Abstract
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide. Although a wide range of therapeutic options is available, the efficacy of these methods and the prognosis of patients with HCC remain very poor. This study was conducted to evaluate the efficacy and safety of viscum fraxini-2 in patients with chemotherapy–naïve, advanced hepatocellular carcinoma. 23 patients with unrespectable HCC who had received no prior systemic chemotherapy with objectively measurable tumors were enrolled on this study. The mistletoe preparation for the study is an aqueous injectable solution. It contains one milliliter of viscum fraxini in dilution stage–2 (15 mg extract of 20 mg mistletoe herb from ash tree, diluted in di-natrium-mono-hydrogen phosphate, ascorbic acid and water) which is equivalent to 10 000 ng/ml injection ampoules. 2 ampoules of viscum fraxini–2 were administered subcutaneously once weekly. As assessed by conventional imaging criteria, 3 (13.1%) patients have achieved complete response, 2 (8.1%) patients have achieved a partial response. 9 (39.1%) had progressive disease while 9 (39.1%) patients didn't have evaluation of response due to early death. The median overall survival time for all patients was 5 months (range 2–38 months), for those who achieved a CR was 29 months (range 12–38 months) and, for those who achieved a PR was 6.5 months (range 6–7 months). The median progression free survival for all patients was 2 months (range 1–38 months), for those who achieved a CR, it was 29 months (range 8–38 months) and for those who achieved a partial response, it was 5 months (range 4–6 months). No hematologic toxicity has been encountered. The spectrum of non-hematologic toxicity was mild. The WHO toxicity criteria grade 3–4 were 34.8% drug related fever, 13.1% erthyma at injection site and 17.4% pain at the site of injection. No drug related discontinuation or toxic deaths have occurred. Viscum fraxini-2 seems to be particularly promising in patients with advanced HCC, it shows antitumor activity and low toxicity profile. Further studies in combination with other active agents are clearly warranted.
Keywords: chemotherapy, hepatocellular carcinoma, phase II, viscum fraxini-2
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide. Although it is far less common in western countries, it is the most common malignant tumor in areas of Africa and Asia (Bosch and Munoz, 1991; Lotze et al, 1993). Although a wide range of therapeutic options is available, the efficacy of these methods and the prognosis of patients with HCC remain very poor. Surgical resection represents the only possibility of cure. However, resection rates for patients with HCC remain low because of a high incidence of associated cirrhosis, the direct invasion of the tumor into the portal or the hepatic veins, or early spread to the entire liver. Many non-surgical local treatments, such as cryosurgery and radiation therapy, have been proposed; however, considerable uncertainty remains about their effectiveness (Venook, 1994). Eventually, in most patients with HCC, the disease progress to a far-advanced stage for which effective local treatment is not available. These findings stress the pressing need for efficacious, systemic chemotherapy for patients with inoperable HCC.
The role of chemotherapy in the treatment of patients with HCC remains controversial. Numerous single chemotherapeutic agents and drug combinations have been given to HCC patients in an attempt to alter their predictably short survival time. Unfortunately, the activity of a single agent is limited, with only a few drugs showing a response rate >10%. Moreover, combination chemotherapy has proven equally disappointing, because additional drugs have resulted in increased toxicity without any increased efficacy compared with single–agent doxorubicin therapy (Whang-Peng and Chao, 1998). Therefore, there is no drug or protocol of treatment that can be recommended as standard therapy for this group of patients. Due to the lack of any effective systemic chemotherapy, there is an urgent need to investigate new drugs.
