Abstract
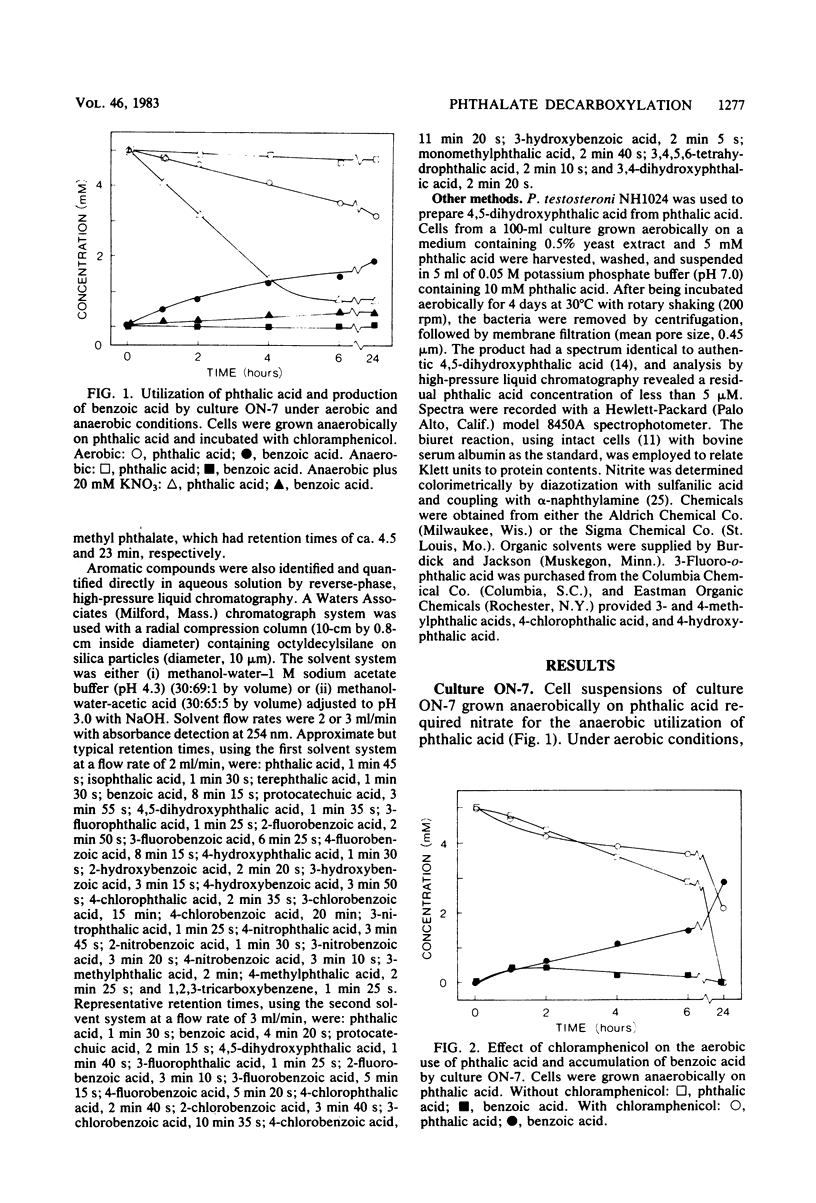
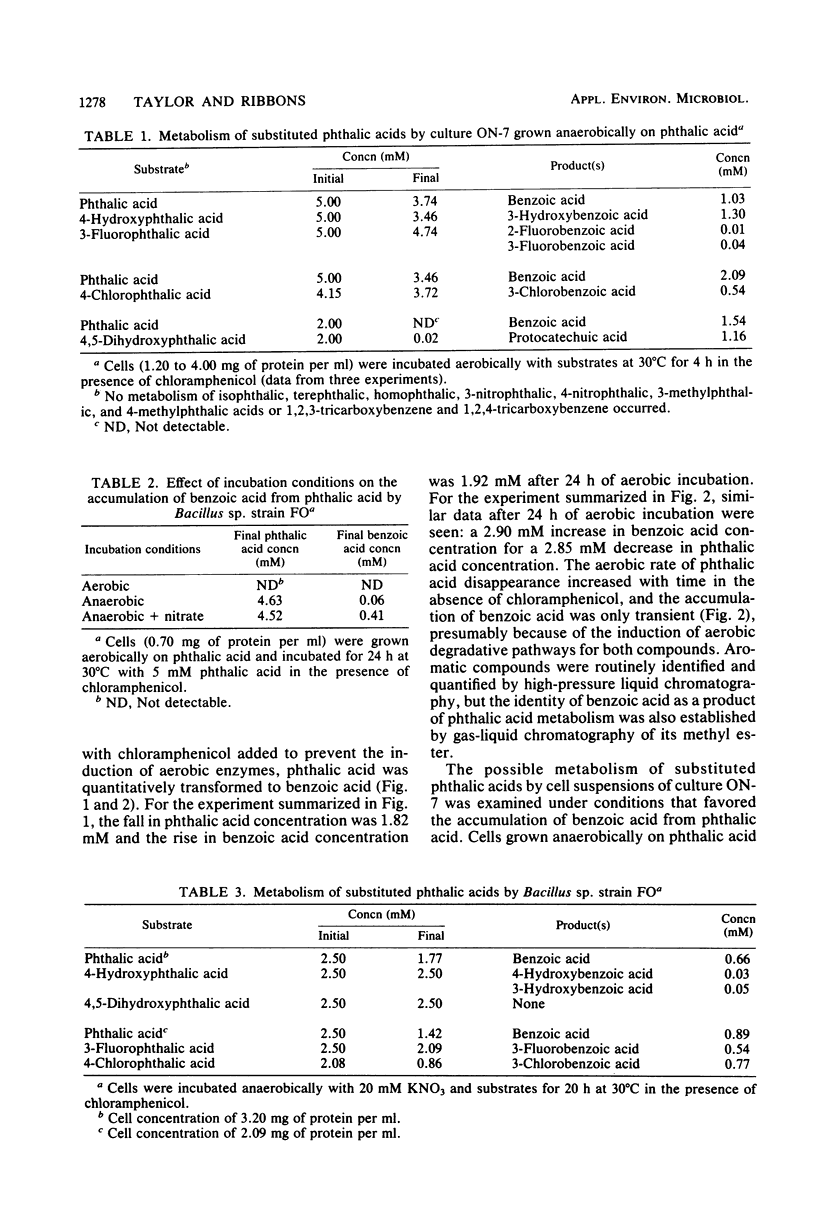
The decarboxylation of phthalic acids was studied with Bacillus sp. strain FO, a marine mixed culture ON-7, and Pseudomonas testosteroni. The mixed culture ON-7, when grown anaerobically on phthalate but incubated aerobically with chloramphenicol, quantitatively converted phthalic acid to benzoic acid. Substituted phthalic acids were also decarboxylated: 4,5-dihydroxyphthalic acid to protocatechuic acid; 4-hydroxyphthalic and 4-chlorophthalic acids to 3-hydroxybenzoic and 3-chlorobenzoic acids, respectively; and 3-fluorophthalic acid to 2-and 3-fluorobenzoic acids. Bacillus sp. strain FO gave similar results except that 4,5-dihydroxyphthalic acid was not metabolized, and both 3- and 4-hydroxybenzoic acids were produced from 4-hydroxyphthalic acid. P. testosteroni decarboxylated 4-hydroxyphthalate (to 3-hydroxybenzoate) and 4,5-dihydroxyphthalate but not phthalic acid and halogenated phthalates. Thus, P. testosteroni and the mixed culture ON-7 possessed 4,5-dihydroxyphthalic acid decarboxylase, previously described in P. testosteroni, that metabolized 4,5-dihydroxyphthalic acid and specifically decarboxylated 4-hydroxyphthalic acid to 3-hydroxybenzoic acid. The mixed culture ON-7 and Bacillus sp. strain FO also possessed a novel decarboxylase that metabolized phthalic acid and halogenated phthalates, but not 4,5-dihydroxyphthalate, and randomly decarboxylated 4-hydroxyphthalic acid. The decarboxylation of phthalic acid is suggested to involve an initial reduction to 1,2-dihydrophthalic acid followed by oxidative decarboxylation to benzoic acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aftring R. P., Chalker B. E., Taylor B. F. Degradation of phthalic acids by denitrifying, mixed cultures of bacteria. Appl Environ Microbiol. 1981 May;41(5):1177–1183. doi: 10.1128/aem.41.5.1177-1183.