kit). A portion (0.5 μg) of each DNA was in vitro transcribed by using T7/T3 RNA polymerase and [α-32P]UTP. The template DNA was degraded with DNase I, and the radiolabeled RNA was purified by using NucTrap probe purification columns. RNase protection assays were performed as described (21, 23) with a mixture of RNase A and RNase T1. Densitometric analyses of the RNase protection gels were performed by using a Phospho-Imager (Bio-Rad). The density of each NOS III band was normalized with the corresponding β-actin or γ-actin band.
NOS III Protein Preparation and Western Blotting.
Protein isolation and Western blotting were done as described (21, 25). In brief, a CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate) extract was prepared from the different types of endothelial cells and was separated by sodium dodecylsulfate polyacrylamide gel electrophoresis (7.5% gels). The proteins were transferred to nitrocellulose membranes (Schleicher & Schüll) by electroblotting (Bio-Rad). Proteins were detected by using a polyclonal anti-NOS III antibody (1:500) and a monoclonal anti-β-tubulin antibody (1:500). After an incubation with the appropriate secondary antibodies conjugated to alkaline phosphatase as described (25), densitometric analyses were performed with a Video-Imager (Bio-Rad). NOS III protein bands were normalized by using the respective β-tubulin protein bands (21).
Determination of NOS III Activity.
NOS III activity was bioassayed by using the stimulation of soluble guanylyl cyclase in RFL-6 rat lung fibroblasts as described (26). Dexamethasone-pretreated endothelial cells or control cells were incubated for 2 min at 37°C in 1 ml of Locke’s solution containing 0.3 mM 3-isobutyl-1-methylxanthine and 20 units/ml superoxide dismutase. Then, the conditioned media were transferred to the RFL-6 cells. After a 2-min incubation at 37°C on the RFL-6 cells, the reaction was stopped with 1 ml of ice-cold 50 mM sodium acetate buffer (pH 4.0) and by rapidly freezing the cells with liquid nitrogen. The cGMP content of the RFL-6 samples was determined by radioimmunoassay as described (26).
Reporter Gene Assay Using the 5′-Flanking Region of the Human NOS III Gene Stably Transfected into EA.hy 926 Cells.
EA.hy 926 cells stably transfected with the plasmid pNOS III-Hu-3500-Luc-neo have been described (23). This plasmid contains 3.5 kilobases of the human NOS III promoter sequence (position −3,470 to +115) cloned before the luciferase reporter gene (23). Extracts of dexamethasone-treated cells and normal control cells were prepared by using the reporter lysis buffer, and luciferase activity was determined by using the luciferase assay system as described (21, 23).
Electrophoretic Mobility Shift Assays.
Binding activities of transcription factors Sp1 and GATA in nuclear extracts from dexamethasone-treated or untreated cells were determined by electrophoretic mobility shift assays as described (21). Ten micrograms of nuclear protein were incubated with 17.5 fmol 32P-labeled double-stranded oligonucleotide containing a consensus Sp1-binding motif (5′-ATTCGATCGGGGCGGGGCGAGC-3′, Promega), the Sp1 element (positions −109 to −91) of the human NOS III promoter (5′-GGATAGGGGCGGGGCGAGG-3′) (27), a consensus GATA-binding motif (5′-CACTTGATAACAGAAAGTGATAACTCT-3′, Santa Cruz Biotechnology), or the GATA site (positions −239 to −218) of the human NOS III promoter (5′-GCTCCCACTTATCAGCCTCAGT-3′) (27). Specificity of binding was determined by adding excess (1.75 pmol) unlabeled oligonucleotide. Gels were autoradiographed on x-ray film.
Statistics.
Data represent means ± SEM. Statistical differences were determined by Wilcoxon signed rank test or by factorial analysis of variance followed by Fisher’s protected least-significant-difference (PLSD) test for comparison of multiple means.
Results
Ingestion of Dexamethasone Increased Blood Pressure and Decreased Serum NO2−/NO3− in Rats.
