Abstract
We addressed the role of plasmacytoid dendritic cells (PDC) in protection against AIDS in nonpathogenic simian immunodeficiency virus (SIVagm) infection in African green monkeys (AGMs). PDC were monitored in blood and lymph nodes (LNs) starting from day 1 postinfection. We observed significant declines in blood during acute infection. However, PDC then returned to normal levels, and chronically infected AGMs showed no decrease of PDC in blood. There was a significant increase of PDC in LNs during acute infection. Blood PDC displayed only weak alpha interferon (IFN-α) responses to TLR9 agonist stimulation before infection. However, during acute infection, both blood and LN PDC showed a transiently increased propensity for IFN-α production. Bioactive IFN-α was detected in plasma concomitant with the peak of viremia, though levels were only low to moderate in some animals. Plasma interleukin 6 (IL-6) and IL-12 were not increased. In conclusion, PDC were recruited to the LNs and displayed increased IFN-α production during acute infection. However, increases in IFN-α were transient. Together with the lack of inflammatory cytokine responses, these events might play an important role in the low level of T-cell activation which is associated with protection against AIDS in nonpathogenic SIVagm infection.
During primary and chronic human immunodeficiency virus type 1 (HIV-1) infection, both subsets of dendritic cells (DC), i.e., myeloid dendritic cells (MDC) and plasmacytoid dendritic cells (PDC), are decreased in the blood (20, 36, 46). The capacity of PDC to produce IFN-α is impaired in acute and chronic HIV-1 infection (13, 23, 29). Long-term nonprogressors display higher numbers of PDC and a higher capacity for their PDC to produce IFN-α than progressors (46). Early profound and persistent depletions of PDC have also been observed in macaques infected with the macaque strain of simian immunodeficiency virus (SIVmac) (4, 40). Different mechanisms have been proposed to explain DC declines, including cell death and homing to lymph nodes (LNs) (4, 32, 40, 50).
Here, we investigated the dynamics and function of PDC in blood and LNs during a nonpathogenic infection, i.e., SIVagm infection in African green monkeys (AGMs). AGMs, like other African nonhuman primates, such as mandrills and mangabeys, are natural hosts for SIV and generally do not progress to AIDS despite displaying high levels of plasma and intestinal viral load (VL) (21). Natural hosts for SIV display low levels of T-cell activation, in contrast to HIV-infected humans and SIVmac-infected macaques (6). Exacerbated chronic T-cell activation might drive CD4 T-cell depletion and AIDS (19, 22). An immunologic activation set point is established early after HIV-1 infection, and this set point is predictive of the rate at which CD4+ T cells are lost over time (11, 49). Innate immune responses acting at the early time points are crucial for T-cell activation profiles. In the present study, we studied whether PDC are recruited to LNs in response to SIVagm and analyzed early cytokine profiles, including alpha interferon (IFN-α) production by blood and LN PDC.
MATERIALS AND METHODS
Animals and infections.
AGMs of the species Chlorocebus sabaeus were housed at Institut Pasteur in Dakar (Senegal) according to institutional and ethical guidelines. The study included 12 noninfected AGMs (00017, 00021, 02001, 02004, 02010, 02015, 02024, 02026, 03005, 03006, 03007, and Thyaliss), 8 naturally infected AGMs (89046, 92017, 93035, 00015, 00018, 01016, 02017, and 03002), and 9 AGMs experimentally infected with SIVagm.sab92018, of which three were studied exclusively in the chronic phase (96030, 97005, and 98011) and the other six were monitored prospectively. The latter were between 2 and 5 years old and consisted of three females (97008, 00020, and 01013) and three males (98013, 01015, and 02003). Three of these AGMs (97008, 98013, and 00020) were sacrificed at day 120 postinfection (p.i.). The inoculum, VLs, and CD4 cell counts of the SIVagm.sab92018-infected monkeys were published previously (26).
SIVagm.sab plasma VL.
Plasma viral RNA was quantified using real-time PCR based on amplification of long terminal repeat RNA as previously described (26).
Preparation of cells.
Whole blood was collected in EDTA-K2 and heparinized Vacutainer (BD) tubes. Biopsies of peripheral LNs were performed by excision. After careful removal of adhering connective and fat tissues, the LN fragments were mechanically disrupted on a sterile nylon mesh and the cells treated with 20 IU/ml of collagenase type VII (Sigma) and 20 IU/ml of DNase I (Sigma). Peripheral blood mononuclear cells (PBMC) and LN mononuclear cells (LNMC) were isolated by Ficoll-Paque (Pharmacia Biotech AB) density gradient centrifugation.
Flow cytometry.
