Abstract
In vivo electroporation (EP) has been shown to augment the immunogenicity of plasmid DNA vaccines, but its mechanism of action has not been fully characterized. In this study, we show that in vivo EP augmented cellular and humoral immune responses to a human immunodeficiency virus type 1 Env DNA vaccine in mice and allowed a 10-fold reduction in vaccine dose. This enhancement was durable for over 6 months, and re-exposure to antigen resulted in anamnestic effector and central memory CD8+ T-lymphocyte responses. Interestingly, in vivo EP also recruited large mixed cellular inflammatory infiltrates to the site of inoculation. These infiltrates contained 45-fold-increased numbers of macrophages and 77-fold-increased numbers of dendritic cells as well as 2- to 6-fold-increased numbers of B and T lymphocytes compared to infiltrates following DNA vaccination alone. These data suggest that recruiting inflammatory cells, including antigen-presenting cells (APCs), to the site of antigen production substantially improves the immunogenicity of DNA vaccines. Combining in vivo EP with plasmid chemokine adjuvants that similarly recruited APCs to the injection site, however, did not result in synergy.
Plasmid DNA vaccines have proven considerably less immunogenic in clinical studies than in preclinical studies (3, 9, 13, 24, 33), demonstrating the need to improve their potency. Various strategies are currently being pursued, including the use of plasmid cytokine and chemokine adjuvants (5, 6, 11, 19, 26, 30), polymer adjuvants (29), novel transcriptional regulatory elements (7), and improved delivery techniques such as in vivo electroporation (EP) (2, 23, 25). In vivo EP involves the administration of electrical pulses to muscle tissue following intramuscular (i.m.) injection of DNA vaccines and has been shown to enhance the immunogenicity of DNA vaccines in a wide variety of small and large animal models (1, 8, 10, 12, 17, 18, 20, 22, 27, 32). It has been suggested that in vivo EP functions in part by increasing myocyte permeability and thereby facilitating plasmid uptake and antigen expression by host cells (2, 14-16, 25, 28, 34).
We have previously reported that there are very few professional antigen-presenting cells (APCs) in muscles after DNA vaccination (6), and we therefore hypothesized that DNA vaccines may be limited by insufficient APCs at the site of antigen production. Consistent with this hypothesis, we observed that plasmid chemokines and growth factors such as plasmid MIP-1α and Flt3L were able to recruit dendritic cells (DCs) and macrophages to the site of inoculation and to enhance DNA vaccine-elicited immune responses (26, 30). Whether APCs are similarly recruited by in vivo EP, however, has not previously been investigated. In addition, the phenotype of cellular immune responses elicited by DNA vaccination with in vivo EP has not been assessed in detail. In the present study, we investigated the magnitude, phenotype, and durability of cellular immune responses elicited in mice by human immunodeficiency virus type 1 (HIV-1) Env DNA vaccination with or without in vivo EP and assessed the extent and nature of cellular inflammatory infiltrates at the site of inoculation.
In vivo EP augments DNA vaccine-elicited immune responses.
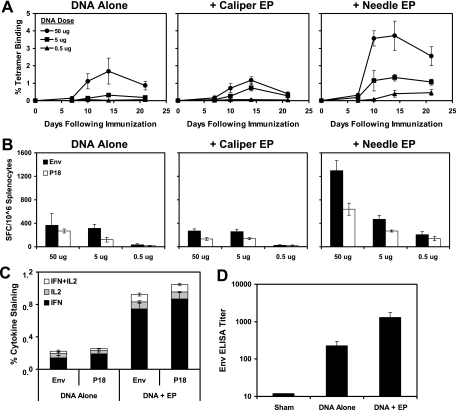
We initiated studies by assessing the immunogenicity of 50, 5, or 0.5 μg of a previously described HIV-1 Env IIIB gp120 DNA vaccine (6, 30) either alone or with two different methods of in vivo EP. BALB/c mice (four animals/group) were anesthetized and immunized i.m. in the quadriceps muscles, and in vivo EP was performed according to the manufacturer's protocols (Inovio Biomedical, San Diego, CA). Caliper EP involved application of electric pulses across intact muscle using surface electrodes with conductive gel after DNA vaccination (6 × 100-μs pulses at 600 V/cm). Needle EP involved delivery of electric pulses from electrodes inserted i.m. flanking the injection site after DNA vaccination (2 × 60-ms pulses at 200 V/cm).
