Abstract
Nonsteroidal anti-inflammatory drugs (NSAIDs) have chemopreventive potential against colorectal carcinomas (CRCs). Inhibition of cyclooxygenase (COX)-2 underlies part of this effect, although COX-2-independent mechanisms may also exist. Nonsteroidal anti-inflammatory drugs appear to inhibit the initial stages of the adenoma–carcinoma sequence, suggesting a link to the APC/β-catenin/TCF pathway (Wnt-signalling pathway). Therefore, the effect of the NSAID sulindac on nuclear (nonphosphorylated) β-catenin and β-catenin/TCF-mediated transcription was investigated. Nuclear β-catenin expression was assessed in pretreatment colorectal adenomas and in adenomas after treatment with sulindac from five patients with familial adenomatous polyposis (FAP). Also, the effect of sulindac sulphide on β-catenin/TCF-mediated transcription was studied. Adenomas of FAP patients collected after treatment with sulindac for up to 6 months showed less nuclear β-catenin expression compared to pretreatment adenomas of the same patients. Sulindac sulphide abrogated β-catenin/TCF-mediated transcription in the CRC cell lines DLD1 and SW480, and decreased the levels of nonphosphorylated β-catenin. As a result, the protein levels of the positively regulated TCF targets Met and cyclin D1 were downregulated after sulindac treatment. This study provides in vivo and in vitro evidence that nuclear β-catenin localisation and β-catenin/TCF-regulated transcription of target genes can be inhibited by sulindac. The inhibition of Wnt-signalling provides an explanation for the COX-2-independent mechanism of chemoprevention by NSAIDs.
Keywords: sulindac, TCF, FAP
Epidemiological data, rodent studies and in vitro experiments have demonstrated that nonsteroidal anti-inflammatory drugs (NSAIDs) have anticolorectal cancer (CRC) activity (Giardiello et al, 1995). Also, in patients with familial adenomatous polyposis (FAP), an autosomal dominantly inherited disorder characterised by the development of numerous colorectal adenomas at a young age, the NSAIDs sulindac and indomethacin can cause regression of adenomas (Giardiello et al, 1993; Nugent et al, 1993; Spagnesi et al, 1994; Hirota et al, 1996; Winde et al, 1997; Picariello et al, 1998). The chemopreventive effect of NSAIDs appears mediated by the induction of apoptosis and cell cycle arrest (Pasricha et al, 1995; DuBois and Smalley, 1996; Piazza et al, 1997; Keller et al, 1999; Shiff and Rigas, 1999). The molecular mechanisms underlying these biological effects are not completely understood. Nonsteroidal anti-inflammatory drugs inhibit the enzymatic activity of cyclooxygenase (COX)-1 and -2, enzymes that convert arachidonic acid into prostaglandins (Shiff and Rigas, 1999). However, COX-independent mechanisms may also play a role, since NSAIDs inhibit the growth of colon cancer cell lines lacking COX-2 expression (Hanif et al, 1996; Zhang et al, 1999; Smith et al, 2000).
Oncogenic activation of the Wnt-signalling pathway by mutations in Adenomatous polyposis coli (APC) or β-catenin, which results in the accumulation and nuclear translocation of β-catenin and in β-catenin/TCF4-regulated transcription of TCF target genes, is mandatory for the initial neoplastic transformation of intestinal epithelium (reviewed in Kinzler and Vogelstein, 1996; Bienz and Clevers, 2000). Previous studies have shown an effect of NSAIDs on the expression and localisation of β-catenin, suggesting nuclear β-catenin as an alternative target for the chemopreventive effect of NSAIDs. Nonsteroidal anti-inflammatory drugs were shown to prevent the nuclear accumulation of β-catenin in chemically induced colon tumours in rats (Brown et al, 2001) and in human colorectal cancer cell lines (Smith et al, 2000; Hawcroft et al, 2002). In addition, indomethacin and aspirin can downregulate the expression of the TCF target gene cyclin D1 in CRC cell lines (Dihlmann et al, 2001; Hawcroft et al, 2002). Together, these data suggest that NSAIDs may exert an antineoplastic effect by inhibiting the Wnt-signalling pathway. Previously, we reported low levels of nuclear β-catenin in sulindac-resistant adenomas (Keller et al, 2001). This could reflect a downregulation of nuclear β-catenin by sulindac or represent an intrinsic feature of resistant adenomas. In the present study, we therefore compared nuclear accumulation of β-catenin in adenomas from FAP patients before and after treatment with sulindac for up to 6 months (Giardiello et al, 1993). In addition, we studied the effects of the active metabolite of sulindac, sulindac sulphide, on Wnt-signalling in human CRC cell lines.
