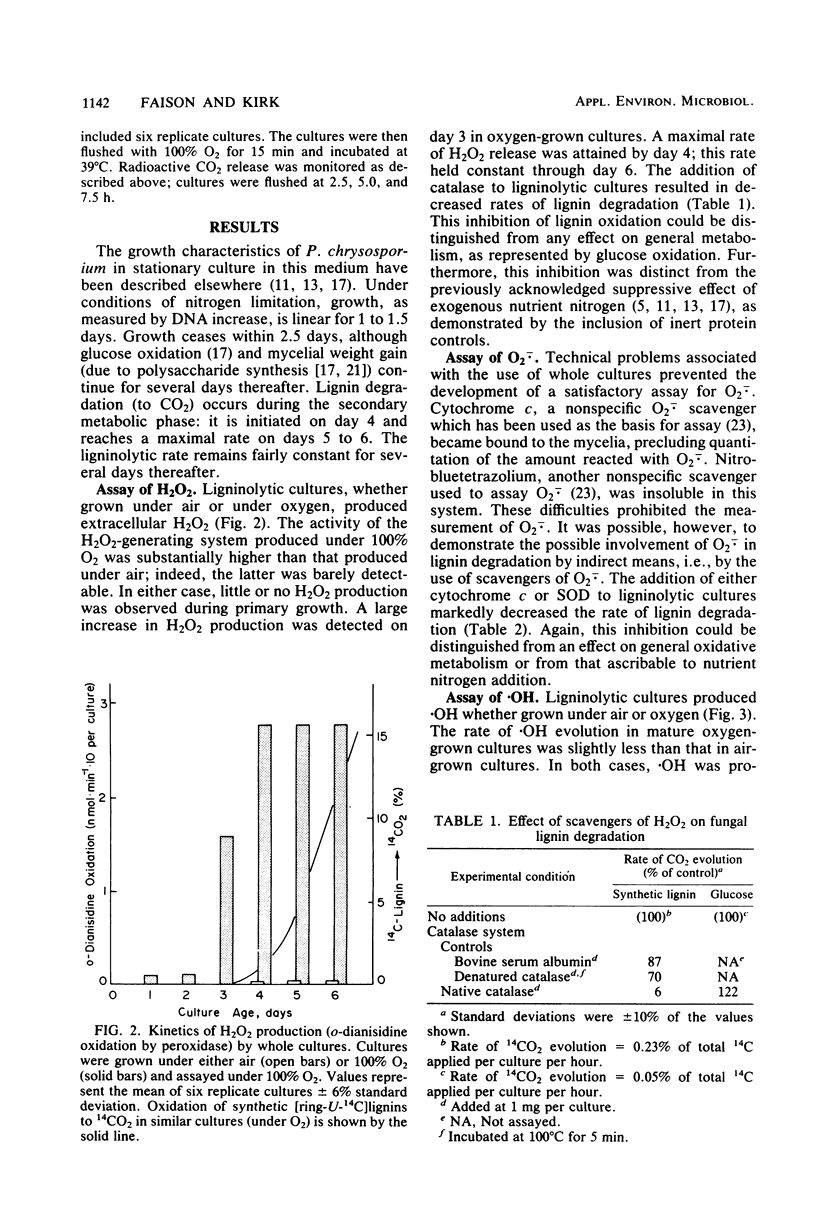
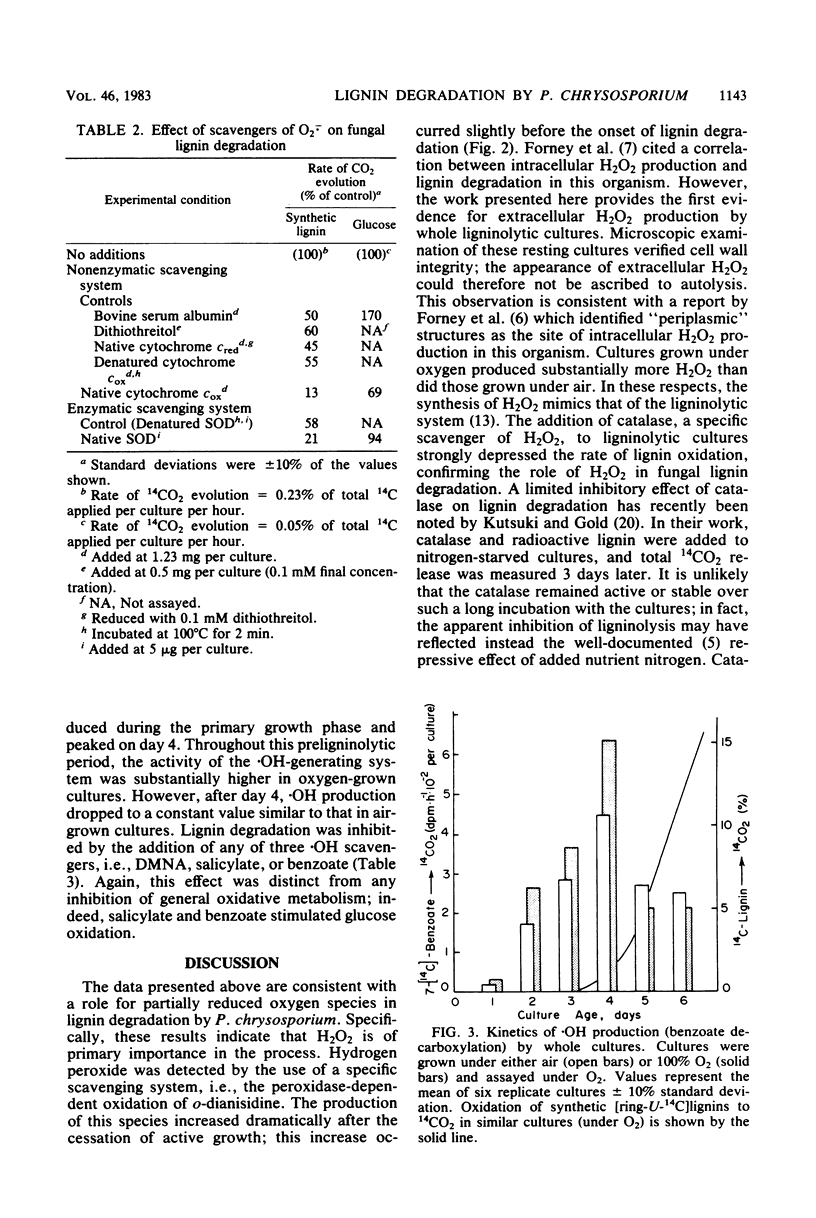
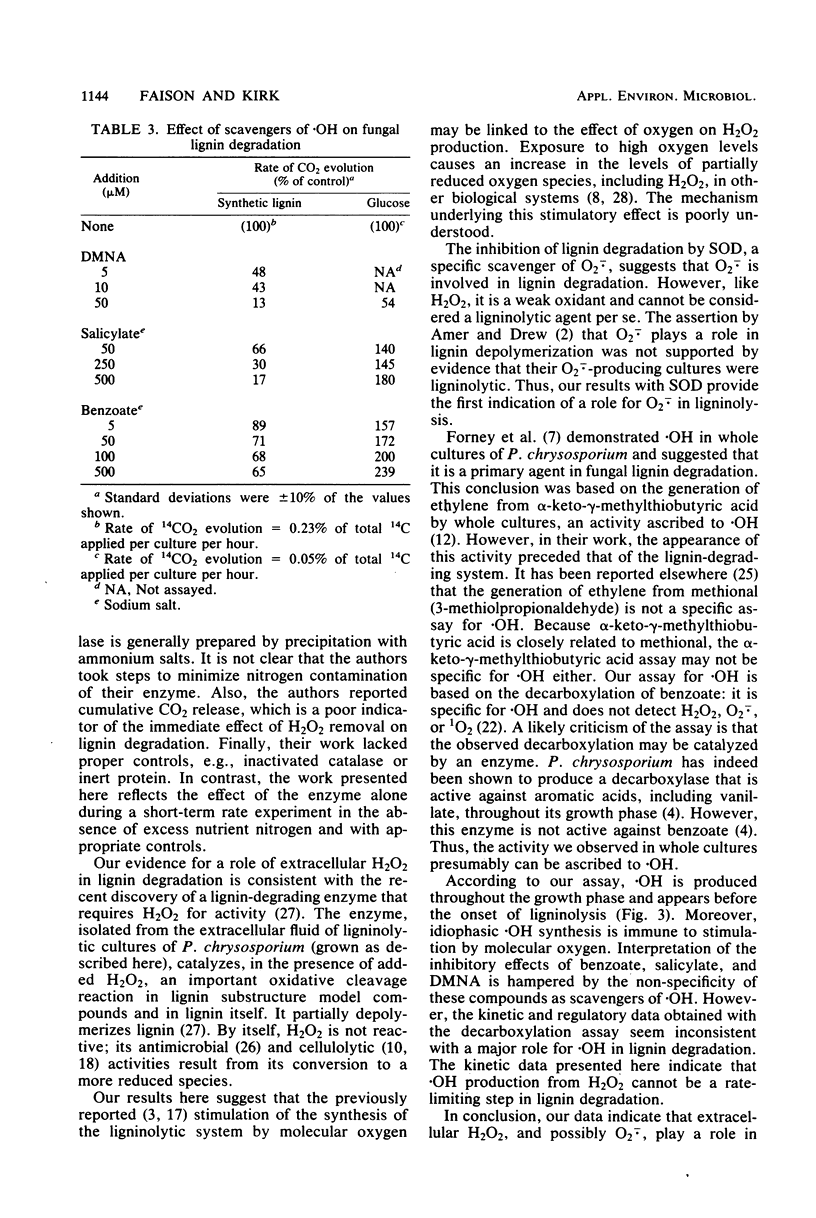
Abstract
The relationship between the production of reduced oxygen species, hydrogen peroxide (H2O2), superoxide (O2∙̅), and hydroxyl radical (·OH), and the oxidation of synthetic lignin to CO2 was studied in whole cultures of the white-rot fungus Phanerochaete chrysosporium Burds. The kinetics of the synthesis of H2O2 coincided with the appearance of the ligninolytic system; also, H2O2 production was markedly enhanced by growth under 100% O2, mimicking the increase in ligninolytic activity characteristic of cultures grown under elevated oxygen tension. Lignin degradation by whole cultures was inhibited by a specific H2O2 scavenger, catalase, implying a role for H2O2 in the degradative process. Superoxide dismutase also inhibited lignin degradation, suggesting that O2∙̅ is also involved in the breakdown of lignin. The production of ·OH was assayed in whole cultures by a benzoate decarboxylation assay. Neither the kinetics of ·OH synthesis nor the final activity of its producing system obtained under 100% O2 correlated with that of the lignin-degrading system. However, lignin degradation was inhibited by compounds which react with ·OH. It is concluded that H2O2, and perhaps O2∙̅, are involved in lignin degradation; because these species are relatively unreactive per se, their role must be indirect. Conclusions about a role for ·OH in ligninolysis could not be reached.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Lev S. S., Kirk T. K. Effects of molecular oxygen on lignin degradation by Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1981 Mar 31;99(2):373–378. doi: 10.1016/0006-291x(81)91755-1. [DOI] [PubMed] [Google Scholar]