Viscum album L. is a semi parasitic plant growing on different host trees (Becker, 1986). The extracted mistletoes are composed of many biologically active substances. The principle of the mistletoe phytotherapeutics can be considered as combined cytotoxic and biological response modifying activities that result from the activities of the plant lectins and other biologically relevant substances (Zarkovic et al, 2001). In 1920, Steiner recommended the mistletoe as a remedy against cancer (Steiner, 1961). In a systematic review on controlled clinical trials, twenty-three studies were identified: 16 randomized, 2 quasi-randomized and 5 non-randomized. Cancer sites included breast, lung, stomach, colon, rectum, head and neck, kidney, bladder, melanoma, glioma, and genital. Among these studies, statistically significant positive outcomes were reported for survival (n=8), tumor remission (n=1), overall quality of life (n=3), and quality of life in relation to side effects during cytoreductive therapy (n=3) (Kienle et al, 2003).
Viscum Fraxini is an aqueous extract of mistletoe (Viscum album L. grown on ash trees) (Koehler, 1992) and it is the preparation with the highest lectin content (Scheer, 1996). We herein, report the results of a phase II study to evaluate its efficacy and safety in the treatment of patients with advanced HCC.
PATIENTS AND METHODS
Eligibility criteria
The eligibility criteria included: (1) pathology proven primary HCC or α-fetoprotein >400 ng/ml with a hepatic tumor highly suggestive of HCC by imaging studies; (2) unresectable tumor and patient was not a candidate for either transcatheter arterial chemoembolization (TACE) or percutaneous ethanol injection (PEI); (3) bi-dimensional measurable disease; (4) no previous systemic chemotherapy; (5) age between 16 years and 75 years; (6) performance status 0–3 WHO, 7) within normal renal, cardiac and hematological profile.
Treatment protocol
Prior to entry into the study, all patients provided a complete history and physical examination, including performance status, concurrent nonmalignant diseases and therapy. Laboratory studies included a complete blood cell counts, differential count, biochemical liver and renal function tests, electrolyte, chest x-rays, α-fetoprotein, triphasic liver computed scan (CT) and Child class evaluation were performed before treatment. The mistletoe preparation for the study is an aqueous injectable solution. It contains one milliliter of viscum fraxini in dilution stage–2 (15 mg extract of 20 mg mistletoe herb from ash tree, diluted in di-natrium-mono-hydrogen phosphate, ascorbic acid and water) which is equivalent to 10 000 ng/ml injection ampoules. 2 ampoules of viscum fraxini–2 were administered subcutaneously once weekly. Patients were seen on a weekly basis during treatment for history taking and physical examination. A complete blood count was determined every week. Renal and liver functions and α-fetoprotein levels were examined every 4 weeks. The tumor was assessed by CT every 8 weeks.
Definition of response
Determination of the tumor response followed standard response criteria established by the World Health Organization (WHO) (Miller et al, 1981). Complete response (CR) was defined as the complete disappearance of all known lesions on radiological grounds for at least 4 weeks. Partial response (PR) was defined as a decrease of 50% or more in the product of two perpendicular diameters of the largest tumor nodule for at least 4 weeks without the appearance of new lesions or progression of lesions. Static disease (SD) was defined as a <50% decrease, or not more than a 25% increase, in the product of two perpendicular diameters of the largest tumor nodule. Progressive disease (PD) was defined as >25% increase in the product of two perpendicular diameters of the largest tumor nodule or one of the measurable lesions, or the appearance of new lesions. Patients who did not survive to reassessment by radiological methods were considered to have undetermined response (UR).
Toxicity
Evaluation of toxicity, classified according to the criteria of the WHO (World Health Organization, 1979) included physical examination prior to every injection, complete blood cell counts and serum assessment of renal and hepatic functions. Each patient after having at least one dose of protocol therapy was evaluable for toxicity.
Statistical analysis
Descriptive statistics are reported as percentages and medians. The overall survival time was calculated from the start of therapy to the date of death or the last visit of the patient. The time to disease progression was defined from the start of therapy to the date of disease progression. Survival curves were constructed using the Kaplan-Meier productlimit method (Kaplan and Meier, 1959).
Study approval
The study was approved by the local ethics committee and all patients signed informed consent before entering the study.