MATERIALS AND METHODS
Patients and adenoma specimens
The study population consisted of five FAP patients who were treated with sulindac 150 mg p.o. twice a day as described previously (Giardiello et al, 1993, 1996; Cruz-Correa et al, 2002). All patients had adenomas at the initiation of treatment (baseline) and showed adenoma regression after 6 months treatment with sulindac. Patients were selected on the basis of availability of both adenomas collected at baseline and during the first 6 months of treatment with sulindac. Patient characteristics are shown in Table 1. In all, 21 polyps from five patients were studied.
Table 1. Patient characteristics.
|
Polyp count |
Polyps studied |
|||||||
|---|---|---|---|---|---|---|---|---|
| Patients | Age (years) | Sex | APC mutation | Colectomy/intact colon | Baseline | 6 months | Baseline | After sulindac |
| I | 32 | F | Not tested | IRA | 19 | 3a | 2 | 1 |
| II | 42 | F | Codon 423 | IRA | 47 | 6a | 2 | 6 |
| III | 52 | F | Not tested | IRA | 10 | 0 | 3 | 2b |
| IV | 25 | F | Codon 1061 | IRA | 7 | 0 | 1 | 2b |
| V | 23 | F | Segments 2–3c | IRA | 10 | 3 | 1 | 1 |
Complete response after 10 months treatment with sulindac.
Studied after 4 months treatment with sulindac.
Assessed with protein truncation test, mutation between codons 1099 and 1217 (overlapping part of segments 2 and 3 of APC protein). Listed are age, sex, mutational status, surgical status, polyp count before and after 6 months sulindac and numbers of polyps studied.
F=female; IRA=ileorectal anastomosis; APC=Adenomatous polyposis coli.
Immunohistochemistry for β-catenin
Immunohistochemistry was performed on 5 μm sections of formalin-fixed, paraffin-embedded samples as described previously (Entius et al, 2000). For antigen retrieval, the slides were boiled for 10 min in citrate buffer followed by an overnight incubation at 4°C with a primary monoclonal antibody against β-catenin, clone 14 (Transduction Laboratories, Lexington, KY, USA). The staining pattern in adjacent normal mucosa was used as an indication for specificity.
Immunostained slides were scored semiquantitatively using a scale from 0 to 3 (0: no expression; 1: <5% positive nuclei; 2: 5–25% positive nuclei; 3: >25% positive nuclei). Also, membranous staining was scored separately as normal or decreased in comparison to adjacent mucosa. Slides were assessed in a coded manner by two independent observers (JJK and GJAO) and discrepancies were solved by consensus. Comparisons of staining patterns between adenomas collected at baseline and during treatment with sulindac were made using the nonparametric Mann–Whitney test and Fisher's exact test. A P<0.05 was considered statistically significant; P-values were two-sided.
Cell culture and TCF reporter analysis
The human CRC cell lines DLD1 and SW480 were grown in RPMI medium supplemented with 10% fetal calf serum, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin (all from Life Technologies, Paisley, UK). Colorectal carcinoma cells were transiently transfected with either 5 μg pTOPflash or pFOPflash reporter plasmids (Upstate Biotechnology, Lake Placid, NY, USA) using lipofectamine (Invitrogen, Paisley, UK). To correct for differences in transfection efficiency, cells were cotransfected with a reporter plasmid containing a GFP gene. Transfection efficiency was determined in live cells by counting, with a fluorescence microscope fitted with phase-contrast optics, the number of GFP-positive cells as well as the total cell number in 10 independent optical fields. The resulting transfection efficiency was used to correct for differences in transfection in each culture well in each experiment. All experiments were performed in triplicate. Cells were grown with or without the addition of sulindac sulphide (100 μM) for 24 or 48 h. In addition, cells were grown with different concentrations of sulindac sulphide (0–100 μM) for 24 h. Sulindac is in vivo metabolised into sulindac sulphide and sulindac sulphone by the intestinal flora. As these bacteria are not present in in vitro experiments, sulindac cannot be converted into its active metabolite. Therefore, we used the active metabolite of sulindac, sulindac sulphide, in our in vitro experiments. The concentration range of sulindac sulphide in this study was equivalent to that used by others (Zhang et al, 1999; Smith et al, 2000; Hawcroft et al, 2002). Sulindac sulphide was prepared as 1000 × stock solution in dimethyl sulphoxide (DMSO). Control cultures contained DMSO at an equivalent dilution, resulting in a final DMSO concentration of 0.1%. After the indicated period of time, cells were counted and equal numbers of cells were lysed in luciferase reporter lysis buffer (Promega, Madison, NY, USA). Cell lysates were monitored for luciferase activity using luciferase assay substrate buffer (Promega, Madison, NY, USA). Light units were recorded in a luminometer.