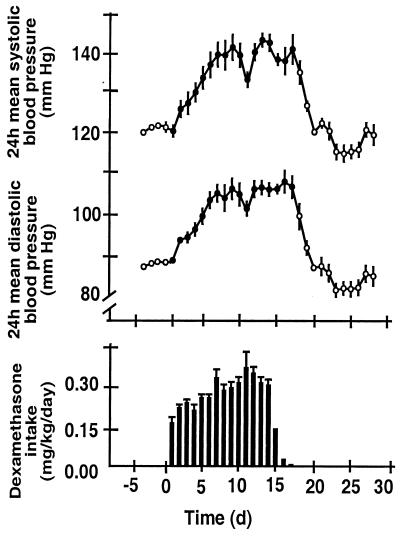
Untreated rats were normotensive with 24-h average blood pressures of 121.3 ± 1.1/88.1 ± 0.9 mmHg (systolic/diastolic). When the dexamethasone treatment was started, the water intake of the rats decreased initially; the target dose of 0.3 mg/kg/day was reached after ≈5 days (Fig. 1). Dexamethasone treatment resulted in an increase in blood pressure that reached a plateau after 1 week of treatment (Fig. 1). When dexamethasone treatment was discontinued [by tapering off the daily dose over 3 days (Fig. 1)], blood pressure returned to normal levels (Fig. 1). Na+ and K+ concentrations in rat plasma were 148 ± 3.8 mM and 6.6 ± 0.43 mM, respectively (n = 6). They remained unchanged after dexamethasone (145 ± 4.4 and 7.35 ± 0.39 mM, respectively). Control 24-h excretion of Na+ and K+ was 0.75 ± 0.07 and 1.39 ± 0.10 mmol/day, respectively (n = 6). After dexamethasone treatment, it was 0.82 ± 0.06 and 1.18 ± 0.13 mmol/day, respectively. However, a 15-day administration of dexamethasone (≈0.3 mg/kg/day) significantly reduced serum NO2−/NO3− levels from 17.4 ± 1.7 to 6.9 ± 0.7 μM (n = 5, P < 0.001). NO2−/NO3− levels returned to normal (17.1 ± 1.4 μM) after 7 days of cessation of dexamethasone treatment. A moderate dose (10 mg/kg/day) of the NOS inhibitor L-NAME, given for 7 days, produced a very similar increase in blood pressure as dexamethasone (from 125.2 ± 0.9/87.6 ± 0.6 mmHg, systolic/diastolic, to 144.2 ± 0.9/98.1 ± 0.8 mmHg). Interestingly, the reduction in plasma NO2−/NO3− produced by this dose of L-NAME was virtually the same as that produced by dexamethasone (NO2−/NO3− levels decreased from 17.4 ± 1.7 to 9.5 ± 1.6 μM; n = 5, P < 0.001).
Figure 1.
Effect of dexamethasone in the drinking water (3 mg/liter) on systolic and diastolic blood pressure of rats (Upper). Dexamethasone intake was monitored continuously with a PC-controlled monitoring system. The exact dose of dexamethasone ingested by the rats in 24 h is shown in the lower panel. Systolic and diastolic blood pressures were measured telemetrically before (○), during (●), and after (○) dexamethasone treatment. Values and bars represent means ± SEM obtained from five rats.
Dexamethasone Treatment Decreased NOS III mRNA Levels in the Aorta and Other Tissues.
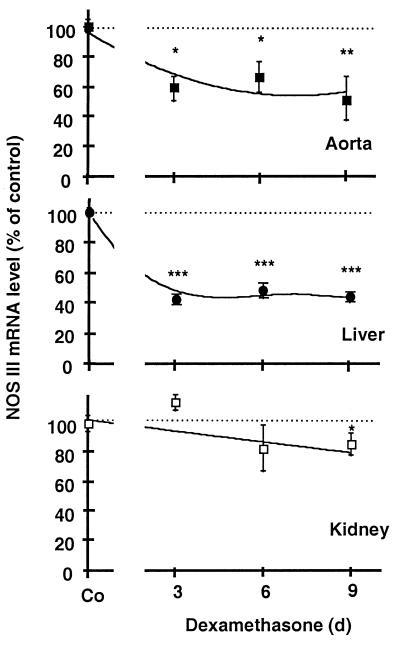
When rats were treated with dexamethasone (0.3 mg/kg/day) as described above and killed on days 3, 6, and 9 of treatment, NOS III mRNA levels in the aorta were found to be reduced by up to 40% (Fig. 2). The dexamethasone treatment also reduced NOS III mRNA levels in the liver and the kidney (Fig. 2) but not in the heart (n = 5, data not shown).
Figure 2.