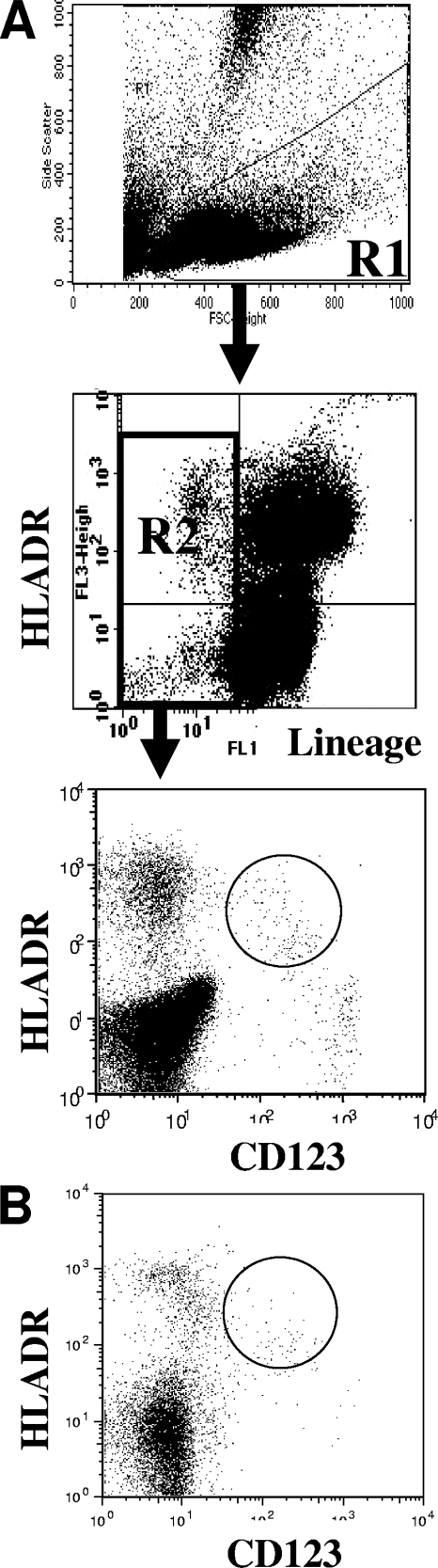
Flow cytometric analyses were performed on freshly isolated cells. CD4+ T cells were quantitated as previously described (26). For PDC quantification, cells were stained with the following monoclonal antibodies: fluorescein isothiocyanate-labeled anti-Lineage (Lin) panel: anti-CD3 (FN18; Biosource)/CD14 (Tük4; Miltenyi)/CD16 (3G8; BD)/CD20 (2H7, BD), peridin chlorophyll protein-labeled anti-HLA-DR (L243; BD), and anti-CD123 (7G3; BD). Cross-reactivity of the anti-human MAb had been validated on AGM monocyte-derived dendritic cells (33, 39). For each sample, 50,000 to 200,000 events were acquired. PDC were defined as Lin− HLA-DR+ CD123+ (Fig. 1) (20, 36).
FIG. 1.
Flow cytometric analysis of AGM PDC. PBMC or LNMC were selected in the R1 gate, excluding debris and polynuclear cells. Lin− cells (CD3− CD20− CD16− CD14−) were selected in the R2 gate. CD123+ HLA-DR+ events were quantified in R3 from R1 × R2 gated events. (A) Blood; (B) LN.
Quantification of bioactive IFN-α.
Plasma IFN-α titers were determined using a functional assay based on protection of Madin-Darby bovine kidney (MDBK) cells against the cytolytic effect of vesicular stomatitis virus (37). MDBK are more sensitive to IFN-α than to IFN-β and are not sensitive to the IFN-γ antiviral effect. The titers were expressed in international units based on the reference standard for human IFN-α (G-023-901-527; NIH, Bethesda, MD). This test measures all subtypes of human IFN-α (37). The detection limit of this assay corresponded to 4 IU/ml, which was 7.75 times lower than a multisubtype human IFN-α enzyme-linked immunosorbent assay kit formulated for serum or plasma (PBL Biomedical Laboratories), at least for AGM plasma IFN-α (data not shown).
In order to assess IFN-α production by blood and LN PDC, fresh mononuclear cells were exposed to herpes simplex virus type 1 (HSV-1) at a multiplicity of infection of 1 (37). Stimulation with HSV-1 generally occurs through TLR9, which is expressed by PDC. It has been shown that after stimulation of human or macaque PBMC with HSV-1, PDC are the major producers of IFN-α (8, 28). We verified that HSV-1 also induces IFN-α production by AGM PDC by flow cytometric intracellular staining (data not shown). After 20 h of culture in the presence of HSV-1, virus was UV inactivated, and IFN-α activity in the supernatants was quantified as described above.
Quantification of IL-6 and IL-12.
The cytokines IL-12 (p40+p70) and IL-6 in the plasma were measured by using monkey enzyme-linked immunosorbent assay kits (U-Cytech). The detection limits were 10 and 2.5 pg/ml for IL-12 and IL-6, respectively.
Statistical analysis.
Statistical analysis was done with one-way analysis of variance or with Kruskal-Wallis analysis of variance when assumptions of normality of the distribution or homogeneity of variances were not verified using R (http://www.R-project.org). The assumptions and the normality of the distribution and variance homogeneity were, respectively, tested with a Shapiro and a Bartlett test. Data were considered significant when P values were <0.05. For each data set, the procedure analysis was as follows. (i) Determine if the values before infection could be pooled by an analysis of homogeneity of the mean. If the means of different time points were not significantly different, all preinfection values could be pooled and constituted the baseline, the latter corresponding to the mean of all preinfection values. (ii) Determine if the values after infection display a significant variance by the methods described above. If the postinfection phase is not homogeneous (at least two time points have different means), determine for each day after infection if the mean is significantly different than the baseline (for each day after infection, H0: μbefore = μday; H1: μbefore ≠ μday) by a nonparametric Wilcoxon-Mann-Whitney test (or a Student t test when the data are normally distributed and when the variances were homogenous).
The Spearman rank test was used to assess the correlation between two continuous variables. Multiple regression was used to evaluate the effect of more than two independent continuous variables on one dependent continuous variable.
RESULTS
Blood PDC levels in uninfected AGMs.
Our goal was to study the dynamics and function of PDC in blood and LNs early after infection in a model of nonpathogenic SIV infection. PDC were quantified by the method used for human cells (20, 36). PDC numbers were first determined in the blood of 18 uninfected AGMs (Table 1). The mean number of blood PDC was 8,703/ml (median, 7,672), and the mean percentage among PBMC was 0.26% (median, 0.25). The levels were generally in the same range as those reported for naïve macaques (4, 9, 25, 40, 47) and healthy humans (7, 13, 14, 20, 46).
TABLE 1.