CD8+ T-lymphocyte responses to the dominant Env P18 epitope (RGPGRAFVTI) (31) were assessed by Dd/P18 tetramer binding assays at multiple time points after immunization as previously described (6, 30). Cellular immune responses to a pool of overlapping Env peptides and the P18 epitope peptide were also assessed by gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays at week 4 after immunization. As shown in Fig. 1A and B, the DNA vaccine alone elicited potent cellular immune responses at the dose of 50 μg and detectable responses at the dose of 5 μg, but no responses were observed at the dose of 0.5 μg. Caliper EP utilizing these experimental conditions had little adjuvant effect. In contrast, needle EP resulted in a significant threefold enhancement of the magnitude of CD8+ T-lymphocyte responses at the dose of 50 μg (P = 0.001 comparing tetramer binding responses on day 14 after immunization using two-tailed t tests). Cellular immune responses were also detected at the lowest dose of 0.5 μg, indicating that needle EP allowed a 10-fold reduction in the DNA vaccine dose. Needle EP also resulted in a significant fourfold augmentation of both IFN-γ+ and IFN-γ+/IL-2+ CD8+ T-lymphocyte responses by intracellular cytokine staining assays (P = 0.0002; Fig. 1C) (21) and a sixfold increase in Env-specific antibody titers by enzyme-linked immunosorbent assay (ELISA) (P = 0.01; Fig. 1D) (6, 30) at week 4 after immunization in mice that received the 50-μg dose. Needle EP was therefore utilized in subsequent studies, although we do not exclude the possibility that caliper EP could be further optimized.
FIG. 1.
Immunogenicity of DNA vaccination with in vivo EP. Groups of BALB/c mice (four animals/group) were immunized with 50, 5, or 0.5 μg of the DNA vaccine expressing Env IIIB gp120 alone or with caliper EP or needle EP. Cellular and humoral immune responses were assessed by Dd/P18 tetramer binding assays at multiple time points after immunization (A) and by Env pooled peptide and P18 epitope peptide IFN-γ ELISPOT assays (B). Env pooled peptide and P18 epitope peptide IFN-γ/IL-2 CD8+ intracellular cytokine staining assays (C) and Env-specific ELISAs (D) were performed at week 4 after immunization in mice that received the 50-μg dose of the DNA vaccine with or without needle EP.
In vivo EP does not augment rAd5 vaccine-elicited immune responses.
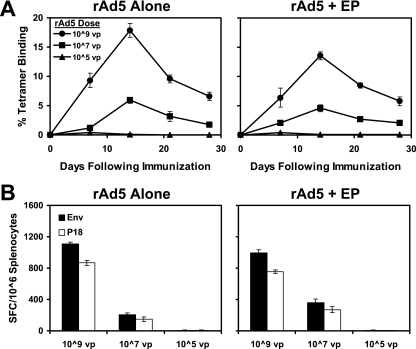
We next evaluated the capacity of in vivo needle EP to enhance the immunogenicity of a recombinant adenovirus serotype 5 (rAd5) vector-based vaccine. As shown in Fig. 2, in vivo EP did not significantly augment responses elicited by 109, 107, or 105 viral particles (vp) rAd5 expressing Env IIIB gp120 (4). These data suggest that in vivo EP may prove more useful at improving DNA vaccines rather than certain live vectors, perhaps as a result of the innate inflammatory responses induced by these viral vectors, together with the fact that in vivo EP would not likely increase the infectivity of viral vectors that enter host cells efficiently via specific cellular receptors.
FIG. 2.
Immunogenicity of rAd5 vaccination with in vivo EP. Groups of BALB/c mice (four animals/group) were immunized with 109, 107, or 105 vp gp120 rAd5 expressing Env IIIB gp120 alone or with needle EP. Cellular immune responses were assessed by Dd/P18 tetramer binding assays (A) and Env pooled peptide and P18 epitope peptide IFN-γ ELISPOT assays (B) at week 4 after immunization.
Comparison of in vivo EP with plasmid chemokine adjuvants.