Western blot analysis
The CRC cells DLD1 and SW480 were grown with different concentrations of sulindac sulphide (0–100 μM) for 24 h. In addition, cells were grown with or without 100 μM sulindac sulphide for 24 or 48 h. Protein extracts were prepared by resuspending the cells (0.5 × 106 cells grown to subconfluence in a T25flask) into lysis buffer (10 mM Tris pH 8.0; 15 mM NaCl; 1% NP40, 10% glycerol; 0.4 mg ml−1 sodium orthovanadate). Protein extracts (50 μg) were separated by SDS–PAGE and blotted onto immobilon-P transfer membranes (Millipore corp., Bedford, USA) by tank blotting. Membranes were blocked in Tris-buffered saline (100 mM Tris-HCl pH 7.5; 150 mM NaCl) containing 0.1% Tween (Sigma, St Louis, MO, USA) and 5% nonfat dry milk, probed with monoclonal antibodies; 8E4 (against nonphosphorylated β-catenin, dilution 1 : 1000) (Alexis Biochemicals, San Diego, CA, USA) and AC-15 (against β-actin, dilution 1 : 3000) (Sigma), or polyclonal antibodies: H-102 (against β-catenin, dilution 1 : 1000), C12 (against Met, dilution 1 : 1000), M-20 (against cyclin D1, dilution 1 : 1000) (all Santa Cruz Biotechnology, Santa Cruz, CA, USA). Proteins were detected with horseradish peroxidase-conjugated secondary antibodies (Dakopatts, Glostrup, Denmark) and a standard chemiluminescence Western blotting protocol (ECL Western blotting, Amersham Pharmacia Biotech Inc., Aylesbury, UK). Blots were analysed by densitometry. Intensities were quantified using NIH Image software (NIH, USA). All signals were normalised for loading in comparison with the appropriate β-actin signal.
RESULTS
β-Catenin expression in adenomas before and after sulindac treatment
In all, 21 adenomas from FAP patients removed before (n=9) and after (n=12) treatment with sulindac for up to 6 months were evaluated. The median treatment duration was 3 months with a range of 2–6 months and a mean of 3.7 months. All adenomas were tubular or tubulovillous lesions, <1 cm, with mild to moderate dysplasia. No morphological differences were noted between adenomas removed before and after treatment. The nuclear accumulation of β-catenin was assessed semiquantitatively on immunostained slides. Immunostained slides were scored semiquantitatively as described in the Materials and methods section and summarised in Table 2 . There was significantly more nuclear accumulation of β-catenin in baseline adenomas compared to adenomas collected after treatment with sulindac (P<0.02)(Figure 1). Membranous β-catenin was decreased in nine out of nine baseline adenomas, and in nine out of 12 adenomas removed after sulindac (P>0.05). In the adjacent mucosa, a predominantly membranous staining without the nuclear accumulation of β-catenin was always observed.
Table 2. Nuclear β-catenin expression in adenomas before and after sulindac treatment.
|
Score |
||||
|---|---|---|---|---|
| No. of adenomas | 0 | 1 | 2 | 3 |
| Before treatment | 0 | 4 | 1 | 4 |
| After treatment* | 4 | 6 | 2 | 0 |
P=0.018.
Figure 1.