PDC levels in blood of AGMs
| AGMs (n) | Median PDC level (range)
|
|
|---|---|---|
| % | Cells/ml | |
| Noninfected (18) | 0.25 (0.07-0.9) | 7672 (2405-22464) |
| Chronically infected (14) | 0.18 (0.06-0.32) | 7704 (2232-15294) |
Early but transient declines of blood PDC during primary SIVagm infection.
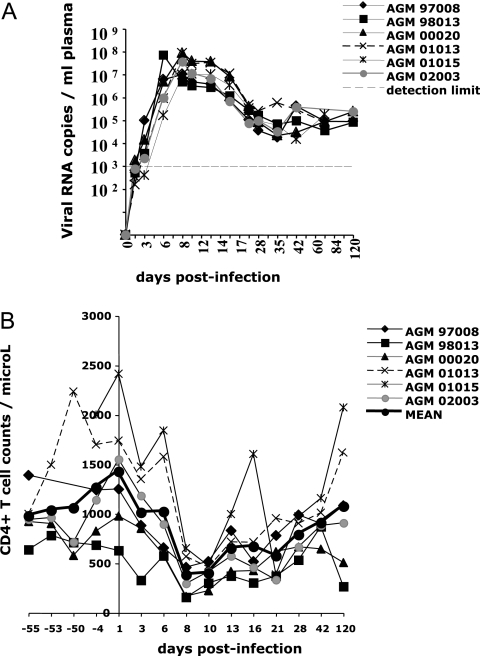
Six of the 18 AGMs described above were then randomly selected for a prospective study. They were infected with wild-type SIVagm.sab92018. As described in previous studies, plasma VLs peaked around day 8 p.i. (Fig. 2A) (26).
FIG. 2.
VL and T CD4+ cell dynamics. (A) Plasma VL in SIVagm-infected AGMs. (B) Blood CD4+ T cell counts before and after SIVagm infection.
Blood CD4+ T cells significantly declined between days 8 and 28 p.i. (P ≤ 0.007), as previously reported (26). Afterwards, they recovered and reached their preinfection baseline levels at day 120 p.i. (Fig. 2B).
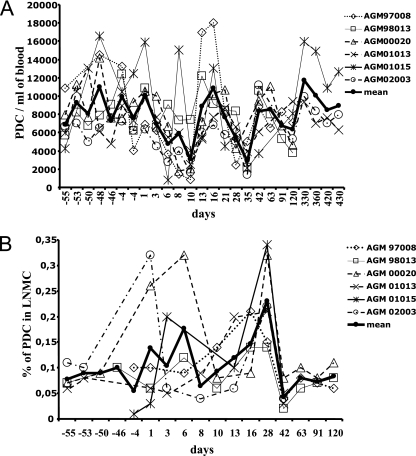
The mean percentage of PDC before infection in these six AGMs was 0.26% and is thus representative of the uninfected animals described above. In order to get a robust baseline, we carried out four to seven measurements for each animal before infection (Fig. 3A). In addition, in order to ensure that the sampling kinetics used did not affect homeostasis, for four of the six animals (00020, 01013, 02003, and 98013) we used exactly the same time intervals for blood collection before infection as between days 1 and 13 p.i.
FIG. 3.
PDC dynamics in blood and LNs of AGMs before and after SIVagm infection. (A) Absolute numbers of PDC in blood; (B) PDC percentages in LN. In order to study narrow intervals, LN were collected from three AGMs (97008, 98013, and 00020) at days 1, 3, 8, 13, 28, 42, 63, 91, and 120 p.i. and from three other AGMs (01013, 01015, and 02003) at days 1, 6, 10, 16, 28, and 42 p.i.
We then evaluated the absolute numbers of PDC in blood. Before infection, we observed large fluctuations in the PDC counts; however, the variance was not significant (P = 0.74). PDC counts significantly declined at days 6, 8, and 10 p.i. (P = 0.0002 at day 10 p.i.) and at weeks 4 and 5 p.i. (P = 0.02 and 0.0004, respectively) (Fig. 3A). The levels of PDC then progressively increased again to reach baseline levels starting from day 42 p.i. (P = 1). Similar results were observed with percentages of circulating PDC (data not shown).
We then evaluated PDC counts in the chronic phase of infection. Three of the six AGMs (01013, 01015, and 02003) were studied at 1 year p.i. (the other three AGMs were sacrificed at day 120 p.i.; see below), and we extended the analysis to 11 additional long-term SIVagm-infected AGMs. The latter consisted of three experimentally and eight naturally infected AGMs (1 to 14 years of infection; median, 4 years; mean, 6.1 years). We detected no significant difference in the frequency or the absolute numbers of PDC in a cross-sectional comparison of the 14 chronically SIVagm-infected AGMs to all noninfected AGMs (Table 1). There was no significant difference between the six experimentally and the eight naturally infected animals regarding the frequency and the absolute numbers of PDC in blood (data not shown).
There was no correlation between the absolute numbers of PDC with CD4+ T cells during the early phase of infection (days 1 to 42 p.i.). During the chronic phase of infection (days 63 to 430 p.i.), however, PDC were positively correlated with CD4+-T-cell counts (rho = 0.60, P = 0.003). By multiple regression analysis, PDC counts were predictive for CD4 T cell counts (P = 0.01).
There was no correlation between PDC counts and VL throughout the follow-up. Nevertheless, when only the first week p.i. was considered, PDC counts were negatively correlated with VL (rho = −0.55, P < 0.009).
Altogether, we observed early declines of PDC in blood. These were transient, and no depletion was detected in the chronic phase of SIVagm infection. PDC counts were negatively correlated with VL during the first week p.i. and positively correlated with CD4+ counts during the chronic phase of SIVagm infection.
Increased frequencies of PDC in LN during primary SIVagm infection.
