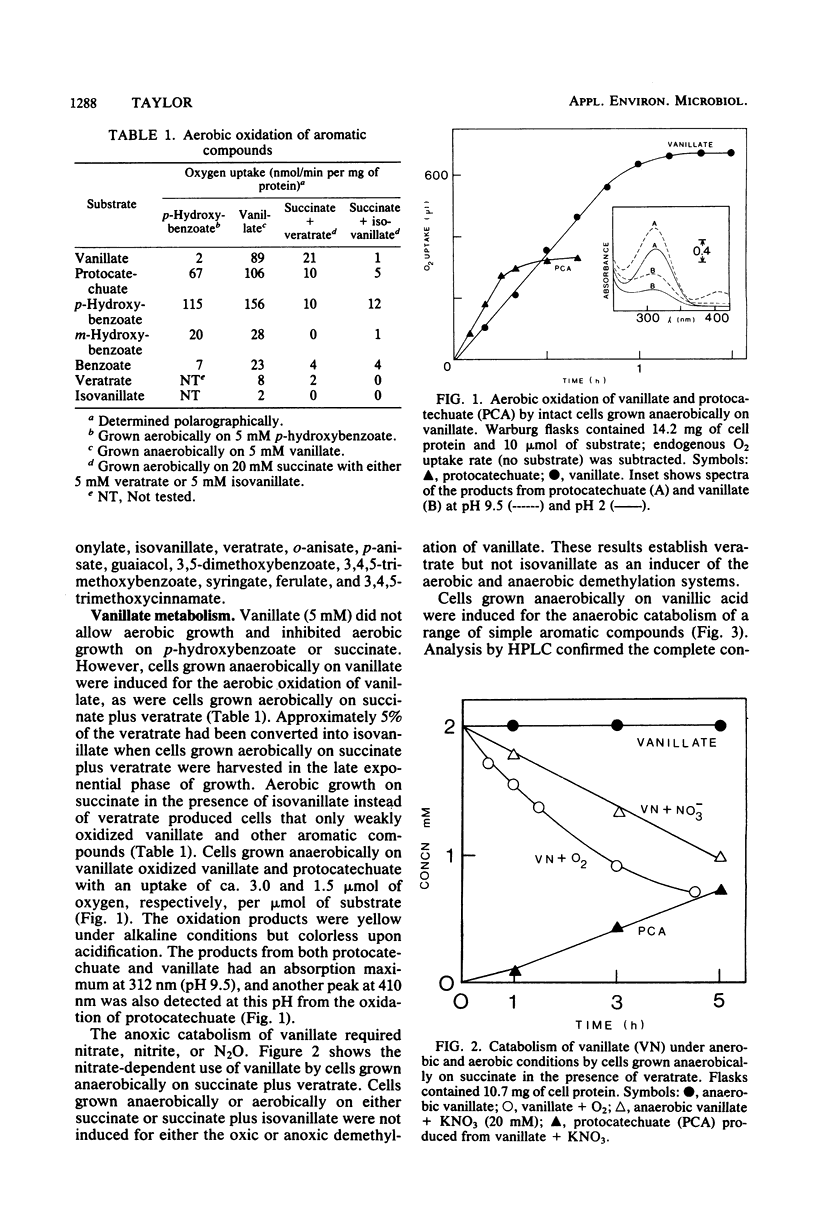
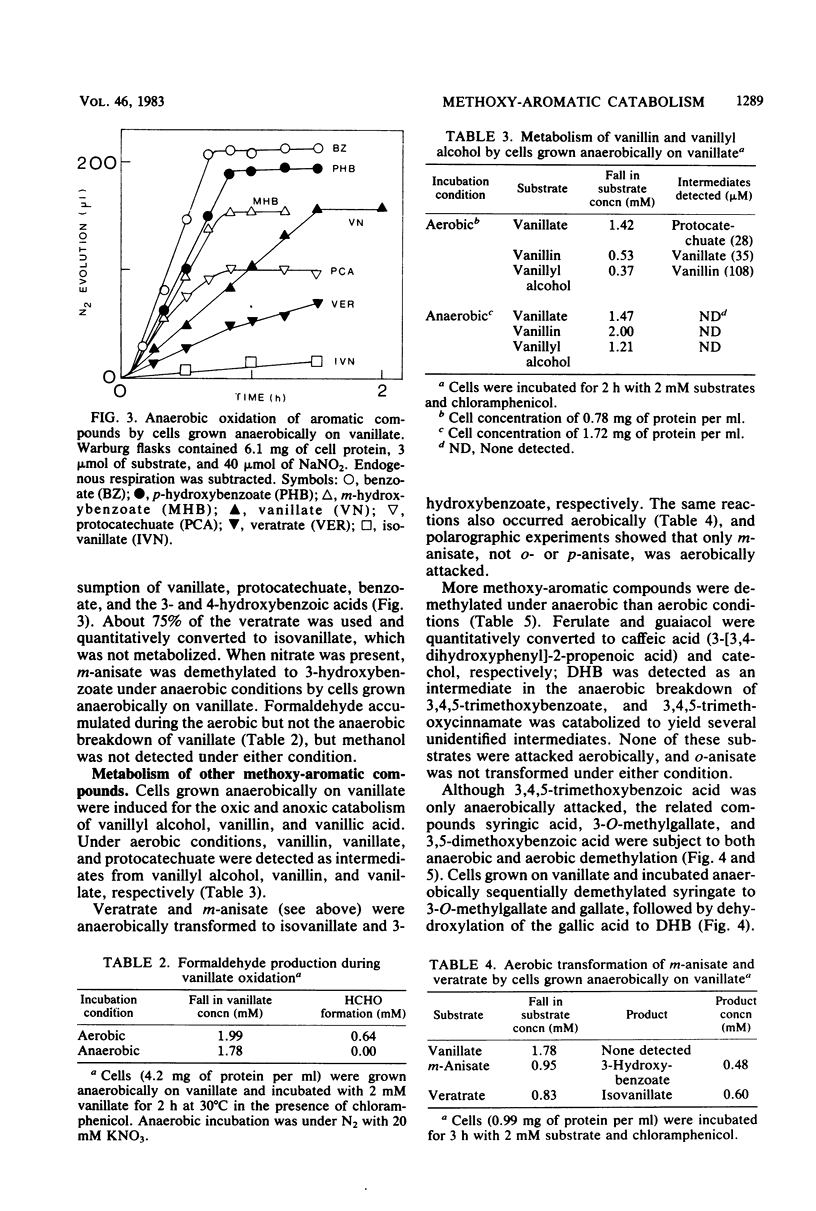
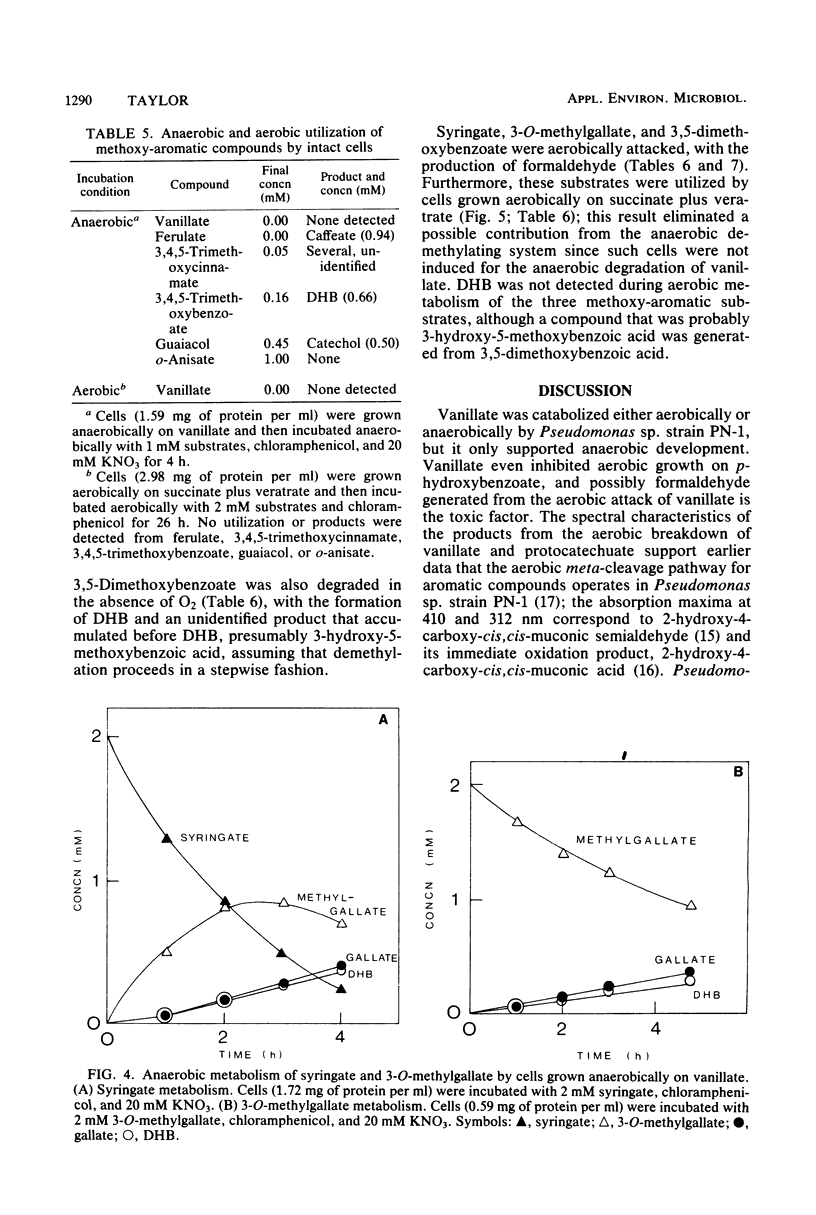
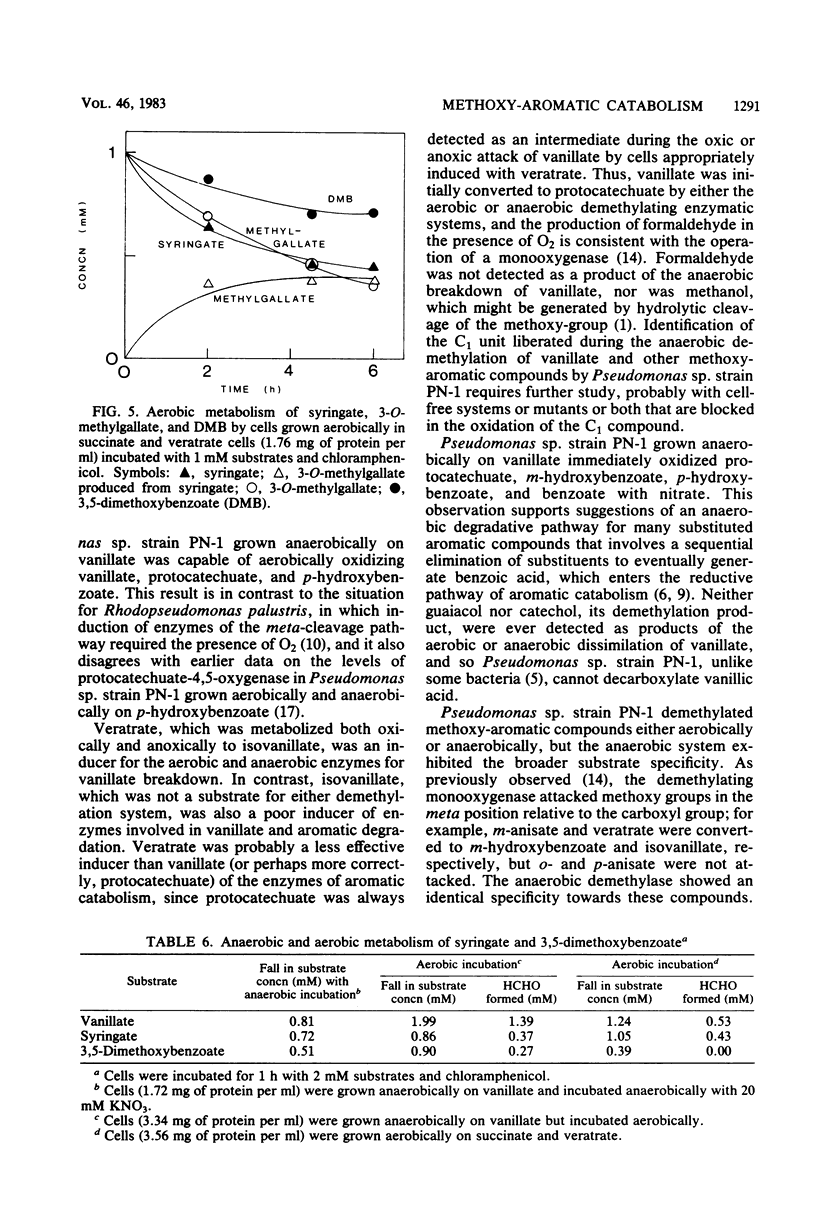
Abstract
Vanillic acid (4-hydroxy-3-methoxybenzoic acid) supported the anaerobic (nitrate respiration) but not the aerobic growth of Pseudomonas sp. strain PN-1. Cells grown anaerobically on vanillate oxidized vanillate, p-hydroxybenzoate, and protocatechuic acid (3,4-dihydroxybenzoic acid) with O2 or nitrate. Veratric acid (3,4-dimethoxybenzoic acid) but not isovanillic acid (3-hydroxy-4-methoxybenzoic acid) induced cells for the oxic and anoxic utilization of vanillate, and protocatechuate was detected as an intermediate of vanillate breakdown under either condition. Aerobic catabolism of protocatechuate proceeded via 4,5-meta cleavage, whereas