- Buswell J. A., Ander P., Pettersson B., Eriksson K. E. Oxidative decarboxylation of vanillic acid by Sporotrichum pulverulentum. FEBS Lett. 1979 Jul 1;103(1):98–101. doi: 10.1016/0014-5793(79)81258-2. [DOI] [PubMed] [Google Scholar]
- Forney L. J., Reddy C. A., Pankratz H. S. Ultrastructural Localization of Hydrogen Peroxide Production in Ligninolytic Phanerochaete chrysosporium Cells. Appl Environ Microbiol. 1982 Sep;44(3):732–736. doi: 10.1128/aem.44.3.732-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney L. J., Reddy C. A., Tien M., Aust S. D. The involvement of hydroxyl radical derived from hydrogen peroxide in lignin degradation by the white rot fungus Phanerochaete chrysosporium. J Biol Chem. 1982 Oct 10;257(19):11455–11462. [PubMed] [Google Scholar]
- Freeman B. A., Topolosky M. K., Crapo J. D. Hyperoxia increases oxygen radical production in rat lung homogenates. Arch Biochem Biophys. 1982 Jul;216(2):477–484. doi: 10.1016/0003-9861(82)90236-3. [DOI] [PubMed] [Google Scholar]
- Jeffries T. W., Choi S., Kirk T. K. Nutritional Regulation of Lignin Degradation by Phanerochaete chrysosporium. Appl Environ Microbiol. 1981 Aug;42(2):290–296. doi: 10.1128/aem.42.2.290-296.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R. L., Reddy C. A. Ethylene production from alpha-oxo-gamma-methylthiobutyric acid is a sensitive measure of ligninolytic activity by Phanerochaete chrysosporium. Biochem J. 1982 Aug 15;206(2):423–425. doi: 10.1042/bj2060423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser P., Kirk T. K., Zeikus J. G. Ligninolytic enzyme system of Phanaerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol. 1978 Sep;135(3):790–797. doi: 10.1128/jb.135.