We wondered whether the transient declines of circulating PDC during the early stages of SIVagm infection could be linked with migration to secondary lymphoid organs. We therefore longitudinally monitored PDC dynamics in the LNs. For each of the six AGMs, one LN biopsy was performed before infection. For four of the six animals (98013, 00020, 01013, and 02003), we performed a second biopsy in order to get the most robust possible preinfection intraindividual baseline (Fig. 3B). Before infection, the mean percentages of PDC among mononucleated cells in LNs of the six AGMs were 0.08% (range, 0.01 to 0.11%) (Fig. 3B). Such levels are consistent with some reports on rhesus macaques (30). The variance among the values before infection was not significant. We then determined LN PDC levels after SIVagm infection starting from day 1 p.i. The PDC showed a significant increase at day 28 p.i. (P = 0.001) (Fig. 3B). The increases at days 6 and 16 were not significant (P = 0.088); however, we sampled only three animals for these time points. These increased proportions were found to have a temporal association with decreases in blood counts. There was indeed a trend toward a significant negative correlation between LN and blood PDC (rho = −0.28, P = 0.09).
During the chronic phase of infection (days 42 to 120 p.i.), no significant increase of PDC was observed in LNs.
Because of the suggested potential important role of the gut in AIDS pathogenesis, we also evaluated DC frequencies in mesenteric LN for three animals (97008, 98013, and 00020) that were sacrificed at day 120 p.i. The mean percentages of PDC in mesenteric LN corresponded to 0.08% and were thus similar to those in peripheral LNs (data not shown).
Altogether, our longitudinal study revealed a significant increase of PDC in LN during primary SIVagm infection.
Increased IFN-α production during primary SIVagm infection.
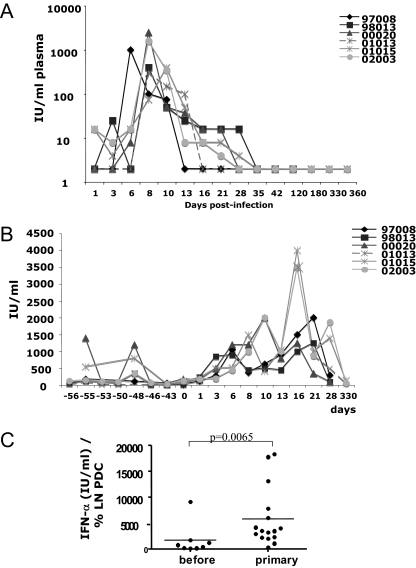
Since PDC were increased in LNs at early stages of SIVagm infection, we addressed the question of the functionality of these cells. One major function of PDC in the setting of a viral infection is the production of large amounts of IFN-α (44). We quantified the levels of bioactive IFN-α in the plasma of the six AGMs. The quantification method was the same as that previously used for humans and in the SIVmac251/rhesus macaque model (23, 24). Plasma IFN-α increased as early as day 1 p.i. in two of six AGMs (02003 and 01015) and was increased in all animals at days 8 and 10 p.i. (Fig. 4A). Plasma IFN-α reached levels of 2,500 IU/ml at day 8 p.i. The peak of IFN-α was coincident with the peak of VL. IFN-α titers then decreased, and from day 35 p.i. on, they remained below detection level in all animals. We analyzed whether plasma IFN-α levels correlate with VL during primary infection (day 1 to day 35 p.i.). There was a significant positive correlation (rho = 0.61, P < 0.0001).
FIG. 4.
Levels and production of bioactive IFN-α. (A) IFN-α levels in plasma. The detection level of the assay was 2 IU/ml. (B) IFN-α concentrations in supernatants of PBMC stimulated with HSV-1 in vitro. Cells were collected before and after infection from all six animals until day 120 p.i. For day 330 p.i., three of six animals were studied (01013, 01015, and 02003). (C) IFN-α production by LN PDC. LNMC were collected from the six longitudinally monitored AGMs at four time points before infection and at seven time points during primary infection. LNMC were stimulated with HSV-1 in vitro, and the concentrations of IFN-α in the supernatants were quantified. Data are ratios of the IFN-α concentration and the percentage of LN PDC before infection and during primary infection.
In order to address the question of whether PDC might contribute to the heightened IFN-α levels in plasma, we analyzed the capacity of AGM blood and LN PDC to produce IFN-α. We quantified IFN-α production after stimulation with a TLR9 agonist. In order to have a robust baseline, we measured eight time points before infection (Fig. 4B). Without stimulation, IFN-α levels were <6 IU/ml (data not shown). After stimulation, the median values ranged from 34 to 350 IU/ml. Two AGMs (00020 and 01015) displayed strong intraindividual variation and occasionally produced high levels (between 800 and 1,400 IU/ml) at two preinfection time points (Fig. 4B). Overall, stimulation with the TLR9 agonist resulted in significantly increased IFN-α production. However, the levels produced were four times lower than those of human controls (23).
After infection, median values of IFN-α produced by blood AGM PDC ranged from 200 to 1,750 IU/ml. Compared to preinfection levels, IFN-α production was significantly increased during primary SIVagm infection between days 3 and 21 p.i. (P ≤ 0.017 for each time point) (Fig. 4B). When days −55 and −48 were omitted from the baseline values, days 1 and 28 p.i. also showed significant increases (P ≤ 0.001). The highest levels of production (peak values up to 4,000 IU/ml) were observed around 1 week after the peak of plasma viremia and of plasma IFN-α. There was no correlation between PDC numbers and their IFN-α production capacity, but there was a significant positive correlation between VL and IFN-α production by PDC (rho = 0.57, P < 0.0001).
