Abstract
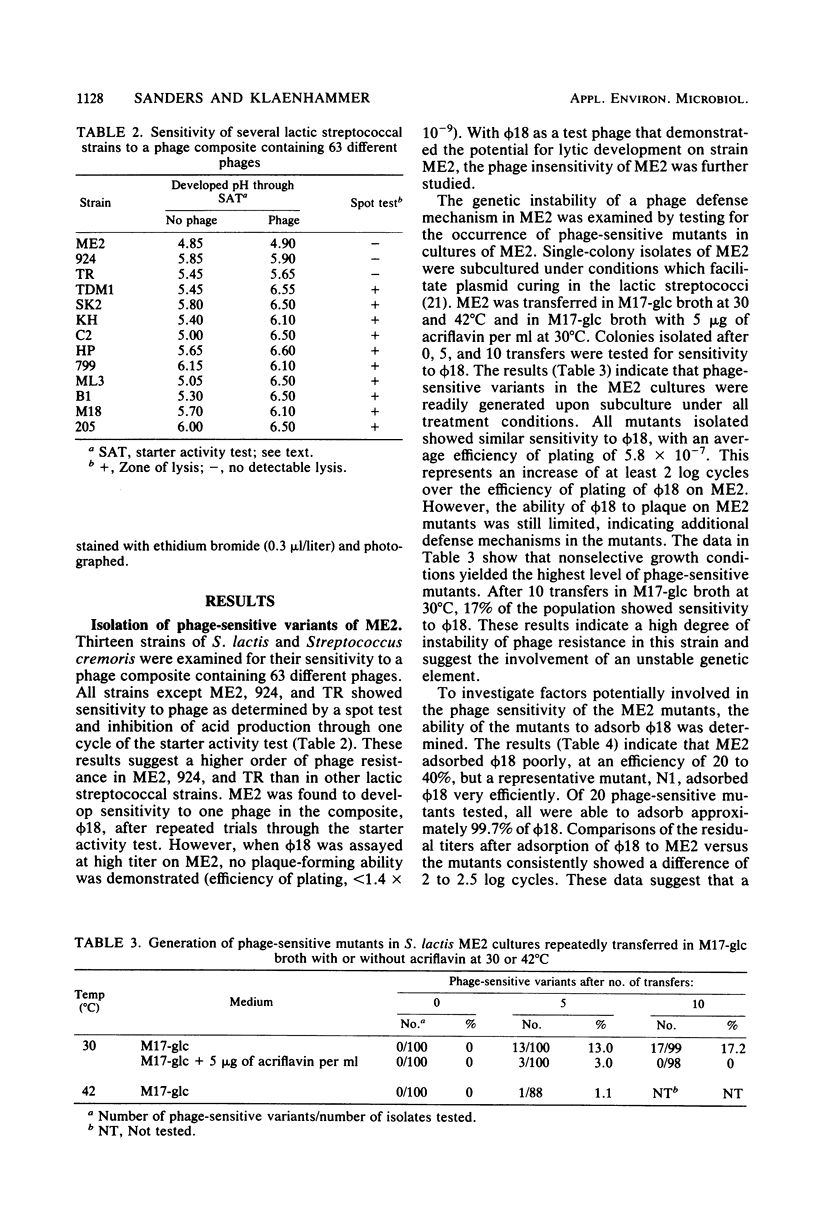
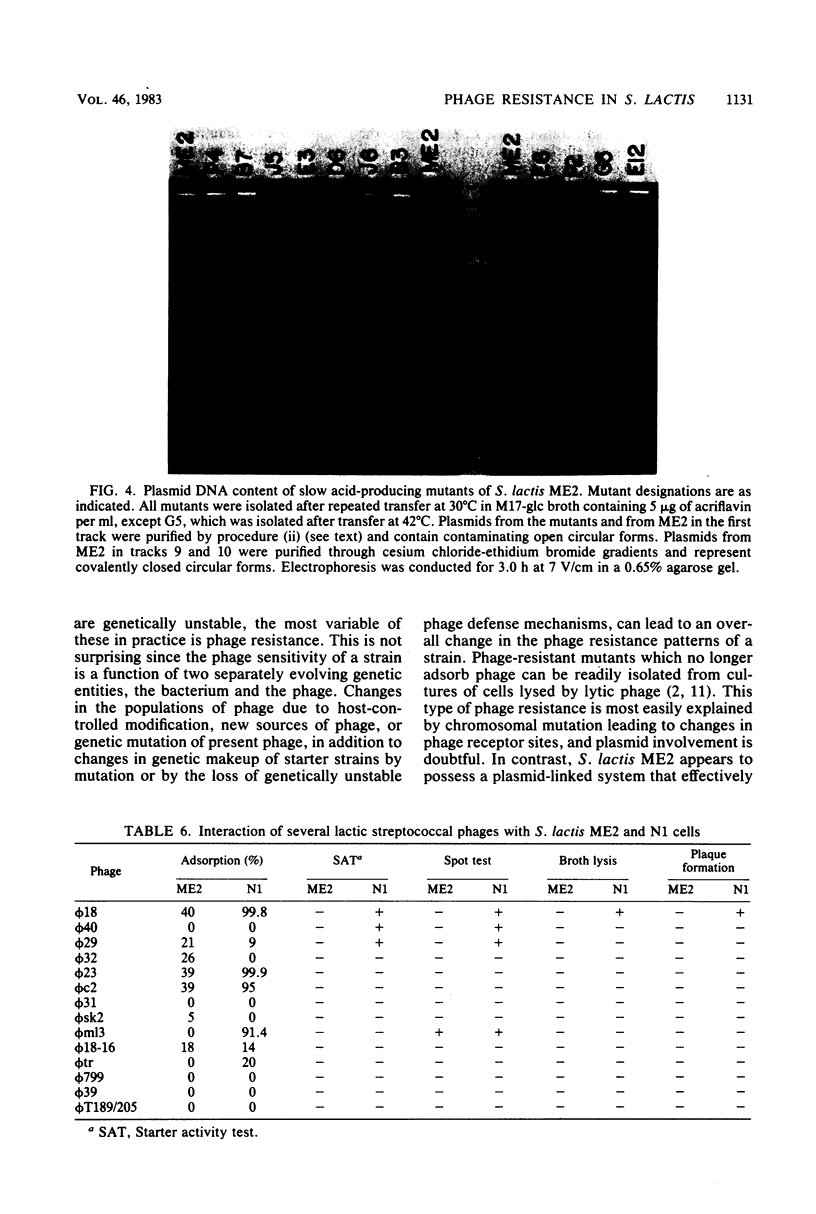
A phage-insensitive strain of Streptococcus lactis, designated ME2, was used as a prototype strain for the study of mechanisms and genetics of phage resistance in the lactic streptococci. Mutants sensitive to a Streptococcus cremoris phage, ϕ18, were isolated at a level of 17% from cultures of ME2 after sequential transfer at 30°C. Phage-sensitive mutants of ME2 were not fully permissive to ϕ18. The efficiency of plating of ϕ18 on the mutants was 5 × 10−7 as compared with <10−9 for ϕ18 on ME2. Further characterization of the mutants showed that they efficiently adsorbed ϕ18 at levels of >99.8%, whereas ME2 adsorbed only 20 to 40% of ϕ18. These results suggest that increased phage susceptibility of the mutants may result from the loss of a mechanism that inhibits phage adsorption. Moreover, the high frequency of spontaneous mutation in ME2 indicates the involvement of an unstable genetic determinant in this phage defense mechanism. ME2 was shown to possess 13 plasmids ranging in size from 1.6 to 34 megadaltons. Of 40 mutants examined that had increased efficiencies of plating, all were missing a 30-megadalton plasmid, pME0030. These data suggest that pME0030 codes for a function that prevents phage adsorption. Further phenotypic characterization of the phage-sensitive mutants showed that some mutants were deficient in the ability to ferment lactose (Lac) and hydrolyze milk proteins (Prt). However, the Lac+ and Prt+ phenotype segregated independently of the phage-sensitivity phenotype. One phage-sensitive adsorption mutant, designated N1, was tested for susceptibility to 14 different phages. N1 showed increased capacity to adsorb 4 and to replicate 2 of these 14 phages, thereby indicating a phage resistance mechanism in ME2 that generalizes to phage interactions other than the specific ϕ18-ME2 phage-host interaction. These data provide evidence for a unique plasmid-linked phage defense mechanism in phage-insensitive strains of lactic streptococci.