RESULTS
Patient characteristics
Base line patient characteristics and clinical features are summarized in Table 1 . Twenty-three patients were entered into the trial. They were 20 males and 3 females, aged 39–75 years with a median of 54 years. Their WHO performance status was 1 in 10 patients (43.5%), 2 in 7 patients (30.4%) and 3 in 6 patients (26.1%). HbsAg and anti HCV were positive in 13 patients (56.5%) and 8 patients (34.8%), respectively, while 2 patients (8.7%) were positive for both. All patients were chemotherapy naı"ve. The median duration of treatment on viscum fraxini-2 is 17 weeks (range 3–152 weeks). Okuda stage I was found in 4 patients (17.4%), stage II in 12 patients (52.2%) and stage III in 7 patients (30.4%). The diagnosis of HCC was based on fine-needle aspiration cytology of liver tumors in 14 patients (60.9%). The remaining 9 patients (39.1%) were diagnosed by marked elevation of α-fetoprotein level and imaging studies indicating advanced HCC. All of the patients had far-advanced HCC at the time of diagnosis. Bilateral lobar affection of the liver was present in 8 patients (34.8%). Extensive one lobe affection was present in 15 patients (65.2%). Distant metastasis were present in 4 patients (17.4%) (2 bone metastasis and 2 lymph node metastasis). Main portal vein thrombosis was detected in two patients (8.7%) while ascites was present in 4 patients (17.4%).
Table 1. Patients Characteristics.
| Characteristic | No. of patients | % |
|---|---|---|
| Male: female (total) | 20 : 3 (23) | |
| Median age in years (range) | 54 (39–75) | |
| Performance status | ||
| 1 | 10 | 43.5 |
| 2 | 7 | 30.4 |
| 3 | 6 | 26.1 |
| Hepatitis | ||
| HbsAg (+) | 13 | 56.5 |
| Anti HCV (+) | 8 | 34.8 |
| Both (+) | 2 | 8.7 |
| Child's classification | ||
| A | 9 | 39.1 |
| B | 6 | 26.1 |
| C | 8 | 34.8 |
| Okuda stage: | ||
| I | 4 | 17.4 |
| II | 12 | 52.2 |
| III | 7 | 30.4 |
| Diagnosis: | ||