We then quantified the ability of LN PDC to produce IFN-α. The median values of IFN-production were 62.5 IU/ml before infection and 525 IU/ml after infection (data not shown). Again, cells collected during primary infection were more prone to produce IFN-α than those obtained before infection (P = 0.01). To investigate whether the increases in IFN-α production by LN PDC are due to their increases in percentages, we plotted the ratio of IFN-α production per PDC percentages in LN (Fig. 4C). The difference in the ratios between cells from before and during primary infection was again significant (P = 0.0065). There was no correlation between levels of PDC and IFN-α production (P = 0.6).
In summary, SIVagm-infected AGMs showed increased levels of IFN-α in plasma during primary infection. Moreover, both blood and LN PDC were capable of producing IFN-α in response to a TLR9 agonist in vitro, and the propensity for IFN-α production was significantly increased during primary SIVagm infection.
No detectable increase of IL-6 and IL-12 in plasma.
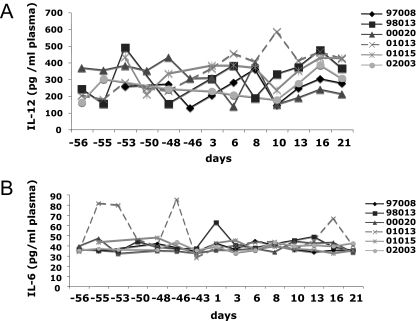
Like IFN-α, many other cytokines are secreted by antigen-presenting cells and involved in the early orientation of T-cell responses. We previously measured tumor necrosis factor alpha (TNF-α) and IL-10 in the plasma of these monkeys (26). Here, we also measured IL-12 and IL-6 because of their respective roles in Th1 induction and Treg inhibition (10, 41). Although there was a trend toward a significant change in plasma IL-12 at 16 p.i. (P = 0.052), overall the levels of plasma IL-12 did not increase during acute SIVagm infection (Fig. 5A). IL-6 in plasma from AGMs did not increase at any time point in response to SIVagm infection (Fig. 5B).
FIG. 5.
IL-6 and IL-12 plasma levels during primary SIVagm infection. (A) IL-12p40+p70 concentrations in plasma; (B) IL-6 concentrations in plasma.
DISCUSSION
We show here that AGMs display a decline of PDC in blood during primary SIVagm infection. We detected two nadirs. The possibility that such oscillations also occur in HIV-1 and SIVmac infections and might have been previously undetected because previous studies were not performed with such early and narrow intervals as this one can not been excluded. The blood PDC levels showed a trend toward a negative correlation with PDC levels in LNs. The PDC depletion in blood might thus be explained, at least in part, by recruitment to lymphoid organs. It is likely that PDC migrate to LN also during early HIV-1 infection, since the density of CD123+ cells in T-cell zones increases in LN from asymptomatic HIV-1-infected patients (16).
During chronic SIVagm infection, we did not observe any sign of PDC depletion. The restoration of PDC to normal levels after the acute phase of SIVagm infection is in sharp contrast with pathogenic HIV/SIVmac infections, where the levels are only partially restored after primary infection (7, 23, 40). During the chronic phase of SIVagm infection, PDC counts were positively correlated with CD4+ T cell counts. Thus, our study supports previous observations in humans suggesting that the degree of recovery of PDC after the primary infection has good prognostic value regarding disease outcome (7, 36, 46).
AGMs may maintain a better ability to generate DC precursors. In line with this, it was reported that SIV-infected sooty mangabeys exhibited preserved functions of bone marrow, at least regarding their T-lymphocyte-regenerative capacity, whereas macaques did not (45, 48). Another possibility is better survival. PDC are susceptible to HIV-1 and SIVmac infection in vivo (15, 40). Interestingly, though, viral replication in PDC occurs only following maturation as induced with CD40L or TNF-α, and cell death of HIV-infected human PDC occurs only when the PDC have contact with activated CD4+ cells (15, 43). Chronically infected AGMs display lower levels of activated lymphocytes and TNF-α than SIVmac-infected macaques (26), and DC might thus be less susceptible to death in AGMs. Finally, the depletions in HIV-1 infections may also be explained by continuous recruitment into lymphoid organs (2, 31).
The proinflammatory cytokines IL-6 and IL-12 were not increased in plasma. These data are in line with our previous results in AGMs, showing no or only weak increases of proinflammatory cytokine (TNF-α, IFN-γ, and MIP-1α) and Th1 transcription factor (t-bet) expressions (26, 38). In contrast, macaques generally show increases in proinflammatory cytokines such as IL-6 and TNF-α (1, 3, 17, 35). Plasma IFN-α levels were significantly increased during primary SIVagm infection and strongly correlated with VL, as is also the case in SIVmac infection (18, 24). However, the increases were very transient, and high levels (above 1,000 IU/ml) were detected at only one time point, which corresponded to the peak of viremia (day 8 p.i.). Moreover, in a recent study that we performed using the same quantification method, the peak IFN-α levels were generally higher in SIVmac-infected macaques than in AGMs (C. Butor, unpublished data). In line with this, PDC from noninfected AGMs produced lower levels of IFN-α after TLR9 stimulation than cells from humans (23).
During primary SIVagm infection, PDC were prone to produce IFN-α. The levels were significantly increased, the highest levels being observed after the peak of plasma IFN-α, which is most likely related to a positive feedback loop of IFN-α (28). The capacity of AGM PDC to produce IFN-α was not correlated with the PDC counts, similar to what has been reported for HIV-1, where a correlation was detected in chronic but not primary infection (36). In both cases, this might be explained either by the presence of other cellular sources of IFN-α or by variable IFN-α production among PDC cells. We do not know whether AGM PDC would also show increased IFN-α production after stimulation with SIV or TLR-7 agonists in vitro. However, plasma IFN-α levels are increased, and the strong correlation with the VL suggests a role for the virus in IFN-α induction in vivo.