Effect of treatment of male rats with dexamethasone on NOS III mRNA expression in different tissues. Rats remained untreated [control (Co)] or were treated for 3, 6, and 9 days with dexamethasone (3 mg/liter in the drinking water, resulting in an approximate dose of 0.3 mg/kg/day). The dexamethasone treatment induced a significant decrease in NOS III mRNA expression in aorta (■) and liver (●) and a moderate decrease in kidney (□). The figure shows densitometric analyses of five independent experiments. Bars represent means ± SEM. Asterisks indicate significant differences from untreated rats (*, P < 0.05; **, P < 0.01; ***, P < 0.001 by ANOVA and Fisher’s PLSD test).
Dexamethasone Treatment Selectively Attenuated Endothelium-Dependent Vasodilatation in Resistance Arterioles.
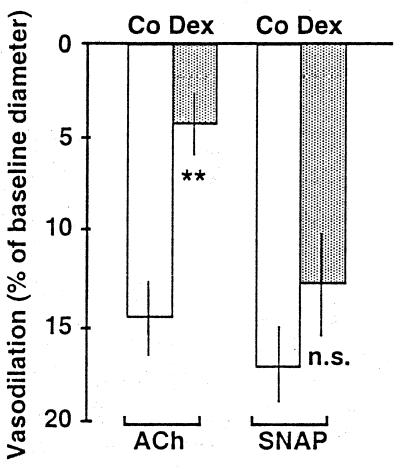
Dexamethasone treatment (≈0.3 mg/kg/day) of C57/BL 6J mice significantly attenuated the vasorelaxation in response to the endothelium-dependent vasodilator acetylcholine (10 μM; Fig. 3). The same dexamethasone treatment had no significant effect on the vasorelaxation of resistance arterioles to superfusion with 10 μM SNAP (Fig. 3).
Figure 3.
Dexamethasone treatment (0.3 mg/kg/day) significantly attenuated endothelium-dependent relaxation of resistance arterioles as assessed by intravital microscopy in awake C57/BL 6J mice. Shown is the vasorelaxation of resistance arterioles as percent of baseline microvessel diameter. The mean baseline diameters were 37.9 ± 6.5 μm in control animals (Co, white bars) and 45.0 ± 9.8 μm in dexamethasone-treated animals (Dex, gray bars). Depicted in the figure are changes in the responses to acetylcholine (ACh, 10 μM) and SNAP (10 μM). Data represent means ± SEM of n = 7 animals per group. Asterisks indicate significant differences from control animals (**, P < 0.01, Wilcoxon rank sum test) n.s., not significant.
Glucocorticoids Decreased NOS III mRNA Levels in Three Types of Endothelial Cells.
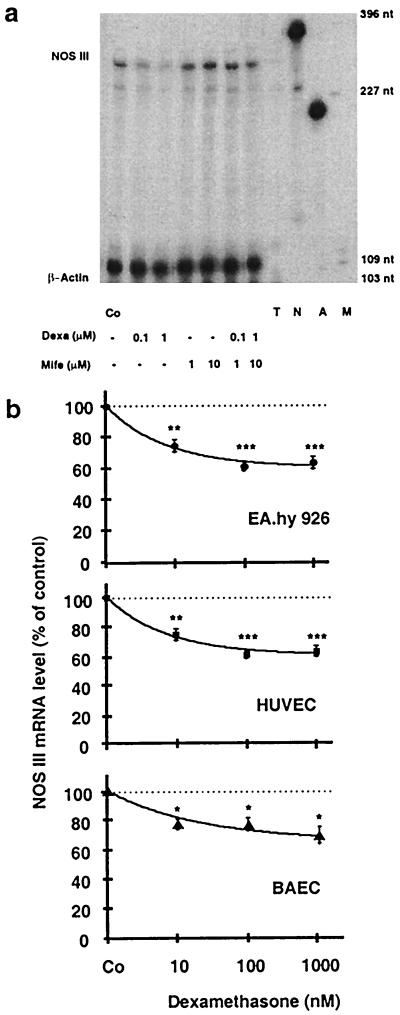
When EA.hy 926 cells, human umbilical vein endothelial cells, or bovine aortic endothelial cells were treated with dexamethasone (10 nM to 1 μM for 18 h), NOS III mRNA decreased to 60–70% of control (Fig. 4). Also, beclomethasone (10 nM to 1 μM) and dihydrocortisol (0.1 to 100 μM) reduced NOS III mRNA expression in EA.hy 926 cells to a similar extent (n = 3 each, data not shown). The inhibition of NOS III mRNA expression by dexamethasone was prevented by the glucocorticoid receptor antagonist mifepristone (Fig. 4a). The antagonist alone (1 to 10 μM) had no effect on NOS III mRNA expression (Fig. 4a).