| Cytology | 14 | 60.9 |
| α-fetoprotein and imaging | 9 | 39.1 |
| Tumor status | ||
| Bilateral lobe affection | 8 | 34.8 |
| Unilateral lobe affection | 15 | 65.2 |
| Distant metastasis | 4 | 17.4 |
| Main Portal vein Thrombosis | 2 | 8.7 |
| Ascites | 4 | 17.4 |
Response
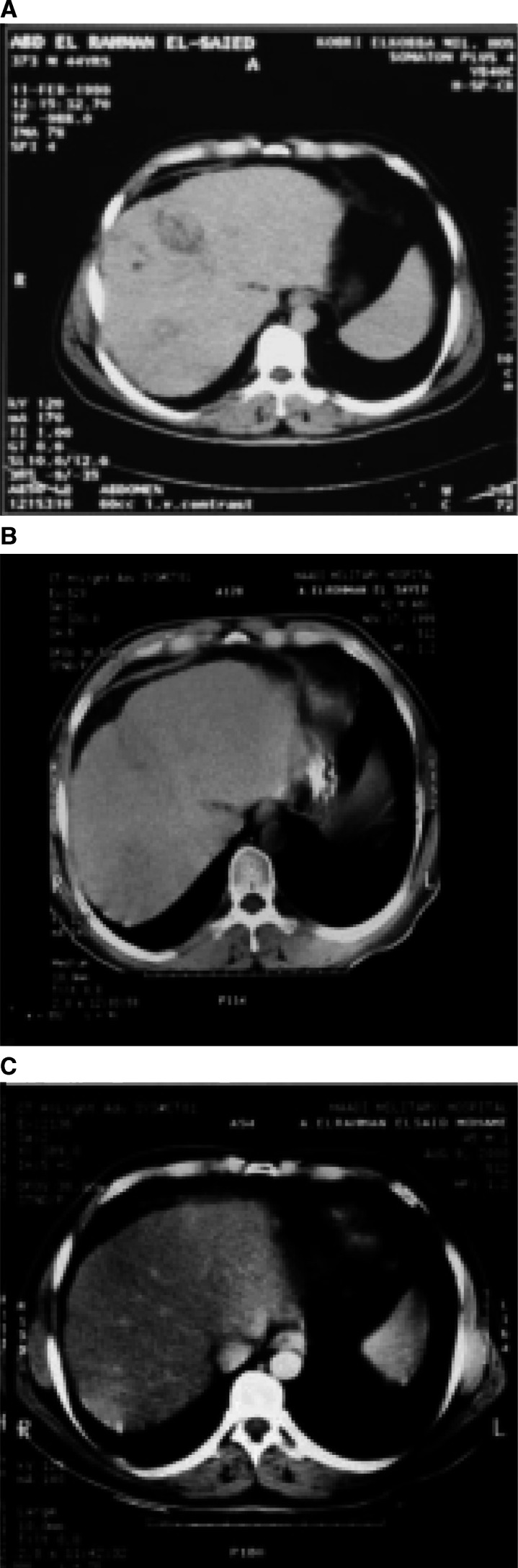
According to conventional radiological response criteria, 3 patients (13.1%) achieved complete response. The first patient achieved CR after 4 months from starting the treatment and remained disease free for 4 months. The second and the third patients achieved CR after 6 months and they are still living disease free for more than 29 and 38 months, respectively (CT scan of the third patients before treatment and after disappearance of the tumor is shown in Figure 1). 2 patients (8.7%) achieved partial 9 response. Progressive disease has been shown in 9 patients (39.1%). 9 patients (39.1%) did not have evaluation of response due to early death and they were classified as UR.
Figure 1.
A case of hepatocellular carcinoma with multiple focal lesions before treatment (A) with regression of the tumor (B) and disappearance of the tumor (C) after 6 months of viscum fraxini-2 therapy.
Survival
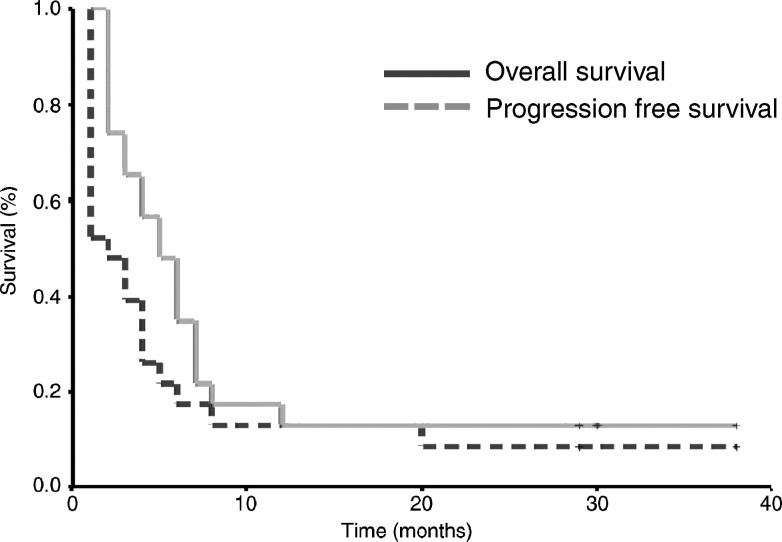
At the time of analysis, 3 (13.1%) patients remained alive including two patients with CR and one patient with slowly progressive disease. The median overall survival time for all patients was 5 months (range 2–38 months), for those who achieved a CR was 29 months (range 12–38 months) and, for those who achieved a PR was 6.5 months (range 6–7 months). The median progression free survival for all patients was 2 months (range 1–38 months), for those who achieved a CR, it was 29 months (range 8–38 months) and for those who achieved a partial response, it was 5 months (range 4–6 months). The Kaplan- Meier actuarial overall survival and PFS curves for all patients are shown in Figure 2.