β-catenin expression in adenomas of FAP patients. Adenomas of FAP patients before (A) and after treatment with sulindac (B) were stained with an antibody against β-catenin. After sulindac treatment, the nuclear β-catenin staining (arrow) is strongly diminished (arrowhead).
Sulindac targets β-catenin/TCF-activated transcription
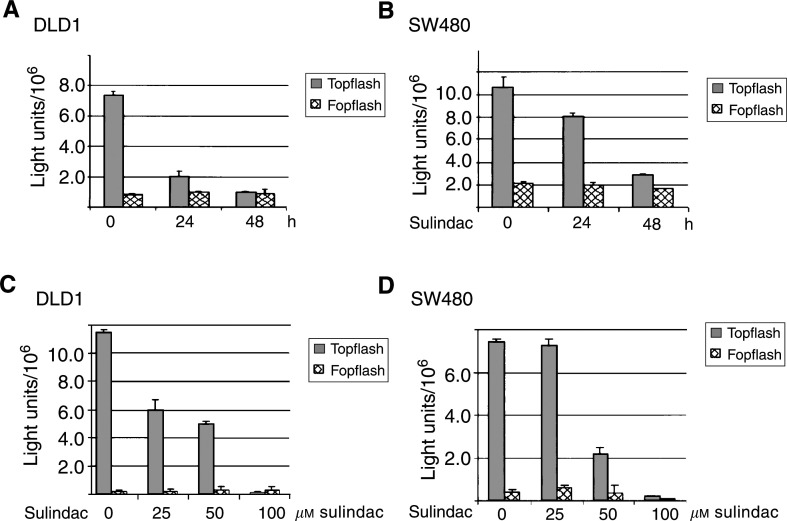
The molecular mechanism of chemopreventive action of sulindac sulphide was analysed in human colorectal cancer cell lines DLD1 and SW480. These cell lines contain a constitutively activated Wnt-signalling pathway caused by mutations in the APC gene and are devoid of COX-2 expression (Boon et al, 2002; and confirmed by Western blotting, data not shown). The inhibition of β-catenin/TCF transcriptional activation by sulindac sulphide was monitored by transfecting a TCF reporter (pTOPflash) or, as a control, a construct containing scrambled TCF binding sites (pFOPflash) in both CRC cell lines. Differences in transfection efficiency were corrected for by cotransfecting the cells with a GFP-encoding reporter plasmid, as described in the Materials and methods section. Following sulindac sulphide (100 μM) treatment for 24 or 48 h, β-catenin/TCF-activated TOPflash activity was abrogated in both DLD1 and SW480 cells (Figure 2A and B). Comparable results were observed by indomethacin treatment of the CRC cell lines (data not shown). The inhibition of TCF-mediated transcription was also seen after treatment of both cell lines with different concentrations (0–100 μM) of sulindac sulphide (Figure 2C and D). The effect of sulindac sulphide treatment on β-catenin/TCF-activated TOPflash activity was already visible after treatment of the cells with 25 μM sulindac sulphide. FOPflash activity remained unchanged in both cell lines, indicating that sulindac sulphide treatment inhibited the Wnt-signalling pathway.
Figure 2.
TCF reporter activity following sulindac treatment of DLD1 and SW480 cells. DLD1 (A) or SW480 cells (B) were treated with 100 μM sulindac sulphide for 24 or 48 h. In parallel, DLD1 (C) or SW480 (D) cells were treated with various concentrations of sulindac sulphide (0–100 μM) for 24 h. Cells were lysed and TOPflash and FOPflash activities were recorded in a luminometer.