Figure 4.
Down-regulation of NOS III mRNA by dexamethasone and reversal of the effect by the glucocorticoid receptor antagonist mifepristone (Mife) in endothelial cells. a shows an autoradiograph of a representative gel of an RNase protection experiment. RNase protection analyses were performed by using antisense RNA probes to human NOS III and β-actin (for standardization) and RNA from EA.hy 926 cells incubated with 0.1 or 1 μM dexamethasone, 1 or 10 μM mifepristone, or a combination of both. T, tRNA control; N, undigested NOS III antisense probe; A, undigested β-actin antisense probe; M, molecular size marker (pGl2-Basic, restricted with HinfI). The gel is representative of three similar experiments. b shows the down-regulation of NOS III mRNA in three types of endothelial cells: EA.hy 926 cells (●), human umbilical vein endothelial cells (HUVEC, ■), and bovine aortic endothelial cells (BAEC, ▴, using bovine antisense RNA probes). The cells either were left untreated [control cells (Co)] or were incubated with dexamethasone (10 nM to 1 μM) for 18 h. Values represent means ± SEM. Asterisks indicate significant differences from untreated cells (*, P < 0.05; **, P < 0.01; ***, P < 0.001 by ANOVA and Fisher’s PLSD test).
Dexamethasone Treatment Reduced NOS III Protein and NO Production in Endothelial Cells.
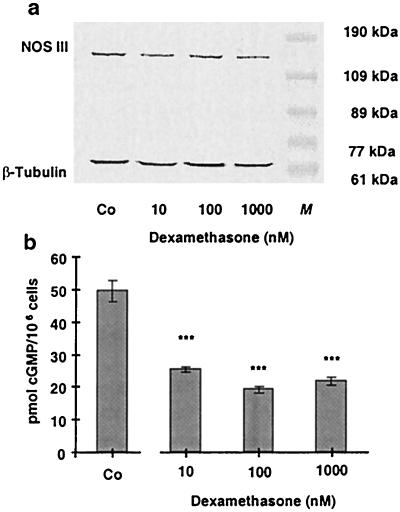
Incubation of EA.hy 926 cells with dexamethasone (10, 100, and 1,000 nM) for 36 h reduced NOS III protein expression to 79.8 ± 4.5, 74.2 ± 3.1, and 68.5 ± 4.1% of control (n = 4; Fig. 5a). Also, NO production by EA.hy 926 cells was reduced after treatment of the cells with dexamethasone for 36 h (Fig. 5b) or with 10 μM dihydrocortisol for 36 h (reduction to 72.6 ± 4.3% of control, n = 3, data not shown).
Figure 5.
Effect of an incubation of EA.hy 926 cells with dexamethasone for 36 h on NOS III protein expression and NOS activity. a shows a Western blot analysis using a polyclonal anti-NOS III- and a monoclonal anti-β-tubulin-antibody (for normalization). Combined cytosolic and solubilized particulate protein fractions were prepared from untreated EA.hy 926 cells [control (Co)] and EA.hy 926 cells incubated with dexamethasone (10–1,000 nmol) for 36 h. M, molecular weight marker. The blot shown is representative of four independent experiments with similar results. b shows the effect of a 36-h incubation with dexamethasone on the NO production of EA.hy 926 cells stimulated for 2 min with Ca2+ ionophore A23187 (10 μM). NO production was measured by the activation of soluble guanylyl cyclase in RFL-6 reporter cells. cGMP content of the RFL-6 cells after a 2-min incubation was determined by radioimmunoassay. Basal cGMP content of the RFL-6 cells (2.12 ± 0.4 pmol/106 cells) was subtracted from all samples. Data represent means ± SEM of three independent experiments. Asterisks indicate significant differences from untreated cells (***, P < 0.001 by ANOVA and Fisher’s PLSD test).
Dexamethasone Treatment Destabilized the NOS III mRNA and Inhibited the Activity of the NOS III Promoter.