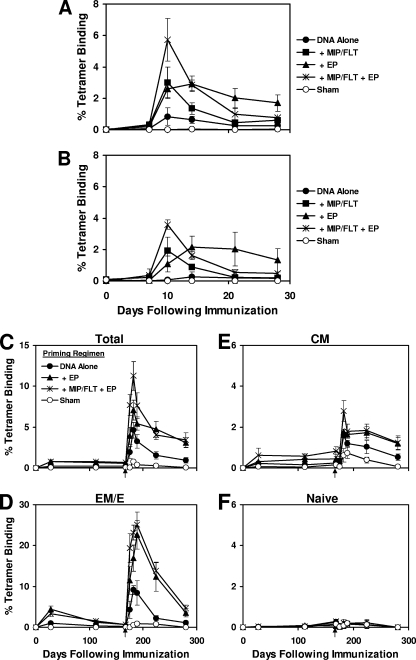
Previous studies have suggested that in vivo EP may function in part by increasing the permeability of myocytes and enhancing plasmid uptake and antigen expression (2, 25, 28, 34). We have previously reported that coadministration of plasmid chemokines and growth factors, particularly the combination of plasmid MIP-1α and Flt3L, recruited APCs to the site of inoculation and enhanced DNA vaccine-elicited immune responses (30). We therefore hypothesized that combining plasmid chemokine adjuvants with in vivo EP might result in synergistic effects by simultaneously recruiting APCs to the injection site and improving transfection of these cells. To explore this hypothesis, groups of mice (four animals/group) were immunized with 50 μg (Fig. 3A) or 5 μg (Fig. 3B) of the gp120 DNA vaccine alone or with plasmid MIP-1α/Flt3L, in vivo EP, or both. The plasmid chemokines and in vivo EP resulted in similar threefold enhancements of peak responses on days 10 to 14 after immunization, although the effects of in vivo EP proved more durable. The combination of plasmid chemokines and in vivo EP led to a transient additive effect on day 10, but these responses contracted quickly, and no long-term synergy was observed by combining these two adjuvant strategies.
FIG. 3.
Durability and phenotype of DNA vaccination with in vivo EP and plasmid chemokine adjuvants. Groups of BALB/c mice (four animals/group) were immunized with 50 μg (A) or 5 μg (B) of gp120 DNA vaccine alone or with plasmid MIP-1α/Flt3L, in vivo EP, or both. CD8+ T-lymphocyte responses were assessed by Dd/P18 tetramer binding assays. In a separate experiment, mice were primed with 50 μg of gp120 DNA vaccine alone, the DNA vaccine with in vivo EP, or the DNA vaccine with both plasmid MIP-1α/Flt3L and in vivo EP. Env P18-specific responses in total (C), EM/E (CD62L−) (D), CM (CD44+ CD62L+) (E), and N (CD44− CD62L+) (F) CD8+ T-lymphocyte subpopulations were evaluated by multiparameter Dd/P18 tetramer binding assays. All groups of mice, including the sham-primed mice, were boosted on day 168 with 50 μg of gp120 DNA vaccine alone (arrows).
We next evaluated the memory phenotypes and recall responses after priming with 50 μg of the gp120 DNA vaccine alone, the DNA vaccine with in vivo EP, or the DNA vaccine with both plasmid MIP-1α/Flt3L and in vivo EP. Env P18-specific responses in total, effector memory/effector (EM/E; CD62L−), central memory (CM; CD44+ CD62L+), and naive (N; CD44− CD62L+) CD8+ T-lymphocyte subpopulations were evaluated by multiparameter tetramer binding assays. As shown in Fig. 3C to F, in vivo EP resulted in a durable 2.9-, 3.7-, and 2.0-fold augmentation of total, EM/E, and CM CD8+ T-lymphocyte responses for 168 days after the priming immunization (P = 0.007, 0.007, and 0.01, respectively). In vivo EP did not skew the relative proportions of memory phenotypes compared to DNA vaccination alone at this time point.
At 6 months after the priming immunization, all groups of mice, including the sham-primed mice, were boosted with 50 μg of the gp120 DNA vaccine without any adjuvants (Fig. 3C to F, arrows). After the boost immunization, a robust 10-fold expansion of EM/E responses and a 3-fold increase in CM responses were detected in all groups. Mice that were primed with in vivo EP 168 days previously exhibited significantly threefold-higher anamnestic EM/E responses and twofold-higher CM responses after the boost immunization compared to mice that received the DNA vaccine alone (P = 0.01), demonstrating the durability of the effects of in vivo EP administered during the priming immunization. The addition of plasmid MIP1α/Flt3L to in vivo EP led to transiently greater peak responses but did not result in long-term synergistic effects.