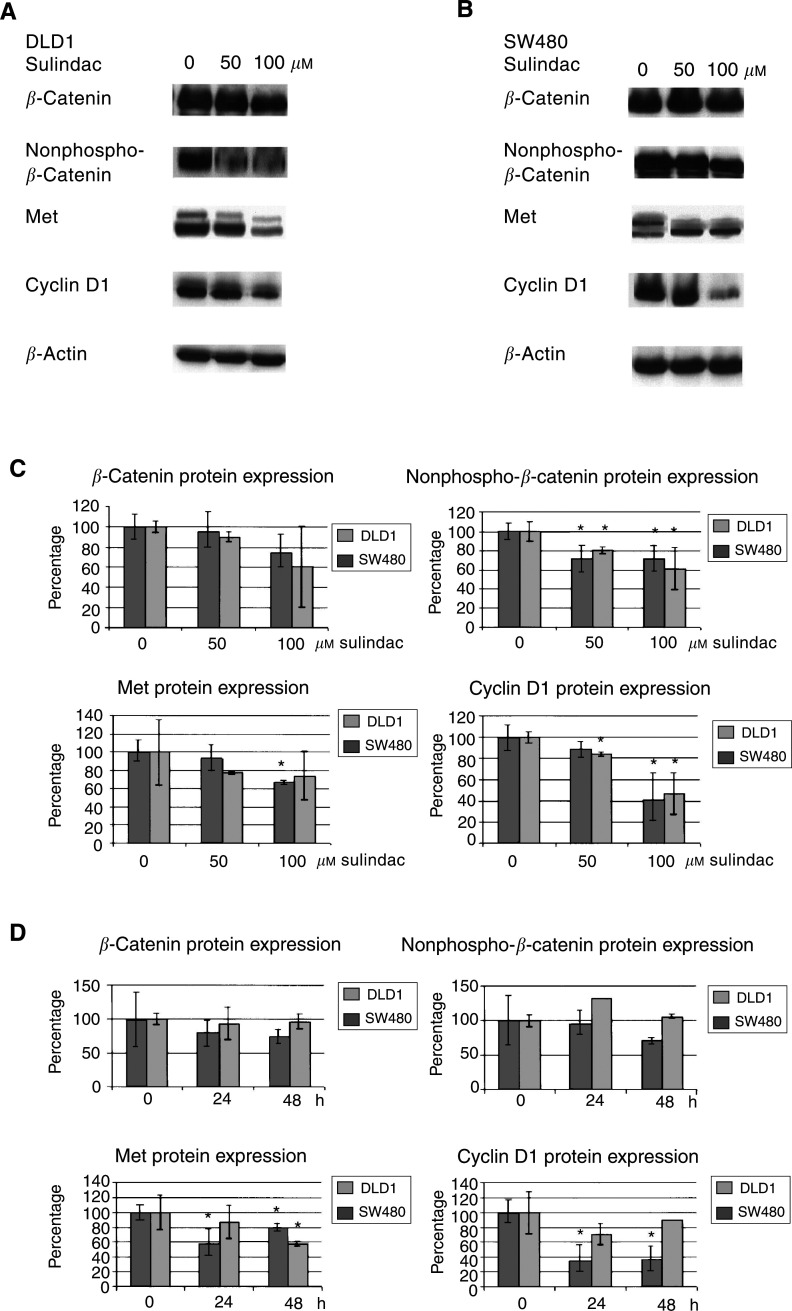
To further support this conclusion, we determined the effect of sulindac sulphide treatment on (i) the phosphorylation status of β-catenin and on (ii) the expression of TCF target genes. In the absence of Wnt signalling, β-catenin is phosphorylated by glycogen synthase kinase-3β (GSK-3β) and targeted for degradation by the ubiquitin–proteasomal pathway. Mutations in the APC gene, present in both DLD1 and SW480 cells, lead to a reduced phosphorylation of β-catenin and to the accumulation of nonphosphorylated β-catenin in the nucleus. Following sulindac sulphide treatment, a decrease in nonphosphorylated β-catenin expression in both cell lines was noted (Figure 3). Furthermore, the expression levels of the TCF target genes Met (Boon et al, 2002) and cyclin D1 (Shtutman et al, 1999) were also downregulated in a time- and dose-dependent manner (Figure 3). A comparable dose- and time-dependent decrease in nonphosphorylated β-catenin and in the expression levels of the TCF target genes Met and cyclin D1 was also noted in response to indomethacin treatment (data not shown).
Figure 3.
Kinetics of the effect of sulindac on TCF-regulated target genes. DLD1 (A) or SW480 cells (B) were treated with various concentrations of sulindac sulphide (0–100 μM) for 24 h. Protein lysates were prepared and Western blot analyses performed with antibodies recognising total β-catenin, nonphosphorylated β-catenin, Met and cyclin D1. Blots were analysed by densitometry and each signal was normalised for loading in comparison with the appropriate β-actin signal (C). Data shown are mean values±s.e.m. from three independent experiments. In parallel, DLD1 or SW480 cells were treated with sulindac sulphide (100 μM) for 24 or 48 h (D).