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Connors W. J., Bleam R. D., Hackett W. F., Zeikus J. G. Preparation and microbial decomposition of synthetic [14C]ligins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2515–2519. doi: 10.1073/pnas.72.7.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Nakatsubo F., Reid I. D. Further study discounts role for singlet oxygen in fungal degradation of lignin model compounds. Biochem Biophys Res Commun. 1983 Feb 28;111(1):200–204. doi: 10.1016/s0006-291x(83)80136-3. [DOI] [PubMed] [Google Scholar]
- Kutsuki H., Gold M. H. Generation of hydroxyl radical and its involvement in lignin degradation by Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1982 Nov 30;109(2):320–327. doi: 10.1016/0006-291x(82)91723-5. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- Nakatsubo F., Reid I. D., Kirk T. K. Involvement of singlet oxygen in the fungal degradation of lignin. Biochem Biophys Res Commun. 1981 Sep 16;102(1):484–491. doi: 10.1016/0006-291x(81)91545-x. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Tang R. H. Ethylene formation from methional. Biochem Biophys Res Commun. 1978 Mar 30;81(2):498–503. doi: 10.1016/0006-291x(78)91562-0. [DOI] [PubMed] [Google Scholar]
- Sagone A. L., Jr, Decker M. A., Wells R. M., Democko C. A new method for the detection of hydroxyl radical production by phagocytic cells. Biochim Biophys Acta. 1980 Feb 21;628(1):90–97. doi: 10.1016/0304-4165(80)90354-2. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Freeman B. A., Crapo J. D. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch Biochem Biophys. 1982 Sep;217(2):411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]