Figure 2.
Acturial overall and progression free survival curve.
Toxicity
All patients were evaluated for toxicity. Drug related fever developed in 8 patients (34.8%). Erythma at injection site developed in 3 patients (13.1%). 4 patients (17.4%) suffered pain at site of injection. 3 patients had to reduce the dose to one ampoule on the subsequent treatment courses. Anti-inflammatory and analgesics were used in only one patient due to severe pain and erythma at injection site. There were no drug related discontinuation or toxic deaths.
DISCUSSION
Hepatocellular carcinoma is one of the most common cancers worldwide (Okuda, 1980). Resection is perhaps the only meaningful chance of cure. Although early diagnosis is becoming more frequent, particularly in those populations subjected to screening programs, resection rates for patients with HCC remain low (Lee et al, 1982 and Okuda et al, 1985). Therefore, the vast majority of patients with HCC are not candidates for curative surgery or other local therapy and systemic chemotherapy may be the only option for their treatment.
The antitumor activities of a number of chemotherapeutic agents have been evaluated in HCC patients, but most yielded poor results and probably are associated with severe side effects. Doxorubicin remains the most active drug against HCC, with a single agent tumor response rate of about 10–20%. However, its toxicity outweighs its benefit (Lai et al, 1988; Lai et al, 1990). Chemotherapeutic agents other than doxorubicin have demonstrated even less activity and progress in treating HCC patients with chemotherapy has been disappointing. New chemotherapeutic drugs, such as paclitaxel, raltrexed, irinotecan, and nolatrexed, have not demonstrated encouraging results. These new drugs exhibit some antitumor activity, but response rates rarely exceed 10% (Rougier et al, 1997; Chao et al, 1998; O'Reilly et al, 1998; Stuart et al, 1999). The possible explanations for the refractioness of HCC to chemotherapy include tumor heterogeneity (Dexter and Leith, 1986), inadequate dosage of anticancer agents (Lai et al, 1990) and the inducible overexpression of the multidrug resistance gene (Huang et al, 1992). Therefore, all patients with advanced HCC should be considered for joining well– designed phase II trials with novel antitumor agents or regimens when patients can tolerate treatment.
Viscum fraxini is an aqueous extract of mistletoe. Controversy exists as to how the plant extracts exert their postulated dual activity of cytotoxicity towards tumor cells and stimulation of immune cells (Janssen et al, 1993).
Immunostimulatory effects of mistletoe extract have been assigned to a low molecular weight oligosaccharide compound (Hamprecht et al, 1987; Mueller et al, 1989). Moreover, the stimulatory effect on natural killer (NK) cell activity appears to result from enhanced production of interferon (INF-γ) and tumor necrosis factor-α (TNF-α) by T cells and macrophages respectively (Mueller and Anderer, 1990). On the other hand, there is some evidence that lectin components are important effector molecules in mistletoe preparations (Luther and Becker, 1987). The lectin components increase the total number and the activities of neutrophils, NK cells and large granular lymphocytes as well as they activate simultaneously monocyte-macrophage and helper cell subpopulations. This was associated with higher levels of cytokines such as interleukins IL-1, IL-6, granulocyte macrophage colony stimulating factor (GM-CSF) and TNF-α (Hajto et al, 1990, Schultze et al, 1991; Beuth et al, 1993).The described immunological effects of mistletoe polysaccharides are supplemented by membrane lipids. As shown by Hartmann et al (1995) isolated vesicles of genuine membrane systems were the most potent stimulating factor for T-cell proliferation of mistletoe extracts (Hartmann et al, 1995).