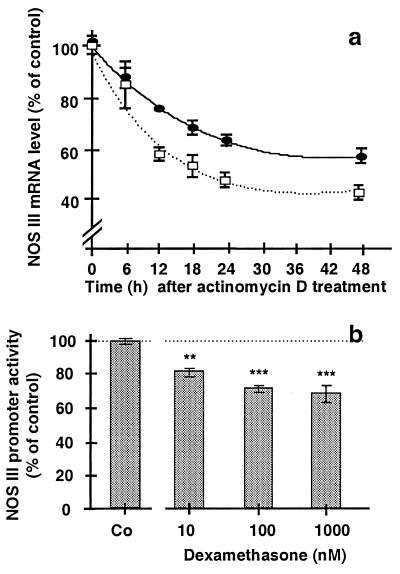
When transcription was blocked with 10 μg/ml actinomycin D added to the incubation medium of EA.hy 926 cells and RNA was prepared from these cells 6 to 48h later, NOS III mRNA levels were found to decline over time (Fig. 6a). The approximate half-life of the mRNA was 48 h. Treatment of EA.hy 926 cells with dexamethasone (100 nM, 18 h) reduced the stability of the NOS III mRNA (Fig. 6a). EA.hy 926 cells stably transfected with the plasmid pNOS III-Hu-3500-Luc-neo showed significant NOS III promoter activity. When the transfected cells were incubated for 18 h with 10–1,000 nM dexamethasone, promoter activity decreased to ≈70% of control (Fig. 6b).
Figure 6.
Effect of dexamethasone on NOS III mRNA stability and NOS III promoter activity in EA.hy 926 cells. In a, EA.hy 926 cells were preincubated without (●) or with 100 nM dexamethasone (□) for 18 h. Then, the inhibitor of transcription actinomycin D (10 μg/ml) was added to the culture medium. RNA was prepared 0, 12, 18, 24, and 48 h thereafter, and NOS III mRNA was determined by RNase protection assays. The NOS III mRNA levels in both groups were set at 100% at the time of addition of actinomycin D (0 h). Values are means ± SEM of three independent experiments. b shows EA.hy 926 cells stably transfected with pNOS III-3500-Hu-Luc-neo (containing a 3.5-kilobase NOS III promoter fragment cloned before a luciferase reporter gene). Stable cells either were kept untreated [control (Co)] or were incubated with dexamethasone (10–1,000 nM) for 24 h. Then, the cells were lysed, and luciferase activity was determined. The luciferase activity was taken as a measure of NOS III promoter activity. Bars represent means ± SEM of three independent experiments. Asterisks indicate significant differences from untreated cells (**, P < 0.01; ***, P < 0.001 by ANOVA and Fisher’s PLSD test).
Effect of Dexamethasone on the Binding of Nuclear Extracts to Transcription Factor-Binding DNA Motifs.
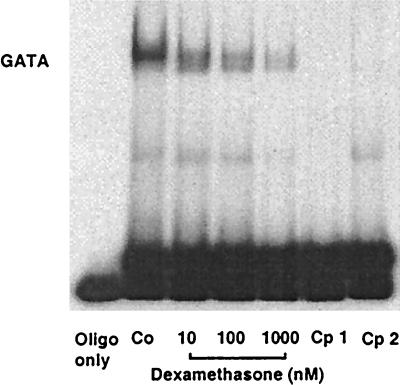
The known sequence of the human NOS III promoter (GenBank accession no. AF032908) contains no bona fide glucocorticoid response element. Therefore, we tested the effect of dexamethasone on the binding activity of other transcription factors known to be important for the activity of the human NOS III promoter, namely Sp1 and GATA (27–29). As shown in Fig. 7, dexamethasone treatment of EA.hy 926 cells reduced the binding activities of nuclear extracts to an oligonucleotide containing the GATA site of the human NOS III promoter (positions −239 to −218) in a concentration-dependent fashion (n = 4). The same results were obtained with an oligonucleotide containing a consensus GATA binding motif (n = 5, data not shown). On the other hand, nuclear extracts from dexamethasone-treated endothelial cells showed unchanged binding activity to oligonucleotides containing the Sp1 element of the human NOS III promoter (positions −109 to −91, n = 4, data not shown) or a consensus Sp1-binding motif (n = 4, data not shown).
Figure 7.