DISCUSSION
In addition to COX-2, a number of alternative targets have been implicated in the chemopreventive action of NSAIDs, including Bcl-2, NF-κB, NAG-1 and PPARδ (Kopp and Ghosh, 1994; He et al, 1999; McEntee et al, 1999; Baek et al, 2001), suggesting that various distinct molecular pathways may play a role in the antitumour effects of these drugs. The present study provides in vivo and in vitro evidence that the β-catenin/TCF-4-signalling pathway is a target of sulindac.
We observed that the nuclear β-catenin expression in adenomas of FAP patients treated with sulindac is strongly decreased in comparison to the β-catenin expression in pretreatment adenomas of the same patients. A similar decrease in nuclear β-catenin has also been reported in rodent intestinal tumours (Brown et al, 2001). Previously, we found less nuclear β-catenin in sulindac-resistant adenomas compared to baseline adenomas (Keller et al, 2001). Those sulindac-resistant adenomas were collected during treatment with sulindac for up to 4 years, from selected sulindac-resistant patients, with a median treatment duration of 20 months, a range of 3–49 months and a mean of 26 months. Therefore, our previous study could not distinguish whether decreased nuclear β-catenin was caused by sulindac or reflected intrinsic features of sulindac-resistant adenomas. The present study compared adenomas from FAP patients removed before and after treatment with sulindac for up to 6 months. At that time point, the maximum efficacy of sulindac on the number and size of adenomas was noted (Giardiello et al, 1993), and in general resistance started to develop afterwards. Thus, it is assumed that the observed decrease of nuclear β-catenin in the present investigation is mostly accounted for by the sulindac treatment, instead of being related to resistance. This corresponds to data from McEntee et al (1999) showing decreased nuclear and cytoplasmic β-catenin in adenomas of ApcMin mice upon treatment with sulindac for several days.
The observation that sulindac treatment leads to a decrease in nuclear β-catenin in vivo is of great interest, since it suggests a direct link between the tumour suppressive effects of sulindac and the key defect of colorectal cancer, that is, deregulated Wnt signalling. Mutations involving components of the Wnt-signalling cascade, specifically in APC or β-catenin, are essential for the initiation of colorectal cancer. In the normal intestinal epithelium, these molecules are part of a multiprotein complex. In this complex, β-catenin is phosphorylated by GSK-3β and targeted for degradation by the ubiquitin–proteasomal pathway. Mutations in APC or β-catenin lead to dissociation of the complex, causing the accumulation of nonphosphorylated β-catenin, which translocates to the nucleus and acts as a transcriptional coactivator of TCF transcription factors (Kinzler and Vogelstein, 1996; Bienz and Clevers, 2000). Our observation that sulindac treatment diminishes the nuclear accumulation of the transcriptional coactivator β-catenin in adenomas of FAP patients in vivo strongly suggests that NSAIDs exert tumour suppressive effects by interfering with TCF-mediated transcription. In line with these in vivo data, we observed that sulindac sulphide treatment of the CRC cell lines DLD1 and SW480 strongly suppresses TCF reporter activity. Furthermore, NSAID treatment decreased the expression of the TCF target genes Met and cyclin D1 in a time- and dose-dependent manner. These suppressive effects were not due to a generalised transcriptional repression, since FOPflash reporter activities remained unchanged after sulindac sulphide treatment in the CRC cells. The effect of NSAIDs is less pronounced on the TCF-target protein levels than on TCF-regulated transcription, as shown in the TCF reporter assay. This effect can be explained by slow protein turnover rates.