Apart from the immunostimulatory effect of mistletoe extract and lectins, it has been reported that they have a direct cytotoxic effect (Ribereau–Gayon et al, 1986; Urech et al, 1996). Lectins are considered essential components of mistletoe aqueous extract responsible for apoptosis (Janssen et al, 1993). Viscotoxins which are a group of cytotoxic polypeptides belonging to the class of thionins (Schrader-Fischer and Apel, 1993) can enhance the permeability of cell membrane (Lankish and Vogt, 1978). So, mistletoe extracts are able to induce both ways of biological cell death; apoptosis and lysis. Moreover, these initial effects subsequently induce immune stimulating reactions.
Taken together, complete mistletoe extract containing membrane lipids like viscum fraxini affects both arms of immune system, the T-cell and the B-cell parts, initially inducing proliferation and activation of immune competent cells as well as expression of cytokines, supporting antitumoral effects.
In the light of these observations, the present study aimed to improve efficacy of systemic chemotherapy. HCC patients with inoperable disease or exrtahepatic disease who were not suitable for regional intra-arterial treatment were enrolled in this study. The treatment resulted in an overall response rate of 21.74% (5/23) with a good impact on survival. While most of Phase II trials of treatment of HCC confirmed that there is no effective drug for HCC and all single systemic anticancer agents produced a response rate of less than 10% (Yoshida et al, 1988; Lin et al, 1993; Okada et al, 1993; Wierzbicki et al, 1994; Falkson and Burger, 1995), Viscum Fraxini-2 in our study showed encouraging results and might represent a good opportunity for treating patients with advanced HCC.
In conclusion, Viscum Fraxini-2 is active in HCC with anti-tumor activity and low toxicity profile. Further studies in combination with other active agents are clearly warranted.
Acknowledgments
This work was presented at the 27th ESMO Congress, 18–22 October, 2002, Nice, France.
References
- Becker H (1986) Botany of European mistletoe (Viscum album L.). Oncology 43: 2–7 [DOI] [PubMed] [Google Scholar]
- Beuth J, KO HL, Tungal L, Steuer MK, Geisel J, Jeljaszewics J, Pulverer G (1993) Das lektin der mistel als immunomodulatoe in der adjuvanten tumortherapie. Detsche zeitschrift fur onkologie 25(3): 73–76 [Google Scholar]
- Bosch FX, Munoz N (1991) Hepatocellular carcinoma in the world: epidemiologic questions in etiology, pathology and treatment of hepatocellular carcinoma in North America. In: Tabor E, DiBisecglie AM, Purcell RH, (eds) Advances in the Applied Biotechnology Series, pp 35–54
- Chao Y, Chan WK, Birkhofer MJ, Hu OPY, Wang SS, Huang YS, Liu M, Whang-Peng J, Chi KH, Lui WY, Lee SD (1998) Phase II and pharmacokinetic study of paclitaxel therapy for unresectable hepatocellular carcinoma. Br J Cancer 78(1): 34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter DL, Leith JT (1986) Tumor heterogeneity and drug resistance. J Clin Oncol 4: 244–257 [DOI] [PubMed] [Google Scholar]
- Falkson G, Burger W (1995) A phase II trial of Vindesine in hepatocellular carcinoma. Oncology 52: 86–87 [DOI] [PubMed] [Google Scholar]
- Hajto T, Hostanska K, Frel K, Rohrdorf C, Gabius HJ (1990) Increased secretion of tumor necrosis factor-α, interleukin-1, and interleukin-6 by human mononuclear cells exposed to B–galactosid–specific lectin from clinically applied mistletoe extract. Cancer Research 50: 3322–3326 [PubMed] [Google Scholar]