Electrophoretic mobility shift assay showing the effect of dexamethasone on the binding activity of nuclear extracts from EA.hy 926 cells to a double-stranded oligonucleotide containing the binding motif for transcription factor GATA of the human NOS III promoter (positions −239 to −218; 5′-GCTCCCACTTATCAGCCTCAGT-3′). Nuclear extracts were prepared from control cells (Co) or from cells incubated for 18 h with dexamethasone (10 nM to 1 μM) and were incubated with the radiolabeled oligonucleotide. Samples were analyzed on native polyacrylamide gels. Lane 1 shows radiolabeled oligonucleotide alone (oligo only). Lanes 5 and 6 (Cp1 and Cp2) show competition experiments with extracts from control (Co) cells and the addition of 100-fold excess of unlabeled oligonucleotide 5′-CACTTACAGAAAGTGATAACTCT-3′ (containing a consensus GATA binding motif), or unlabeled oligonucleotide 5′-GCTCCCACTTATCAGCCTCAGT-3′ (containing the GATA site of the human NOS III promoter), respectively. The gel shown is representative of four gels yielding the same results.
Discussion
Because NOS III is a vasoprotective enzyme (8), changes in its expression and/or activity may have important consequences for vascular homeostasis. The NO produced by NOS III actively decreases vascular resistance, as demonstrated with NOS III inhibitors and mice lacking the NOS III gene (2). Cushing’s syndrome is associated with hypertension, and, also, synthetic glucocorticoids—when administered for prolonged periods of time—are known to increase blood pressure (11). Although cortisol-induced hypertension is characterized by Na+ retention and volume expansion, studies with synthetic glucocorticoids or Na+ restriction suggest that the hypertension is, to a substantial degree, independent of Na+ and volume (11). Also, in the current study, the blood pressure-elevating effects of dexamethasone are unlikely to be attributable to marked Na+ and water retention because no change in plasma or urine Na+ or K+ concentrations were noted. Evidence for separate mechanisms of glucocorticoid- and mineralocorticoid-induced hypertension comes from experiments with Wistar-Furth rats. This strain does not develop hypertension when treated with salt and mineralocorticoids. Wistar-Kyoto rats developed hypertension when treated with either deoxycorticosterone acetate/NaCl or dexamethasone whereas Wistar-Furth rats developed hypertension only after dexamethasone treatment (30).
Increase in cardiac output is not essential for the glucocorticoid-induced rise in blood pressure, but the precise role of increases in peripheral resistance as a primary mechanism has not been determined. Increased pressor responsiveness, particularly to catecholamines and angiotensin II, is a prominent feature, but its mechanism is poorly understood (10, 11). In vitro studies have shown that the number of angiotensin II type 1 receptors of vascular smooth muscle cells is increased by glucocorticoids (11). Also, a reduced activity of depressor systems, such as the kallikrein-kinin system, vasodilator prostaglandins and NO, has been discussed (11).
In the current study, we have demonstrated that glucocorticoids lead to a substantial down-regulation of endothelial NOS mRNA and protein. This has been shown in vitro in three types of cultured endothelial cells from two species (Figs. 4 and 5) and in the aorta and some other rat tissues ex vivo (Fig. 2). The down-regulation was prominent in the liver, which is a highly vascularized organ that contains a large number of endothelial cells. The finding that NOS III mRNA expression was not reduced in the heart may be explained by the fact that, in this organ, most of the NOS III is present in cardiomyocytes (31), where its expression may be regulated differently from endothelial cells. The decreased protein expression of NOS III resulted in a reduced NO production, as demonstrated by a smaller stimulation of soluble guanylyl cyclase in RFL-6 cells in vitro (Fig. 5b) and lower serum levels of NO2−/NO3− in rats in vivo. This was paralleled by an increase in blood pressure. A similar increase in blood pressure was achieved with a moderate dose of the NOS inhibitor L-NAME, which produced a similar reduction in plasma NO2−/NO3− concentrations. The pathophysiological relevance of the dexamethasone effect also was demonstrated in vivo by intravital microscopy, in which resistance arterioles of dexamethasone-treated mice exhibited significantly attenuated vasorelaxation in response to the endothelium-dependent vasodilator acetylcholine but not to the endothelium-independent vasodilator SNAP (Fig. 3).