TLR9 stimulated PDC are able to induce CD4+ and CD8+ Treg (34). Human MDC treated with IFN-α and IL-10 have been shown to become tolerogenic and able to induce regulatory CD4+ T cells in vitro (5, 27, 42). On the other hand, IL-6, which is increased in macaques but not in AGMs, can prevent CD4+ CD25+ Treg regulatory functions (12). It is thus possible that, due to the particular cytokine environments during early SIVagm infection, MDC do not migrate massively to LNs and/or that the DC are conditioned to preferentially induce regulatory T cells. In fact, our previous data suggested the presence of CD4+ and CD8+ regulatory T cells during SIVagm infection (26).
In summary, our study reveals declines in levels of PDC in blood during primary SIVagm infection. The declines are only transient, as normal levels are maintained afterwards. Conversely to blood, PDC frequencies were increased in AGM LNs during primary SIVagm infection. Blood and LN PDC showed a heightened propensity to produce IFN-α during primary infection. Plasma IFN-α levels were increased during primary infection, but the peak levels were often low and always transient. The preservation of functional PDC in the absence of strong proinflammatory responses might be at the origin of control of generalized T-cell activation and AIDS.
Acknowledgments
We thank M. Ndiaye and M. Touré for assistance with animal care. We are indebted to Anne Badel and Anne Camproux for their expert contributions in statistical analysis. We thank Lisa An for help with VL assays, Sandrine Kahi and Lene Vimeux for help with DC flow cytometry setups, Véronique Mayau for help with data analysis and editing, Jamie Robinson for revision of the English, D. Scott-Algara for critical reading of the manuscript, and all members of the Dendritic Cells Working Group from the ANRS (AC19) for helpful discussions.
This study was supported by grants from the French Agency for AIDS Research (ANRS). M.J.-Y.P. received scholarships from the MNERT and Sidaction, and L.M. and D.K. received fellowships from ANRS.
Footnotes
Published ahead of print on 2 April 2008.
REFERENCES
- 1.Abel, K., D. M. Rocke, B. Chohan, L. Fritts, and C. J. Miller. 2005. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J. Virol. 7912164-12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behbahani, H., A. Landay, B. K. Patterson, P. Jones, J. Pottage, M. Agnoli, J. Andersson, and A. L. Spetz. 2000. Normalization of immune activation in lymphoid tissue following highly active antiretroviral therapy. J. Acquir. Immune. Defic. Syndr. 25150-156. [DOI] [PubMed] [Google Scholar]
- 3.Benveniste, O., B. Vaslin, R. Le Grand, P. Fouchet, V. Omessa, F. Theodoro, P. Fretier, P. Clayette, F. Boussin, and D. Dormont. 1996. Interleukin 1 beta, interleukin 6, tumor necrosis factor alpha, and interleukin 10 responses in peripheral blood mononuclear cells of cynomolgus macaques during acute infection with SIVmac251. AIDS Res. Hum. Retrovir. 12241-250. [DOI] [PubMed] [Google Scholar]
- 4.Brown, K. N., A. Trichel, and S. M. Barratt-Boyes. 2007. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J. Immunol. 1786958-6967. [DOI] [PubMed] [Google Scholar]
- 5.Carbonneil, C., H. Saidi, V. Donkova-Petrini, and L. Weiss. 2004. Dendritic cells generated in the presence of interferon-alpha stimulate allogeneic CD4+ T-cell proliferation: modulation by autocrine IL-10, enhanced T-cell apoptosis and T regulatory type 1 cells. Int. Immunol. 161037-1052. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti, L. A., S. R. Lewin, L. Zhang, A. Gettie, A. Luckay, L. N. Martin, E. Skulsky, D. D. Ho, C. Cheng-Mayer, and P. A. Marx. 2000. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J. Virol. 741209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chehimi, J., D. E. Campbell, L. Azzoni, D. Bacheller, E. Papasavvas, G. Jerandi, K. Mounzer, J. Kostman, G. Trinchieri, and L. J. Montaner. 2002. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J. Immunol. 1684796-4801. [DOI] [PubMed] [Google Scholar]
- 8.Chung, E., S. B. Amrute, K. Abel, G. Gupta, Y. Wang, C. J. Miller, and P. Fitzgerald-Bocarsly. 2005. Characterization of virus-responsive plasmacytoid dendritic cells in the rhesus macaque. Clin. Diagn. Lab. Immunol. 12426-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coates, P. T., S. M. Barratt-Boyes, L. Zhang, V. S. Donnenberg, P. J. O'Connell, A. J. Logar, F. J. Duncan, M. Murphey-Corb, A. D. Donnenberg, A. E. Morelli, C. R. Maliszewski, and A. W. Thomson. 2003. Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood 1022513-2521. [DOI] [PubMed] [Google Scholar]
- 10.Dalod, M., T. P. Salazar-Mather, L. Malmgaard, C. Lewis, C. Asselin-Paturel, F. Briere, G. Trinchieri, and C. A. Biron. 2002. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 195517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeks, S. G., C. M. Kitchen, L. Liu, H. Guo, R. Gascon, A. B. Narvaez, P. Hunt, J. N. Martin, J. O. Kahn, J. Levy, M. S. McGrath, and F. M. Hecht. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104942-947. [DOI] [PubMed] [Google Scholar]