- Hamprecht K, Handgretinger R, Voetsch W, Anderer FA (1987) Mediation of human NK-activity by components in extracts of viscum album. Int J Immunopharmacol 9: 199–209 [DOI] [PubMed] [Google Scholar]
- Hartmann S, Scheffler A, Lessmann M (1995) A vesicle fraction of mistletoe induces T cell proliferation of sensitized lymphocytes in patients treated with mistletoe extracts. Beitrag Zur Tagung Der Immunologischen Gesellschaft: Regensburg [Google Scholar]
- Huang C, Wu M, Xu G, LiDZ, Cheng H, Tu ZX, Jiang HO, Gu JR (1992) Overexpression of the MDRI gene and P-glycoprotein in human hepatocellular carcinoma. J Natl Cancer Inst 84: 262–264 [DOI] [PubMed] [Google Scholar]
- Janssen O, Scheffler A, Kabelitz D (1993) In vitro effects of mistletoe extracts and mistletoe lectins: Cytotoxicity towards tumor cells due to the induction of programmed cell death (apoptosis). Arzneimittel Forschung, Drug–Research 43(11): 1221–1227 [PubMed] [Google Scholar]
- Kaplan EM, Meier P (1959) Non parametric estimation from incomplete observations. J Am Stat Assoc 53: 457–481 [Google Scholar]
- Kienle GS, Berrino F, Bussing A, Portalupi E, Rosenzweig S, Kiene H (2003) Mistletoe in cancer - a systematic review on controlled clinical trials. Eur J Med Res 8(3): 109–119 [PubMed] [Google Scholar]
- Koehler R (1992) Verfahren und vorrichtung zur herstellung wassriger kolloide. Europaisches Patent Nr. 0310984
- Lai ECS, Choi TK, Cheng CH, Mok FP, Fan ST, Tan ES, Wong J (1990) Doxorubicin for unresectable hepatocellular carcinoma. A prospective study on the addition of verapamil. Cancer 66: 1685–1687 [DOI] [PubMed] [Google Scholar]
- Lai CI, Wu PC, Chan GCB, Lok ASF, Lin HJ (1988) Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. Cancer 62: 479–483 [DOI] [PubMed] [Google Scholar]
- Lankish PG, Vogt W (1978) Potentiation of hemolysis by the combined action of phospholipase A and a basic peptide containing s-s bonds (viscotoxin B). Experientia 27(2): 122–123 [DOI] [PubMed] [Google Scholar]
- Lee NW, Wong J, Ong GB (1982) The surgical management of primary carcinoma of the liver. World J Surg 6: 66–75 [DOI] [PubMed] [Google Scholar]
- Lin J, Shiu W, Leung WT, Tao M, Leung N, Lau WY, Li AK (1993) Phase II study of high dose Ifosfamide in hepatocellular carcinoma. Cancer Chemotherapy Pharmacol 31: 338–339 [DOI] [PubMed] [Google Scholar]
- Lotze MT, Flickinger JC, Carr BJ (1993) Hepatobiliary system. In: DeVita VT, Hellman S, Rosenberg SA, (eds) Cancer: principles and practice of oncology, ed 4. pp 883–914. Philadelphia: J.B. Lippincott [Google Scholar]
- Luther P, Becker H (1987) Die Mistel. Berlin: Spring Verlag [Google Scholar]
- Miller AB, Hoogstraten B, Ataquet M (1981) Reporting results of cancer treatment. Cancer 47: 207–214 [DOI] [PubMed] [Google Scholar]
- Mueller EA, Anderer FA (1990) Synergistic action of a plant rhamnogalacturonan enhancing antitumor cytotoxicity of human natural killer and lymphokin–activated killer cells: chemical specificity of target cell recognition. Cancer Research 50: 3646–3651 [PubMed] [Google Scholar]
- Mueller EA, Hamprecht K, Anderer FA (1989) Biochemical characterization of a component in extracts of viscum album enhancing human NK cytotoxicity. Immunopharmacology 17: 11–18 [DOI] [PubMed] [Google Scholar]
- Okada S, Okazaki N, Nose H, Shimada Y, Yoshimori M, Aoki K (1993) A phase 2 study of cisplatin in patients with hepatocellular carcinoma. Oncology 50: 22–26 [DOI] [PubMed] [Google Scholar]