Sulindac sulphide treatment led to only a moderate decrease in total β-catenin levels. This finding was recently also reported by Smith et al (2000), although Dihlmann et al (2001) reported no appreciable effect on β-catenin levels, despite the change in TCF-mediated transcription. Although we have no explanation for this discrepancy, the validity of our finding is strongly supported by the observation that the decrease in nonphosphorylated β-catenin (which represents the transcriptionally active portion of β-catenin) was more pronounced than that of the total β-catenin pool, suggesting a selective effect of sulindac sulphide on the pool of β-catenin that is involved in Wnt signalling. Since the CRC cells used in this study carry an APC mutation, preventing the formation of the APC/β-catenin multiprotein complex, it seems unlikely that the NSAID-induced decrease in nonphosphorylated β-catenin is regulated by GSK-3β activity. Other pathways of β-catenin downregulation might involve caspase-mediated cleavage (Schmeiser et al, 1998) or the inhibition of guanosine 3′,5′-cyclic monophosphate (cGMP) phosphodiesterase, leading to increased cGMP levels and downregulation of β-catenin, possibly via protein kinase G phosphorylation (Thompson et al, 2000). Also, NSAIDs may directly inhibit the translocation of β-catenin to the nucleus.
Taken together, our findings demonstrate that sulindac suppresses TCF-mediated transcription, presumably by preventing the nuclear accumulation of active β-catenin. Our observations provide new insights in the pathways involved in chemoprevention of NSAIDs and could advance the design of better chemopreventive drugs.
Acknowledgments
This work was supported in part by Grants UVA 98-1712, UVA 97-75 and UVA2003-2841 of the Dutch Cancer Society, The Netherlands Digestive Disease Foundation, the Clayton Fund, the Rangos Fund, Merck Company and NIH Grant CA53801.
References
- Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE (2001) Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol 59: 901–908 [PubMed] [Google Scholar]
- Bienz M, Clevers H (2000) Linking colorectal cancer to Wnt signaling. Cell 103: 311–320 [DOI] [PubMed] [Google Scholar]
- Boon EM, van der Neut R, van de Wetering M, Clevers H, Pals ST (2002) Wnt signaling regulates expression of the receptor tyrosine kinase met in colorectal cancer. Cancer Res 62: 5126–5128 [PubMed] [Google Scholar]
- Brown WA, Skinner SA, Vogiagis D, O'Brien PE (2001) Inhibition of beta-catenin translocation in rodent colorectal tumors: a novel explanation for the protective effect of nonsteroidal antiinflammatory drugs in colorectal cancer. Dig Dis Sci 46: 2314–2321 [DOI] [PubMed] [Google Scholar]
- Cruz-Correa M, Hylind LM, Romans KE, Booker SV, Giardiello FM (2002) Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology 122: 641–645 [DOI] [PubMed] [Google Scholar]
- Dihlmann S, Siermann A, von Knebel Doeberitz M (2001) The nonsteroidal anti-inflammatory drugs aspirin and indomethacin attenuate beta-catenin/TCF-4 signaling. Oncogene 20: 645–653 [DOI] [PubMed] [Google Scholar]
- DuBois RN, Smalley WE (1996) Cyclooxygenase, NSAIDs, and colorectal cancer. J Gastroenterology 31: 898–906 [DOI] [PubMed] [Google Scholar]
- Entius MM, Keller JJ, Drillenburg P, Kuypers KC, Giardiello FM, Offerhaus GJ (2000) Microsatellite instability and expression of hMLH-1 and hMSH-2 in sebaceous gland carcinomas as markers for Muir-Torre syndrome. Clin Cancer Res 6: 1784–1789 [PubMed] [Google Scholar]
- Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ (1993) Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 328: 1313–1316 [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Offerhaus GJ, DuBois RN (1995) The role of nonsteroidal anti-inflammatory drugs in colorectal cancer prevention. Eur J Cancer 31A: 1071–1076 [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Offerhaus JA, Tersmette AC, Hylind LM, Krush AJ, Brensinger JD, Booker SV, Hamilton SR (1996) Sulindac induced regression of colorectal adenomas in familial adenomatous polyposis: evaluation of predictive factors. Gut 38: 578–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanif R, Pittas A, Feng Y, Koutsos MI, Qiao L, Staiano-Coico L, Shiff SI, Rigas B (1996) Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol 52: 237–245 [DOI] [PubMed] [Google Scholar]
