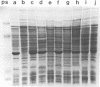
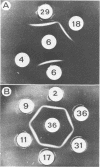
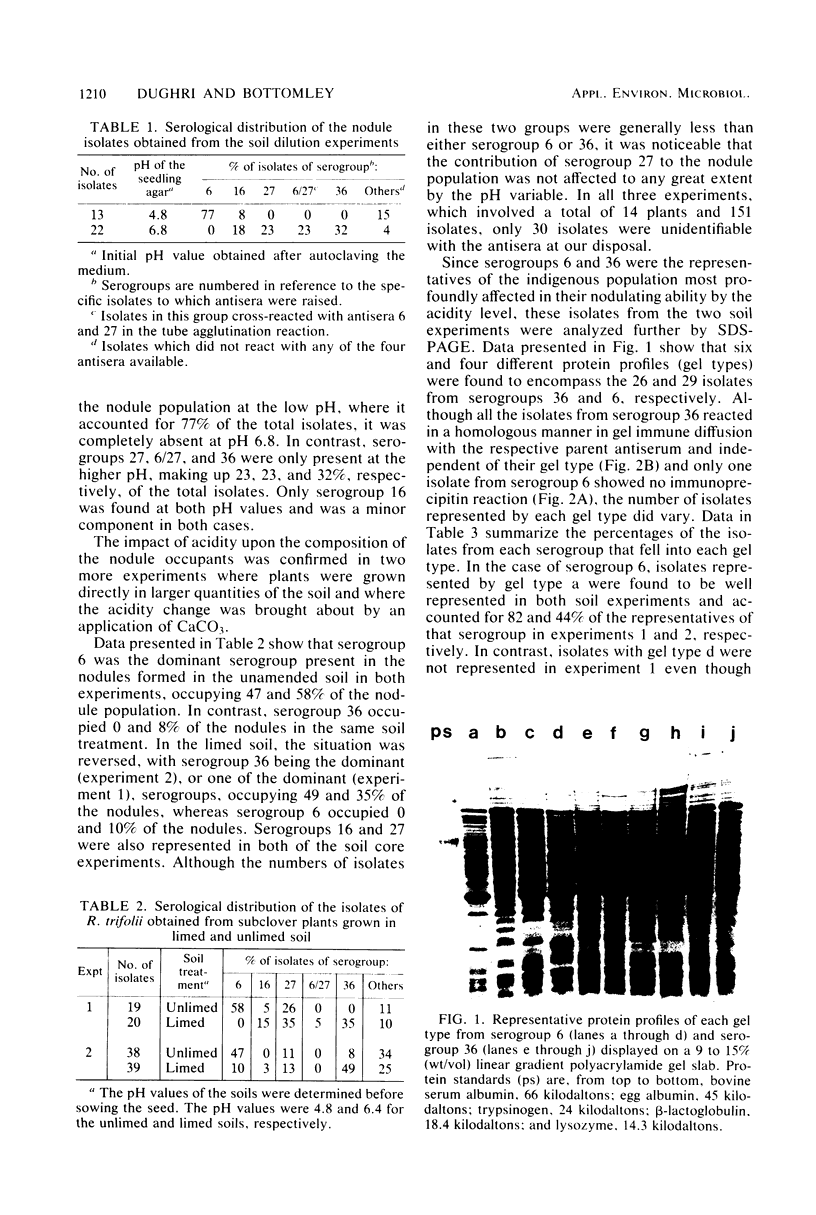
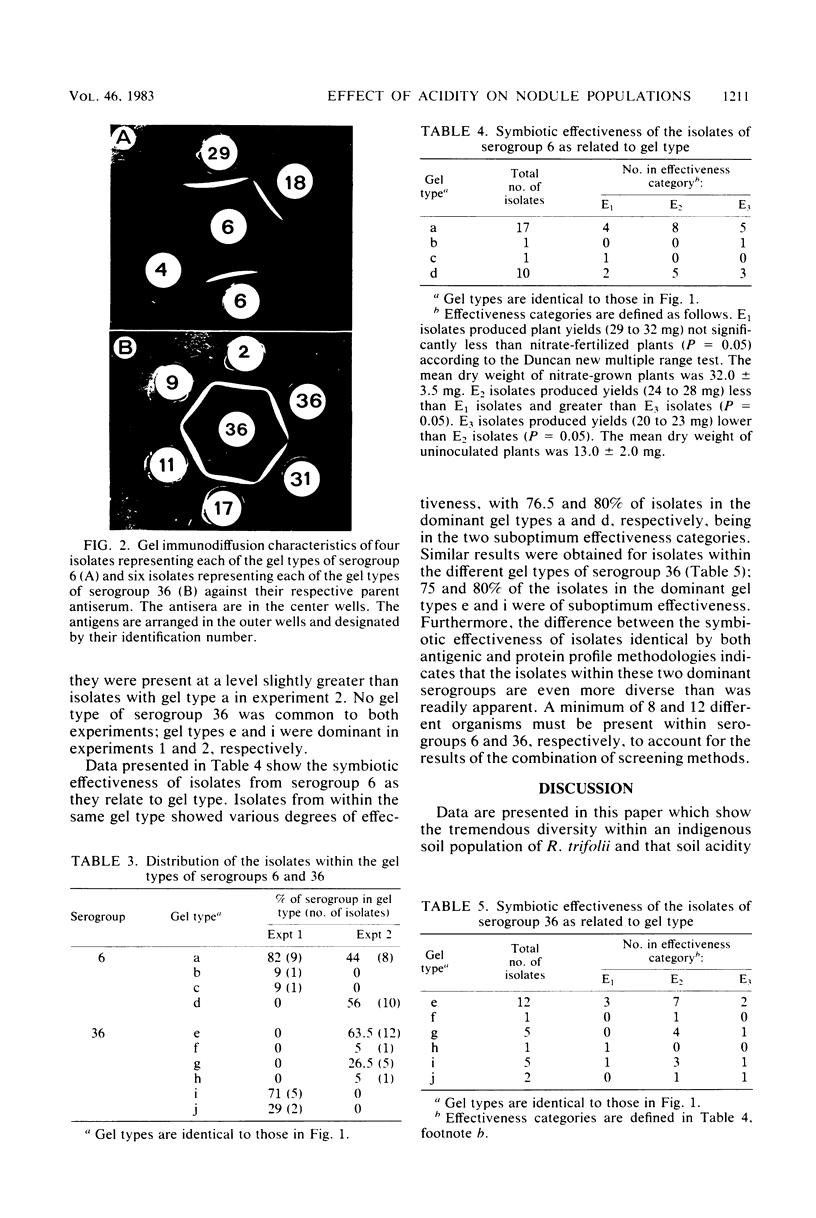
Abstract
Acidity affected which members of an indigenous soil population of Rhizobium trifolii nodulated Trifolium subterraneum L. cv. Mt. Barker. In three experiments involving plants grown either in mineral salts agar adjusted to pH 4.8 or 6.8 and inoculated with a soil suspension or grown directly in samples of unamended soil (pH 4.8) or soil amended with CaCO3 (pH 6.4), 121 of 151 isolates of R. trifolii were placed into four serogroups. Seventy-nine of these isolates were placed into two serogroups (6 and 36) whose nodulating ability was affected by the pH of the plant root environment. Representatives of serogroup 6 occupied the greatest percentage of the nodules at the low pH in both mineral salts agar (77%) and in unlimed soil (47 and 57%). The same serogroup was a minor nodule occupant at the higher pH in mineral salts agar (0%) and in limed soil (0 and 10%). In contrast, serogroup 36 was virtually absent in nodules formed at the low pH, whereas it was the dominant serogroup at the higher pH in both mineral salts agar (32%) and in limed soil (35 and 49%). Despite the isolates from within each serogroup being antigenically identical, separation of cellular proteins by sodium dodecyl sulfate-polyacrylamide gradient gel electrophoresis revealed four