- Okuda K (1980) Primary liver cancer in Japan. Cancer (Phila.) 45: 2663–26726155197 [Google Scholar]
- Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K (1985) Natural history of hepatocellular carcinoma and prognosis in relation to treatment: study of 850 patients. Cancer (phila.) 56: 918–928 [DOI] [PubMed] [Google Scholar]
- O'Reilly E, Stuart K, Kemeny N, Steger C, Raeburn I, Sanz-Altamira P (1998) A phase II trial of irinotecan (CPT-II) in patients with advanced hepatocellular carcinoma. Proc Am Soc Clin Oncol 267: 1026 [Google Scholar]
- Ribereau–Gayon G, Jung ML, Baudino S, Salle JP (1986) Effects of mistletoe (Viscum album L.) extracts on cultured tumor cells. Experientia 42: 594–599 [DOI] [PubMed] [Google Scholar]
- Rougier P, Ducreux M, Kerr D, Carr BI, Francois E, Ednis A, Seymour L (1997) A phase II study of raltitrexed (Tomudex) in patients with hepatocellular carcinoma. Ann Oncol 8: 500–502 [DOI] [PubMed] [Google Scholar]
- Scheer R (1996) Sicherung der pharmazeutischen Qualitat von mistelpraparaten. In: R. Scheer, H. Becker, PA Berg (Ed) Grundlagen der Misteltherapie, pp 139–150. Stuttgart: Hippokrates Verlage [Google Scholar]
- Schrader-Fischer G, Apel K (1993) The anticyclic timing of leaf senescence in the parasitic plant viscum album is closely correlated with the selective degradation of sulfur-rich viscotoxin. Plant physiology 101: 745–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze JL, Stettin A, Berg PA (1991) Demonstration of specifically sensitized lymphocytes in patients treated with an aqueous mistletoe extract (Viscum album L.). Klinische wochenschrift 69: 397–403 [DOI] [PubMed] [Google Scholar]
- Steiner R (1961) Vortrag vom 2. 4. 1920; Geisteswissenschaft und Medizin GA 312, pp 246–262. Dornach: Verlag der Rudolf Steiner NachlaBverwaltung [Google Scholar]
- Stuart K, Tessitore J, Rudy J, Clendennin N, Johnston A (1999) A phase II trial of nolatrexed dihydrochloride in patients with advanced hepatocellular carcinoma. Cancer 86: 410–414 [PubMed] [Google Scholar]
- Urech K, Schaller G, Giannattasio M (1996) Bioassay zur bestmmung von viscotoxinen. In: Scheer R, Becker H, Berg PA (eds) Grundlagen der mistletherapie, pp 111–118. Stutgart: Hippokrates Verlag [Google Scholar]
- Venook AP (1994) Treatment of hepatocellular carcinoma: Too many options? J Clin Oncol 12: 1232–1234 [DOI] [PubMed] [Google Scholar]
- Whang-Peng J, Chao Y (1998) Clinical trials of hepatocellular carcinoma in Taiwan. Hepatogastroenterology 45(24): 1937–1943 [PubMed] [Google Scholar]
- Wierzbicki R, Ezzat A, Abdel-Warith A, Ayoub A, Kagevi I, Fadda M, Siech J, Abdul Kareem M, Amin T, Yazigi A (1994) Phase II trial of chronic daily VP 16 administration in unresectable hepatocellular carcinoma (HCC). Ann Oncol 5: 466–467 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1979) WHO Handbook for Reporting Results of Cancer Treatment. Geneva: WHO Offset Publications [Google Scholar]
- Yoshida T, Okazaki N, Yoshino M, Ohkura H, Miyamoto K, Shimado Y (1988) Phase II trial of mitoxantrone in patients with hepatocellular carcinoma. Eur J Cancer Clin Oncol 24: 1897–1898 [DOI] [PubMed] [Google Scholar]
- Zarkovic N, Vukovic T, Loncaric I, Miletic M, Zarkovic K, Borovic S, Cipak A, Sabolovic S, Konitzer M, Mang S (2001) An overview on anticancer activities of the vsicum album extract Isorel. Cancer Biother Radiopharm 16: 55–62 [DOI] [PubMed] [Google Scholar]