- Hawcroft G, D’Amico M, Albanese C, Markham AF, Pestell RG, Hull MA (2002) Indomethacin induces differential expression of beta-catenin, gamma-catenin and T-cell factor target genes in human colorectal cancer cells. Carcinogenesis 23: 107–114 [DOI] [PubMed] [Google Scholar]
- He TC, Chan TA, Vogelstein B, Kinzler KW (1999) PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell 99: 335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota C, Iida M, Aoyagi K, Matsumoto T, Tada S, Yao T, Fujishima M (1996) Effect of indomethacin suppositories on rectal polyposis in patients with familial adenomatous polyposis. Cancer 78: 1660–1665 [PubMed] [Google Scholar]
- Keller JJ, Offerhaus GJ, Drillenburg P, Caspers E, Musler A, Ristimaki A, Giardiello FM (2001) Molecular analysis of sulindac-resistant adenomas in familial adenomatous polyposis. Clin Cancer Res 7: 4000–4007 [PubMed] [Google Scholar]
- Keller JJ, Offerhaus GJ, Polak M, Goodman SN, Zahurak ML, Hylind LM, Hamilton SR, Giardiello FM (1999) Rectal epithelial apoptosis in familial adenomatous polyposis patients treated with sulindac. Gut 45: 822–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B (1996) Lessons from hereditary colorectal cancer. Cell 87: 159–170 [DOI] [PubMed] [Google Scholar]
- Kopp E, Ghosh S (1994) Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 265: 956–959 [DOI] [PubMed] [Google Scholar]
- McEntee MF, Chiu CH, Whelan J (1999) Relationship of beta-catenin and Bcl-2 expression to sulindac-induced regression of intestinal tumors in Min mice. Carcinogenesis 20: 635–640 [DOI] [PubMed] [Google Scholar]
- Nugent KP, Farmer KC, Spigelman AD, Williams CB, Phillips RK (1993) Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg 80: 1618–1619 [DOI] [PubMed] [Google Scholar]
- Pasricha PJ, Bedi A, O’Connor K, Rashid A, Akhtar AJ, Zahurak ML, Piantadosi S, Hamilton SR, Giardiello FM (1995) The effects of sulindac on colorectal proliferation and apoptosis in familial adenomatous polyposis. Gastroenterology 109: 994–998 [DOI] [PubMed] [Google Scholar]
- Piazza GA, Rahm AK, Finn TS, Fryer BH, Li H, Stoumen AL, Pamukcu R, Ahnen DJ (1997) Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res 57: 2452–2459 [PubMed] [Google Scholar]
- Picariello L, Brandi ML, Formigli L, Orlandini SZ, Dolara P, Caderni G, Raimondi L, Tonelli F (1998) Apoptosis induced by sulindac sulfide in epithelial and mesenchymal cells from human abdominal neoplasms. Eur J Pharmacol 360: 105–112 [DOI] [PubMed] [Google Scholar]
- Schmeiser K, Hammond EM, Roberts S, Grand RJ (1998) Specific cleavage of gamma catenin by caspases during apoptosis. FEBS Lett 433: 51–57 [DOI] [PubMed] [Google Scholar]
- Shiff SJ, Rigas B (1999) The role of cyclooxygenase inhibition in the antineoplastic effects of nonsteroidal antiinflammatory drugs (NSAIDs). J Exp Med 190: 445–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A (1999) The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA 96: 5522–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Hawcroft G, Hull MA (2000) The effect of non-steroidal anti-inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur J Cancer 36: 664–674 [DOI] [PubMed] [Google Scholar]
- Spagnesi MT, Tonelli F, Dolara P, Caderni G, Valanzano R, Anastasi A, Bianchini F (1994) Rectal proliferation and polyp occurrence in patients with familial adenomatous polyposis after sulindac treatment. Gastroenterology 106: 362–366 [DOI] [PubMed] [Google Scholar]
- Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, Sperl G, Ahnen D, Pamukcu R (2000) Exisulind induction of apoptosis involves guanosine 3′,5′-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res 60: 3338–3342 [PubMed] [Google Scholar]
- Winde G, Schmid KW, Brandt B, Muller O, Osswald H (1997) Clinical and genomic influence of sulindac on rectal mucosa in familial adenomatous polyposis. Dis Colon Rectum 40: 1156–1168 discussion 1168-9 [DOI] [PubMed] [Google Scholar]
- Zhang X, Morham SG, Langenbach R, Young DA. (1999) Malignant transformation and antineoplastic actions of nonsteroidal antiinflammatory drugs (NSAIDs) on cyclooxygenase-null embryo fibroblasts. J Exp Med 190: 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]