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autian J. Toxicity and health threats of phthalate esters: review of the literature. Environ Health Perspect. 1973 Jun;4:3–26. doi: 10.1289/ehp.73043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L., Olson P. P. Microbial catabolism of vanillate: decarboxylation to guaiacol. Appl Environ Microbiol. 1978 Oct;36(4):539–543. doi: 10.1128/aem.36.4.539-543.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. W., Ribbons D. W. Metabolism of dibutylphthalate and phthalate by Micrococcus sp. strain 12B. J Bacteriol. 1982 Jul;151(1):48–57. doi: 10.1128/jb.151.1.48-57.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. W., Ribbons D. W. Metabolism of dimethylphthalate by Micrococcus sp. strain 12B. J Bacteriol. 1982 Jul;151(1):465–467. doi: 10.1128/jb.151.1.465-467.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. W., Ribbons D. W. Utilization of phthalate esters by micrococci. Arch Microbiol. 1982 Aug;132(2):185–188. doi: 10.1007/BF00508728. [DOI] [PubMed] [Google Scholar]
- Evans W. C. Biochemistry of the bacterial catabolism of aromatic compounds in anaerobic environments. Nature. 1977 Nov 3;270(5632):17–22. doi: 10.1038/270017a0. [DOI] [PubMed] [Google Scholar]
- Healy J. B., Young L. Y., Reinhard M. Methanogenic decomposition of ferulic Acid, a model lignin derivative. Appl Environ Microbiol. 1980 Feb;39(2):436–444. doi: 10.1128/aem.39.2.436-444.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser P., Pujar B. G., Eaton R. W., Ribbons D. W. Biodegradation of the phthalates and their esters by bacteria. Environ Health Perspect. 1976 Dec;18:159–166. doi: 10.1289/ehp.7618159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T., Hayashi E. Phthalate and 4-hydroxyphthalate metabolism in Pseudomonas testosteroni: purification and properties of 4,5-dihydroxyphthalate decarboxylase. Appl Environ Microbiol. 