In vivo EP recruits large cellular infiltrates to the site of inoculation.
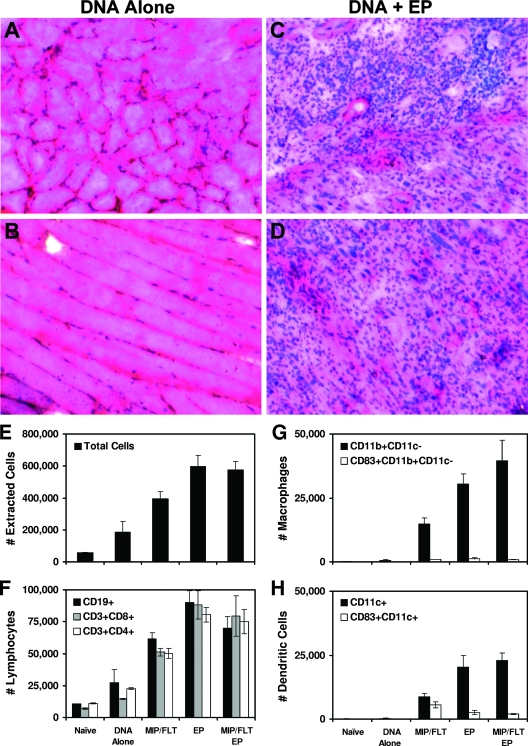
We next evaluated the extent and nature of cellular infiltrates at the site of inoculation after DNA vaccination alone or with plasmid MIP-1α/Flt3L, in vivo EP, or both. As shown in Fig. 4A and B, only mild inflammation was observed in sections of injected muscles on day 7 after DNA vaccination alone, a finding consistent with our prior studies (6, 26, 30). In contrast, as depicted in Fig. 4C and D, large cellular infiltrates were detected after DNA vaccination with in vivo EP. These infiltrates consisted of large clusters of mixed inflammatory cells both in perivascular spaces and throughout muscle tissue and consisted of both polymorphonuclear and mononuclear cells. The extent of these infiltrates was greater than that observed with plasmid MIP-1α/Flt3L (30).
FIG. 4.
Cellular infiltrates after DNA vaccination with in vivo EP. Groups of BALB/c mice (four animals/group) were immunized with 50 μg of gp120 DNA vaccine alone (A and B) or with in vivo EP (C and D). Cellular infiltrates were assessed by hematoxylin and eosin staining of 5-μm transverse (A and C) and longitudinal (B and D) sections of injected muscle harvested on day 7 after immunization. Representative sections are shown. Cells were then extracted from muscles injected with the various regimens and assessed by flow cytometry to determine the numbers of total cells (E), CD19+ and CD3+ lymphocytes (F), CD3− CD19− CD11b+ CD11c− macrophages (G), and CD3− CD19− CD11c+ DCs (H).
To evaluate the nature and composition of these infiltrates, we extracted cells from injected muscles on day 7 after immunization and evaluated them by multiparameter flow cytometry (30). Compared to DNA vaccination alone, DNA vaccination with in vivo EP led to a substantial increase in total extracted cells (P = 0.02; Fig. 4E), as well as two- to sixfold increases in CD19+ B lymphocytes, CD3+ CD4+ T lymphocytes, and CD3+ CD8+ T lymphocytes (P = 0.02, 0.002, and 0.004, respectively; Fig. 4F). Interestingly, in vivo EP also led to dramatic 45-fold increases in CD3− CD19− CD11b+ CD11c− macrophages and 77-fold increases in CD3− CD19− CD11c+ DCs compared to DNA vaccination alone (P = 0.006 and 0.007, respectively; Fig. 4G and H), although the absolute number of recruited professional APCs was lower than the number of recruited lymphocytes and acute inflammatory cells. In vivo EP also led to larger DC infiltrates compared to plasmid MIP-1α/Flt3L, although a smaller percentage of DCs recruited by in vivo EP expressed the activation marker CD83 (Fig. 4H). The infiltrates observed with the combination of plasmid MIP-1α/Flt3L and EP, however, were only marginally greater than those induced by EP alone. These data are consistent with the lack of substantial immunologic synergy observed with these two adjuvant modalities (Fig. 3). No differences in cell populations were observed in blood, draining lymph nodes, and spleen in mice after DNA vaccination with or without in vivo EP (data not shown), suggesting that the mechanism of in vivo EP likely involves local rather than systemic factors. The cellular inflammatory infiltrates in all groups proved transient and were largely resolved by day 14 after immunization (data not shown).