- 12.Fehervari, Z., and S. Sakaguchi. 2004. Control of Foxp3+ CD25+CD4+ regulatory cell activation and function by dendritic cells. Int. Immunol. 161769-1780. [DOI] [PubMed] [Google Scholar]
- 13.Feldman, S., D. Stein, S. Amrute, T. Denny, Z. Garcia, P. Kloser, Y. Sun, N. Megjugorac, and P. Fitzgerald-Bocarsly. 2001. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin. Immunol. 101201-210. [DOI] [PubMed] [Google Scholar]
- 14.Finke, J. S., M. Shodell, K. Shah, F. P. Siegal, and R. M. Steinman. 2004. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J. Clin. Immunol. 24647-652. [DOI] [PubMed] [Google Scholar]
- 15.Fong, L., M. Mengozzi, N. W. Abbey, B. G. Herndier, and E. G. Engleman. 2002. Productive infection of plasmacytoid dendritic cells with human immunodeficiency virus type 1 is triggered by CD40 ligation. J. Virol. 7611033-11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foussat, A., L. Bouchet-Delbos, D. Berrebi, I. Durand-Gasselin, A. Coulomb-L'Hermine, R. Krzysiek, P. Galanaud, Y. Levy, and D. Emilie. 2001. Deregulation of the expression of the fractalkine/fractalkine receptor complex in HIV-1-infected patients. Blood 981678-1686. [DOI] [PubMed] [Google Scholar]
- 17.Giavedoni, L. D. 2005. Simultaneous detection of multiple cytokines and chemokines from nonhuman primates using Luminex technology. J. Immunol. Methods 30189-101. [DOI] [PubMed] [Google Scholar]
- 18.Giavedoni, L. D., M. C. Velasquillo, L. M. Parodi, G. B. Hubbard, and V. L. Hodara. 2000. Cytokine expression, natural killer cell activation, and phenotypic changes in lymphoid cells from rhesus macaques during acute infection with pathogenic simian immunodeficiency virus. J. Virol. 741648-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giorgi, J. V., L. E. Hultin, J. A. McKeating, T. D. Johnson, B. Owens, L. P. Jacobson, R. Shih, J. Lewis, D. J. Wiley, J. P. Phair, S. M. Wolinsky, and R. Detels. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179859-870. [DOI] [PubMed] [Google Scholar]
- 20.Grassi, F., A. Hosmalin, D. McIlroy, V. Calvez, P. Debre, and B. Autran. 1999. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. AIDS 13759-766. [DOI] [PubMed] [Google Scholar]
- 21.Gueye, A., O. M. Diop, M. J. Ploquin, C. Kornfeld, A. Faye, M. C. Cumont, B. Hurtrel, F. Barre-Sinoussi, and M. C. Muller-Trutwin. 2004. Viral load in tissues during the early and chronic phase of non-pathogenic SIVagm infection. J. Med. Primatol. 3383-97. [DOI] [PubMed] [Google Scholar]
- 22.Hazenberg, M. D., S. A. Otto, B. H. van Benthem, M. T. Roos, R. A. Coutinho, J. M. Lange, D. Hamann, M. Prins, and F. Miedema. 2003. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 171881-1888. [DOI] [PubMed] [Google Scholar]
- 23.Kamga, I., S. Kahi, L. Develioglu, M. Lichtner, C. Maranon, C. Deveau, L. Meyer, C. Goujard, P. Lebon, M. Sinet, and A. Hosmalin. 2005. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J. Infect. Dis. 192303-310. [DOI] [PubMed] [Google Scholar]
- 24.Khatissian, E., M. G. Tovey, M. C. Cumont, V. Monceaux, P. Lebon, L. Montagnier, B. Hurtrel, and L. Chakrabarti. 1996. The relationship between the interferon alpha response and viral burden in primary SIV infection. AIDS Res. Hum. Retrovir. 121273-1278. [DOI] [PubMed] [Google Scholar]
- 25.Koopman, G., H. Niphuis, A. G. Haaksma, A. M. Farese, D. B. Casey, L. E. Kahn, D. Mann, T. J. MacVittie, S. L. Woulfe, and J. L. Heeney. 2004. Increase in plasmacytoid and myeloid dendritic cells by progenipoietin-1, a chimeric Flt-3 and G-CSF receptor agonist, in SIV-Infected rhesus macaques. Hum. Immunol. 65303-316. [DOI] [PubMed] [Google Scholar]
- 26.Kornfeld, C., M. J. Ploquin, I. Pandrea, A. Faye, R. Onanga, C. Apetrei, V. Poaty-Mavoungou, P. Rouquet, J. Estaquier, L. Mortara, J. F. Desoutter, C. Butor, R. Le Grand, P. Roques, F. Simon, F. Barre-Sinoussi, O. M. Diop, and M. C. Muller-Trutwin. 2005. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J. Clin. Investig. 1151082-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levings, M. K., R. Sangregorio, F. Galbiati, S. Squadrone, R. de Waal Malefyt, and M. G. Roncarolo. 2001. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J. Immunol. 1665530-5539. [DOI] [PubMed] [Google Scholar]
- 28.Liu, Y. J. 2005. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23275-306. [DOI] [PubMed] [Google Scholar]
- 29.Lopez, C., P. A. Fitzgerald, and F. P. Siegal. 1983. Severe acquired immune deficiency syndrome in male homosexuals: diminished capacity to make interferon-alpha in vitro associated with severe opportunistic infections. J. Infect. Dis. 148962-966. [DOI] [PubMed] [Google Scholar]
- 30.Lore, K. 2004. Isolation and immunophenotyping of human and rhesus macaque dendritic cells. Methods Cell Biol. 75623-642. [DOI] [PubMed] [Google Scholar]
- 31.Lore, K., A. Sonnerborg, C. Brostrom, L. E. Goh, L. Perrin, H. McDade, H. J. Stellbrink, B. Gazzard, R. Weber, L. A. Napolitano, Y. van Kooyk, and J. Andersson. 2002. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS 16683-692. [DOI] [PubMed] [Google Scholar]