and six different gel types within serogroups 6 and 36, respectively. Isolates represented by one or two gel types dominated the contribution of each serogroup to the nodule population. Further evidence for differences between isolates within each gel type were revealed from measurements of symbiotic effectiveness.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fox E. L. Physical training: methods and effects. Orthop Clin North Am. 1977 Jul;8(3):533–548. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Noel K. D., Brill W. J. Diversity and Dynamics of Indigenous Rhizobium japonicum Populations. Appl Environ Microbiol. 1980 Nov;40(5):931–938. doi: 10.1128/aem.40.5.931-938.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes V. G., Schmidt E. L. Population Densities of Rhizobium japonicum Strain 123 Estimated Directly in Soil and Rhizospheres. Appl Environ Microbiol. 1979 May;37(5):854–858. doi: 10.1128/aem.37.5.854-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert F. M., Schmidt E. L. Population Changes and Persistence of Rhizobium phaseoli in Soil and Rhizospheres. Appl Environ Microbiol. 1983 Feb;45(2):550–556. doi: 10.1128/aem.45.2.550-556.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINCENT J. M., WATERS L. M. The influence of the host on competition amongst clover root-nodule bacteria. J Gen Microbiol. 1953 Dec;9(3):357–370. doi: 10.1099/00221287-9-3-357. [DOI] [PubMed] [Google Scholar]