After DNA vaccination in the absence of adjuvants, antigen is primarily expressed in muscle tissue (35). The typical paucity of professional APCs at the site of inoculation and antigen production may therefore represent a critical limitation of DNA vaccines. Our data demonstrate that in vivo EP recruited large mixed cellular infiltrates that contained a proportionally concentrated number of APCs to the site of inoculation. It is likely that the increased numbers of APCs at the site of antigen production contributed substantially to the enhanced immunogenicity of the DNA vaccine. Consistent with this model, the lack of synergy between plasmid chemokines and in vivo EP may reflect their similar mechanisms of action. It is also possible that local cellular inflammation after in vivo EP may have provided an improved cytokine milieu that favored antigen presentation and immune priming.
In vivo EP significantly enhanced the magnitude and durability of DNA vaccine-elicited cellular and humoral immune responses. Qualitative aspects of CD8+ T-lymphocyte responses elicited by DNA vaccination with or without in vivo EP, however, appeared similar in terms of cytokine secretion profiles (Fig. 1C) and memory phenotypes (Fig. 3C to F). Our data extend previous studies by evaluating the durability and phenotype of CD8+ T-lymphocyte responses, as well as the extent and nature of cellular inflammatory infiltrates after DNA vaccination with in vivo EP. We propose that in vivo EP may function by a combination of mechanisms, including not only recruitment of APCs but also improved transfection of cells and increased antigen expression as previously reported (2, 14-16, 25, 28, 34). The potency of in vivo EP thus may reflect its capacity to facilitate multiple steps of the immune priming process.
Acknowledgments
We thank Bonnie Ewald, Diana Lynch, Matt Denholtz, Faye Stephens, Norman Letvin, Kathy Furr, and Michelle Lifton for generous advice, assistance, and reagents. The HIV-1 Env overlapping peptides were obtained from the UK Centralized Facility for AIDS Reagents.
We acknowledge support from NIH grants AI058727 (D.H.B.) and P30 AI060354.
Footnotes
Published ahead of print on 19 March 2008.
REFERENCES
- 1.Ahlen, G., J. Soderholm, T. Tjelle, R. Kjeken, L. Frelin, U. Hoglund, P. Blomberg, M. Fons, I. Mathiesen, and M. Sallberg. 2007. In vivo electroporation enhances the immunogenicity of hepatitis C virus nonstructural 3/4A DNA by increased local DNA uptake, protein expression, inflammation, and infiltration of CD3+ T cells. J. Immunol. 1794741-4753. [DOI] [PubMed] [Google Scholar]
- 2.Aihara, H., and J. Miyazaki. 1998. Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 16867-870. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H. 2006. Rational design of gene-based vaccines. J. Pathol. 208283-289. [DOI] [PubMed] [Google Scholar]
- 4.Barouch, D. H., P. F. McKay, S. M. Sumida, S. Santra, S. S. Jackson, D. A. Gorgone, M. A. Lifton, B. K. Chakrabarti, L. Xu, G. J. Nabel, and N. L. Letvin. 2003. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines. J. Virol. 778729-8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290486-492. [DOI] [PubMed] [Google Scholar]
- 6.Barouch, D. H., S. Santra, K. Tenner-Racz, P. Racz, M. J. Kuroda, J. E. Schmitz, S. S. Jackson, M. A. Lifton, D. C. Freed, H. C. Perry, M. E. Davies, J. W. Shiver, and N. L. Letvin. 2002. Potent CD4+ T-cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. J. Immunol. 168562-568. [DOI] [PubMed] [Google Scholar]