The molecular mechanism of NOS III down-regulation by glucocorticoids seems to involve both mRNA destabilization (Fig. 6a) and reduced transcription of the NOS III gene (Fig. 6b). The glucocorticoid receptor is a ligand-activated transcription factor, but the known 3.5 kilobases of the human NOS III promoter sequence lacks bona fide glucocorticoid response elements. Therefore, electrophoretic mobility shift assays were performed with oligonucleotides that contained consensus binding sequences for transcription factors Sp1 and GATA, whose importance for NOS III promoter activity have been demonstrated (27, 29). In these experiments, dexamethasone reduced only the GATA binding activities of nuclear extracts of EA.hy 926 cells in a concentration-dependent fashion to almost undetectable levels (Fig. 7). Sp1 binding remained unchanged. A mutation of the GATA binding site at position −230 of the human NOS III promoter has been shown to reduce promoter activity to ≈70% of control (27). This corresponds well to the loss in promoter activity seen after dexamethasone treatment of endothelial cells (Fig. 6b).
There is precedent for negative interaction of the activated glucocorticoid receptor with other transcription factors. For example, the inhibition of expression of the collagen gene by dexamethasone is brought about by a direct interaction of the glucocorticoid receptor with the AP-1 components c-JUN and c-FOS (32). Touray et al. demonstrated that activated glucocorticoid receptors can form stable complexes with c-JUN or c-FOS in intact cells (33). Also, for the inhibition by glucocorticoids of the IL-1α-induced IL-8 expression in human T98G glioblastoma cells, the activated glucocorticoid receptor interferes with the binding of the essential transcription factor NF-κB to its cis-element, thereby suppressing transcription of the IL-8 gene (34). A similar mechanism is likely to be operative in the suppression of the inducible NOS II in human A549/8 cells (22). Thus, it seems conceivable that, in endothelial cells, the activated glucocorticoid receptor prevents the binding of transcription factor GATA to its cognate cis-element on the NOS III promoter.
In conclusion, we have demonstrated that dexamethasone and other glucocorticoids down-regulate the expression of the endothelial NOS III to 60–70% of control. This may contribute to the blood pressure increases seen during systemic administration of glucocorticoids.
Acknowledgments
This study was supported by the Collaborative Research Center SFB 553, Project A1 (to U.F.) from the Deutsche Forschungsgemeinschaft (Bonn).
Abbreviations
- L-NAME
NG-nitro-l-arginine methylester
- NOS III
endothelial-type nitric oxide synthase
- SNAP
S-nitroso-N-acetyl-d,l-penicillamine
- PLSD test
protected least-significant-difference test
References
- 1.Rees D D, Palmer R M, Moncada S. Proc Natl Acad Sci USA. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang P L, Huang Z H, Mashimo H, Bloch K D, Moskowitz M A, Bevan J A, Fishman M C. Nature (London) 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 3.Alheid U, Frölich J C, Förstermann U. Thromb Res. 1987;47:561–571. doi: 10.1016/0049-3848(87)90361-6. [DOI] [PubMed] [Google Scholar]
- 4.Radomski M W, Palmer R M, Moncada S. Biochem Biophys Res Commun. 1987;148:1482–1489. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- 5.Arndt H, Smith C W, Granger D N. Hypertension. 1993;21:667–673. doi: 10.1161/01.hyp.21.5.667. [DOI] [PubMed] [Google Scholar]
- 6.Garg U C, Hassid A. J Clin Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakaki T, Nakayama M, Kato R. Eur J Pharmacol. 1990;189:347–353. doi: 10.1016/0922-4106(90)90031-r. [DOI] [PubMed] [Google Scholar]