1978 Aug;36(2):264–269. doi: 10.1128/aem.36.2.264-269.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T., Hayashi E. Phthalate metabolism in Pseudomonas testosteroni: accumulation of 4,5-dihydroxyphthalate by a mutant strain. J Bacteriol. 1977 Jul;131(1):42–48. doi: 10.1128/jb.131.1.42-48.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall D. B. Phthalate esters: Occurrence and biological effects. Residue Rev. 1975;54:1–41. doi: 10.1007/978-1-4612-9857-1_1. [DOI] [PubMed] [Google Scholar]
- Peppercorn M. A., Goldman P. Caffeic acid metabolism by gnotobiotic rats and their intestinal bacteria. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1413–1415. doi: 10.1073/pnas.69.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M., Hegeman G. D. Metabolism of benzoic acid by bacteria. Accumulation of (-)-3,5-cyclohexadiene-1,2-diol-1-carboxylic acid by mutant strain of Alcaligenes eutrophus. Biochemistry. 1971 Jun 22;10(13):2530–2536. doi: 10.1021/bi00789a017. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Metabolism of benzoic acid by bacteria: 3,5-cyclohexadiene-1,2-diol-1-carboxylic acid is an intermediate in the formation of catechol. J Bacteriol. 1971 Oct;108(1):89–94. doi: 10.1128/jb.108.1.89-94.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons D. W., Evans W. C. Oxidative metabolism of phthalic acid by soil pseudomonads. Biochem J. 1960 Aug;76(2):310–318. doi: 10.1042/bj0760310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheline R. R. The metabolism of drugs and other organic compounds by the intestinal microflora. Acta Pharmacol Toxicol (Copenh) 1968;26(4):332–342. doi: 10.1111/j.1600-0773.1968.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Hoare D. S. Acetate assimilation by Nitrobacter agilis in relation to its "obligate autotrophy". J Bacteriol. 1968 Mar;95(3):844–855. doi: 10.1128/jb.95.3.844-855.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