- 7.Barouch, D. H., Z. Y. Yang, W. P. Kong, B. Korioth-Schmitz, S. M. Sumida, D. M. Truitt, M. G. Kishko, J. C. Arthur, A. Miura, J. R. Mascola, N. L. Letvin, and G. J. Nabel. 2005. A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates. J. Virol. 798828-8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchan, S., E. Gronevik, I. Mathiesen, C. A. King, F. K. Stevenson, and J. Rice. 2005. Electroporation as a “prime/boost” strategy for naked DNA vaccination against a tumor antigen. J. Immunol. 1746292-6298. [DOI] [PubMed] [Google Scholar]
- 9.Calarota, S., G. Bratt, S. Nordlund, J. Hinkula, A. C. Leandersson, E. Sandstrom, and B. Wahren. 1998. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet 3511320-1325. [DOI] [PubMed] [Google Scholar]
- 10.Dobano, C., G. Widera, D. Rabussay, and D. L. Doolan. 2007. Enhancement of antibody and cellular immune responses to malaria DNA vaccines by in vivo electroporation. Vaccine 256635-6645. [DOI] [PubMed] [Google Scholar]
- 11.Egan, M. A., S. Y. Chong, S. Megati, D. C. Montefiori, N. F. Rose, J. D. Boyer, M. K. Sidhu, J. Quiroz, M. Rosati, E. B. Schadeck, G. N. Pavlakis, D. B. Weiner, J. K. Rose, Z. R. Israel, S. A. Udem, and J. H. Eldridge. 2005. Priming with plasmid DNAs expressing interleukin-12 and simian immunodeficiency virus gag enhances the immunogenicity and efficacy of an experimental AIDS vaccine based on recombinant vesicular stomatitis virus. AIDS Res. Hum. Retrovir. 21629-643. [DOI] [PubMed] [Google Scholar]
- 12.Folgori, A., S. Capone, L. Ruggeri, A. Meola, E. Sporeno, B. B. Ercole, M. Pezzanera, R. Tafi, M. Arcuri, E. Fattori, A. Lahm, A. Luzzago, A. Vitelli, S. Colloca, R. Cortese, and A. Nicosia. 2006. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat. Med. 12190-197. [DOI] [PubMed] [Google Scholar]
- 13.Graham, B. S., R. A. Koup, M. Roederer, R. T. Bailer, M. E. Enama, Z. Moodie, J. E. Martin, M. M. McCluskey, B. K. Chakrabarti, L. Lamoreaux, C. A. Andrews, P. L. Gomez, J. R. Mascola, and G. J. Nabel. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J. Infect. Dis. 1941650-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gronevik, E., I. Mathiesen, and T. Lomo. 2005. Early events of electroporation-mediated intramuscular DNA vaccination potentiate Th1-directed immune responses. J. Gene Med. 71246-1254. [DOI] [PubMed] [Google Scholar]
- 15.Gronevik, E., S. Tollefsen, L. I. Sikkeland, T. Haug, T. E. Tjelle, and I. Mathiesen. 2003. DNA transfection of mononuclear cells in muscle tissue. J. Gene Med. 5909-917. [DOI] [PubMed] [Google Scholar]
- 16.Gronevik, E., F. V. von Steyern, J. M. Kalhovde, T. E. Tjelle, and I. Mathiesen. 2005. Gene expression and immune response kinetics using electroporation-mediated DNA delivery to muscle. J. Gene Med. 7218-227. [DOI] [PubMed] [Google Scholar]
- 17.Hirao, L. A., L. Wu, A. S. Khan, A. Satishchandran, R. Draghia-Akli, and D. B. Weiner. 2008. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine 26440-448. [DOI] [PubMed] [Google Scholar]
- 18.Hooper, J. W., J. W. Golden, A. M. Ferro, and A. D. King. 2007. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine 251814-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutzler, M. A., T. M. Robinson, M. A. Chattergoon, D. K. Choo, A. Y. Choo, P. Y. Choe, M. P. Ramanathan, R. Parkinson, S. Kudchodkar, Y. Tamura, M. Sidhu, V. Roopchand, J. J. Kim, G. N. Pavlakis, B. K. Felber, T. A. Waldmann, J. D. Boyer, and D. B. Weiner. 2005. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J. Immunol. 175112-123. [DOI] [PubMed] [Google Scholar]
- 20.Li, Z., H. Zhang, X. Fan, Y. Zhang, J. Huang, Q. Liu, T. E. Tjelle, I. Mathiesen, R. Kjeken, and S. Xiong. 2006. DNA electroporation prime and protein boost strategy enhances humoral immunity of tuberculosis DNA vaccines in mice and non-human primates. Vaccine 244565-4568. [DOI] [PubMed] [Google Scholar]