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies F. L., Gasson M. J. Reviews of the progress of dairy science: genetics of lactic acid bacteria. J Dairy Res. 1981 Jun;48(2):363–376. doi: 10.1017/s0022029900021798. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Glenn J., McCorquodale D. J. Inhibition of bacteriophage replication by extrachromosomal genetic elements. Microbiol Rev. 1981 Mar;45(1):52–71. doi: 10.1128/mr.45.1.52-71.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. ROLES OF CITRATE AND ACETOIN IN THE METABOLISM OF STREPTOCOCCUS DIACETILACTIS. J Bacteriol. 1963 Dec;86:1301–1307. doi: 10.1128/jb.86.6.1301-1307.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins A. R., Sandine W. E. Incidence and properties of temperate bacteriophages induced from lactic streptococci. Appl Environ Microbiol. 1977 Jan;33(1):184–191. doi: 10.1128/aem.33.1.184-191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W. R., Collins E. B., Barrett E. L. Frequencies of Bacteriophage-Resistant and Slow Acid-Producing Variants of Streptococcus cremoris. Appl Environ Microbiol. 1983 May;45(5):1481–1485. doi: 10.1128/aem.45.5.1481-1485.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L., Baldwin K. A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978 Mar;35(3):592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Robbins P. W. Mechanism of epsilon-15 conversion studies with a bacterial mutant. J Mol Biol. 1967 Dec 28;30(3):445–455. doi: 10.1016/0022-2836(67)90361-0. [DOI] [PubMed] [Google Scholar]
- Lowrie R. J. Lysogenic strains of group N lactic streptococci. Appl Microbiol. 1974 Jan;27(1):210–217. doi: 10.1128/am.27.1.210-217.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Induction of prophage in Streptococcus lactis C2 by ultraviolet irradiation. Appl Microbiol. 1973 Apr;25(4):682–684. doi: 10.1128/am.25.4.682-684.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Plasmid distribution and evidence for a proteinase plasmid in Streptococcus lactis C2-1. Appl Microbiol. 1975 Apr;29(4):546–548. doi: 10.1128/am.29.4.546-548.