- 8.Förstermann U, Closs E I, Pollock J S, Nakane M, Schwarz P, Gath I, Kleinert H. Hypertension. 1994;23:1121–1131. doi: 10.1161/01.hyp.23.6.1121. [DOI] [PubMed] [Google Scholar]
- 9.Förstermann U, Boissel J-P, Kleinert H. FASEB J. 1998;12:773–790. [PubMed] [Google Scholar]
- 10.Walker B R, Edwards C R. Br Med Bull. 1994;50:342–355. doi: 10.1093/oxfordjournals.bmb.a072895. [DOI] [PubMed] [Google Scholar]
- 11.Saruta T. Hypertens Res. 1996;19:1–8. doi: 10.1291/hypres.19.1. [DOI] [PubMed] [Google Scholar]
- 12.Ray K P, Farrow S, Daly M, Talabot F, Searle N. Biochem J. 1997;328:707–715. doi: 10.1042/bj3280707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Provencher P H, Saltis J, Funder J W. J Steroid Biochem Mol Biol. 1995;52:219–225. doi: 10.1016/0960-0760(94)00168-l. [DOI] [PubMed] [Google Scholar]
- 14.Lemmer B, Witte K, Makabe T, Ganten D, Mattes A. J Cardiovasc Pharmacol. 1994;23:311–314. [PubMed] [Google Scholar]
- 15.Witte K, Zuther P, Lemmer B. Chronobiol Int. 1997;14:561–574. doi: 10.3109/07420529709001447. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt H H H W, Warner T D, Nakane M, Förstermann U, Murad F. Mol Pharmacol. 1992;41:615–624. [PubMed] [Google Scholar]
- 17.de Wit C, Esser N, Lehr H A, Bolz S S, Pohl U. Am J Physiol. 1999;276:H1527–H1534. doi: 10.1152/ajpheart.1999.276.5.H1527. [DOI] [PubMed] [Google Scholar]
- 18.Edgell C J, McDonald C C, Graham J B. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Förstermann U, Pollock J S, Schmidt H H, Heller M, Murad F. Proc Natl Acad Sci USA. 1991;88:1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 21.Kleinert H, Wallerath T, Euchenhofer C, Ihrig-Biedert I, Li H, Förstermann U. Hypertension. 1998;31:582–588. doi: 10.1161/01.hyp.31.2.582. [DOI] [PubMed] [Google Scholar]
- 22.Kleinert H, Euchenhofer C, Ihrig-Biedert I, Förstermann U. Mol Pharmacol. 1996;49:15–21. [PubMed] [Google Scholar]
- 23.Li H, Oehrlein S A, Wallerath T, Ihrig-Biedert I, Wohlfart P, Ulshöfer T, Jessen T, Herget T, Förstermann U, Kleinert H. Mol Pharmacol. 1998;53:630–637. doi: 10.1124/mol.53.4.630. [DOI] [PubMed] [Google Scholar]
- 24.Brown C W, McHugh K M, Lessard J L. Nucleic Acids Res. 1990;18:5312. doi: 10.1093/nar/18.17.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallerath T, Gath I, Aulitzky W E, Pollock J S, Kleinert H, Förstermann U. Thromb Haemostasis. 1997;77:163–167. [PubMed] [Google Scholar]
- 26.Ishii K, Sheng H, Warner T D, Förstermann U, Murad F. Am J Physiol. 1991;261:H598–H603. doi: 10.1152/ajpheart.1991.261.2.H598. [DOI] [PubMed] [Google Scholar]
- 27.Zhang R, Min W, Sessa W C. J Biol Chem. 1995;270:15320–15326. doi: 10.1074/jbc.270.25.15320. [DOI] [PubMed] [Google Scholar]
- 28.Wariishi S, Miyahara K, Toda K, Ogoshi S, Doi Y, Ohnishi S, Mitsui Y, Yui Y, Kawai C, Shizuta Y. Biochem Biophys Res Commun. 1995;216:729–735. doi: 10.1006/bbrc.1995.2682. [DOI] [PubMed] [Google Scholar]
- 29.Tang J L, Zembowicz A, Xu X M, Wu K K. Biochem Biophys Res Commun. 1995;213:673–680. doi: 10.1006/bbrc.1995.2184. [DOI] [PubMed] [Google Scholar]
- 30.Ullian M E, Islam M M, Robinson C J, Fitzgibbon W R, Tobin E T, Paul R V. Am J Physiol. 1997;272:H1454–H1461. doi: 10.1152/ajpheart.1997.272.3.H1454. [DOI] [PubMed] [Google Scholar]
- 31.Balligand J L, Kobzik L, Han X Q, Kaye D M, Belhassen L, Ohara D S, Kelly R A, Smith T W, Michel T. J Biol Chem. 1995;270:14582–14586. doi: 10.1074/jbc.270.24.14582. [DOI] [PubMed] [Google Scholar]
- 32.Pfahl M. Endocr Rev. 1993;14:651–658. doi: 10.1210/edrv-14-5-651. [DOI] [PubMed] [Google Scholar]
- 33.Touray M, Ryan F, Jaggi R, Martin F. Oncogene. 1991;6:1227–1234. [PubMed] [Google Scholar]
- 34.Mukaida N, Morita M, Ishikawa Y, Rice N, Okamoto S, Kasahara T, Matsushima K. J Biol Chem. 1994;269:13289–13295. [PubMed] [Google Scholar]