- 21.Liu, J., B. A. Ewald, D. M. Lynch, A. Nanda, S. M. Sumida, and D. H. Barouch. 2006. Modulation of DNA vaccine-elicited CD8+ T-lymphocyte epitope immunodominance hierarchies. J. Virol. 8011991-11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luckay, A., M. K. Sidhu, R. Kjeken, S. Megati, S. Y. Chong, V. Roopchand, D. Garcia-Hand, R. Abdullah, R. Braun, D. C. Montefiori, M. Rosati, B. K. Felber, G. N. Pavlakis, I. Mathiesen, Z. R. Israel, J. H. Eldridge, and M. A. Egan. 2007. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J. Virol. 815257-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luxembourg, A., C. F. Evans, and D. Hannaman. 2007. Electroporation-based DNA immunization: translation to the clinic. Expert Opin. Biol. Ther. 71647-1664. [DOI] [PubMed] [Google Scholar]
- 24.MacGregor, R. R., J. D. Boyer, K. E. Ugen, K. E. Lacy, S. J. Gluckman, M. L. Bagarazzi, M. A. Chattergoon, Y. Baine, T. J. Higgins, R. B. Ciccarelli, L. R. Coney, R. S. Ginsberg, and D. B. Weiner. 1998. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J. Infect. Dis. 17892-100. [DOI] [PubMed] [Google Scholar]
- 25.Mathiesen, I. 1999. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther. 6508-514. [DOI] [PubMed] [Google Scholar]
- 26.McKay, P. F., D. H. Barouch, S. Santra, S. M. Sumida, S. S. Jackson, D. A. Gorgone, M. A. Lifton, and N. L. Letvin. 2004. Recruitment of different subsets of antigen-presenting cells selectively modulates DNA vaccine-elicited CD4+ and CD8+ T lymphocyte responses. Eur. J. Immunol. 341011-1020. [DOI] [PubMed] [Google Scholar]
- 27.Otten, G. R., M. Schaefer, B. Doe, H. Liu, J. Z. Megede, J. Donnelly, D. Rabussay, S. Barnett, and J. B. Ulmer. 2006. Potent immunogenicity of an HIV-1 gag-pol fusion DNA vaccine delivered by in vivo electroporation. Vaccine 244503-4509. [DOI] [PubMed] [Google Scholar]
- 28.Rizzuto, G., M. Cappelletti, D. Maione, R. Savino, D. Lazzaro, P. Costa, I. Mathiesen, R. Cortese, G. Ciliberto, R. Laufer, N. La Monica, and E. Fattori. 1999. Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc. Natl. Acad. Sci. USA 966417-6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415331-335. [DOI] [PubMed] [Google Scholar]
- 30.Sumida, S. M., P. F. McKay, D. M. Truitt, M. G. Kishko, J. C. Arthur, M. S. Seaman, S. S. Jackson, D. A. Gorgone, M. A. Lifton, N. L. Letvin, and D. H. Barouch. 2004. Recruitment and expansion of dendritic cells in vivo potentiate the immunogenicity of plasmid DNA vaccines. J. Clin. Investig. 1141334-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi, H., Y. Nakagawa, C. D. Pendleton, R. A. Houghten, K. Yokomuro, R. N. Germain, and J. A. Berzofsky. 1992. Induction of broadly cross-reactive cytotoxic T cells recognizing an HIV-1 envelope determinant. Science 255333-336. [DOI] [PubMed] [Google Scholar]
- 32.Tjelle, T. E., R. Salte, I. Mathiesen, and R. Kjeken. 2006. A novel electroporation device for gene delivery in large animals and humans. Vaccine 244667-4670. [DOI] [PubMed] [Google Scholar]
- 33.Wang, R., D. L. Doolan, T. P. Le, R. C. Hedstrom, K. M. Coonan, Y. Charoenvit, T. R. Jones, P. Hobart, M. Margalith, J. Ng, W. R. Weiss, M. Sedegah, C. de Taisne, J. A. Norman, and S. L. Hoffman. 1998. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282476-480. [DOI] [PubMed] [Google Scholar]
- 34.Widera, G., M. Austin, D. Rabussay, C. Goldbeck, S. W. Barnett, M. Chen, L. Leung, G. R. Otten, K. Thudium, M. J. Selby, and J. B. Ulmer. 2000. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J. Immunol. 1644635-4640. [DOI] [PubMed] [Google Scholar]
- 35.Wolff, J. A., R. W. Malone, P. Williams, W. Chong, G. Acsadi, A. Jani, and P. L. Felgner. 1990. Direct gene transfer into mouse muscle in vivo. Science 2471465-1468. [DOI] [PubMed] [Google Scholar]