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa H. H., Collins E. B. Role of galactose or glucose-1-phosphate in preventing the lysis of Streptococcus diacetilactis. J Bacteriol. 1968 Feb;95(2):592–602. doi: 10.1128/jb.95.2.592-602.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H., Siak J. S., Gray R. H. Characteristics of PRD1, a plasmid-dependent broad host range DNA bacteriophage. J Virol. 1974 Sep;14(3):689–699. doi: 10.1128/jvi.14.3.689-699.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D. Isolation and properties of a phage receptor substance from the plasma membrane of Streptococcus lactis ML 3. J Gen Virol. 1971 Oct;13(1):59–71. doi: 10.1099/0022-1317-13-1-59. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The adsorption of phage to group N streptococci. The specificity of adsorption and the location of phage receptor substances in cell-wall and plasma-membrane fractions. J Gen Virol. 1968 Jul;3(1):103–119. doi: 10.1099/0022-1317-3-1-103. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Klaenhammer T. R. Evidence for Plasmid Linkage of Restriction and Modification in Streptococcus cremoris KH. Appl Environ Microbiol. 1981 Dec;42(6):944–950. doi: 10.1128/aem.42.6.944-950.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Klaenhammer T. R. Restriction and modification in group N streptococci: effect of heat on development of modified lytic bacteriophage. Appl Environ Microbiol. 1980 Sep;40(3):500–506. doi: 10.1128/aem.40.3.500-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer H. E., Sederoff R. R. Improved estimation of DNA fragment lengths from Agarose gels. Anal Biochem. 1981 Jul 15;115(1):113–122. doi: 10.1016/0003-2697(81)90533-9. [DOI] [PubMed] [Google Scholar]
- Sinha R. P. Alteration of Host Specificity to Lytic Bacteriophages in Streptococcus cremoris. Appl Environ Microbiol. 1980 Aug;40(2):326–332. doi: 10.1128/aem.40.2.326-332.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UETAKE H., LURIA S. E., BURROUS J. W. Conversion of somatic antigens in Salmonella by phage infection leading to lysis or lysogeny. Virology. 1958 Feb;5(1):68–91. doi: 10.1016/0042-6822(58)90006-0. [DOI] [PubMed] [Google Scholar]