- 32.Meyers, J. H., J. S. Justement, C. W. Hallahan, E. T. Blair, Y. A. Sun, A. O'Shea, M. G. Roby, S. Kottilil, S. Moir, C. M. Kovacs, T. W. Chun, and A. S. Fauci. 2007. Impact of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells. PLoS One 2e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortara, L., M. J. Ploquin, A. Faye, D. Scott-Algara, B. Vaslin, C. Butor, A. Hosmalin, F. Barre-Sinoussi, O. M. Diop, and M. C. Muller-Trutwin. 2006. Phenotype and function of myeloid dendritic cells derived from African green monkey blood monocytes. J. Immunol. Methods 308138-155. [DOI] [PubMed] [Google Scholar]
- 34.Moseman, E. A., X. Liang, A. J. Dawson, A. Panoskaltsis-Mortari, A. M. Krieg, Y. J. Liu, B. R. Blazar, and W. Chen. 2004. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 1734433-4442. [DOI] [PubMed] [Google Scholar]
- 35.Orandle, M. S., K. C. Williams, A. G. MacLean, S. V. Westmoreland, and A. A. Lackner. 2001. Macaques with rapid disease progression and simian immunodeficiency virus encephalitis have a unique cytokine profile in peripheral lymphoid tissues. J. Virol. 754448-4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pacanowski, J., S. Kahi, M. Baillet, P. Lebon, C. Deveau, C. Goujard, L. Meyer, E. Oksenhendler, M. Sinet, and A. Hosmalin. 2001. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood 983016-3021. [DOI] [PubMed] [Google Scholar]
- 37.Palmer, P., M. G. Tovey, F. Raschilas, L. Brassart, J. F. Meritet, R. Porcher, and P. Lebon. 2007. Type I interferon subtypes produced by human peripheral mononuclear cells from one normal donor stimulated by viral and non-viral inducing factors. Eur. Cytokine. Netw. 18108-114. [DOI] [PubMed] [Google Scholar]
- 38.Ploquin, M. J., J. F. Desoutter, P. R. Santos, I. Pandrea, O. M. Diop, A. Hosmalin, C. Butor, F. Barre-Sinoussi, and M. C. Muller-Trutwin. 2006. Distinct expression profiles of TGF-β1 signaling mediators in pathogenic SIVmac and non-pathogenic SIVagm infections. Retrovirology 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ploquin, M. J., O. M. Diop, N. Sol-Foulon, L. Mortara, A. Faye, M. A. Soares, E. Nerrienet, R. Le Grand, Y. Van Kooyk, A. Amara, O. Schwartz, F. Barre-Sinoussi, and M. C. Muller-Trutwin. 2004. DC-SIGN from African green monkeys is expressed in lymph nodes and mediates infection in trans of simian immunodeficiency virus SIVagm. J. Virol. 78798-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeves, R. K., and P. N. Fultz. 2007. Disparate effects of acute and chronic infection with SIVmac239 or SHIV-89.6P on macaque plasmacytoid dendritic cells. Virology 365356-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romagnani, S. 2006. Regulation of the T cell response. Clin. Exp. Allergy. 361357-1366. [DOI] [PubMed] [Google Scholar]
- 42.Rutella, S., G. Bonanno, L. Pierelli, A. Mariotti, E. Capoluongo, A. M. Contemi, F. Ameglio, A. Curti, D. G. De Ritis, M. T. Voso, A. Perillo, S. Mancuso, G. Scambia, R. M. Lemoli, and G. Leone. 2004. Granulocyte colony-stimulating factor promotes the generation of regulatory DC through induction of IL-10 and IFN-alpha. Eur. J. Immunol. 341291-1302. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt, B., I. Scott, R. G. Whitmore, H. Foster, S. Fujimura, J. Schmitz, and J. A. Levy. 2004. Low-level HIV infection of plasmacytoid dendritic cells: onset of cytopathic effects and cell death after PDC maturation. Virology 329280-288. [DOI] [PubMed] [Google Scholar]
- 44.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 2841835-1837. [DOI] [PubMed] [Google Scholar]
- 45.Silvestri, G., D. L. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Non-pathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18441-452. [DOI] [PubMed] [Google Scholar]
- 46.Soumelis, V., I. Scott, F. Gheyas, D. Bouhour, G. Cozon, L. Cotte, L. Huang, J. A. Levy, and Y. J. Liu. 2001. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood 98906-912. [DOI] [PubMed] [Google Scholar]
- 47.Teleshova, N., J. Kenney, J. Jones, J. Marshall, G. Van Nest, J. Dufour, R. Bohm, J. D. Lifson, A. Gettie, and M. Pope. 2004. CpG-C immunostimulatory oligodeoxyribonucleotide activation of plasmacytoid dendritic cells in rhesus macaques to augment the activation of IFN-gamma-secreting simian immunodeficiency virus-specific T cells. J. Immunol. 1731647-1657. [DOI] [PubMed] [Google Scholar]
- 48.Thiebot, H., B. Vaslin, S. Derdouch, J. M. Bertho, F. Mouthon, S. Prost, G. Gras, P. Ducouret, D. Dormont, and R. Le Grand. 2005. Impact of bone marrow hematopoiesis failure on T-cell generation during pathogenic simian immunodeficiency virus infection in macaques. Blood 1052403-2409. [DOI] [PubMed] [Google Scholar]
- 49.van Asten, L., F. Danisman, S. A. Otto, J. A. Borghans, M. D. Hazenberg, R. A. Coutinho, M. Prins, and F. Miedema. 2004. Pre-seroconversion immune status predicts the rate of CD4 T cell decline following HIV infection. AIDS 181885-1893. [DOI] [PubMed] [Google Scholar]
- 50.Walzer, T., M. Dalod, E. Vivier, and L. Zitvogel. 2005. Natural killer cell-dendritic cell crosstalk in the initiation of immune responses. Expert Opin. Biol Ther. 5(Suppl 1)S49-S59. [DOI] [PubMed] [Google Scholar]