anaerobically it was probably dehydroxylated to benzoic acid. Formaldehyde was identified as a product of aerobic demethylation, indicating a monooxygenase mechanism, but was not detected during anaerobic demethylation. The aerobic and anaerobic systems had similar but not identical substrate specificities. Both utilized m-anisic acid (3-methoxybenzoic acid) and veratrate but not o- or p-anisate and isovanillate. Syringic acid (4-hydroxy-3,5-dimethoxybenzoic acid), 3-O-methylgallic acid (3-methoxy-4,5-dihydroxybenzoic acid), and 3,5-dimethoxybenzoic acid were attacked under either condition, and formaldehyde was liberated from these substrates in the presence of O2. The anaerobic demethylating system but not the aerobic enzyme was also active upon guaiacol (2-methoxyphenol), ferulic acid (3-[4-hydroxy-3-methoxyphenyl]-2-propenoic acid), 3,4,5-trimethoxycinnamic acid (3-[3,4,5-trimethoxyphenyl]-2-propenoic acid), and 3,4,5-trimethoxybenzoic acid. The broad specificity of the anaerobic demethylation system suggests that it probably is significant in the degradation of lignoaromatic molecules in anaerobic environments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balba M. T., Evans W. C. The methanogenic fermentation of omega-phenylalkane carboxylic acids [proceedings]. Biochem Soc Trans. 1979 Apr;7(2):403–405. doi: 10.1042/bst0070403. [DOI] [PubMed] [Google Scholar]
- Brooks J., Pace J. The manometric estimation of nitrite in solution and in tissue. Biochem J. 1940 Mar;34(3):260–267. doi: 10.1042/bj0340260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L., Olson P. P. Microbial catabolism of vanillate: decarboxylation to guaiacol. Appl Environ Microbiol. 1978 Oct;36(4):539–543. doi: 10.1128/aem.36.4.539-543.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C. Biochemistry of the bacterial catabolism of aromatic compounds in anaerobic environments. Nature. 1977 Nov 3;270(5632):17–22. doi: 10.1038/270017a0. [DOI] [PubMed] [Google Scholar]
- Hackett W. F., Connors W. J., Kirk T. K., Zeikus J. G. Microbial decomposition of synthetic C-labeled lignins in nature: lignin biodegradation in a variety of natural materials. Appl Environ Microbiol. 1977 Jan;33(1):43–51. doi: 10.1128/aem.33.1.43-51.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy J. B., Young L. Y. Anaerobic biodegradation of eleven aromatic compounds to methane. Appl Environ Microbiol. 1979 Jul;38(1):84–89. doi: 10.1128/aem.38.1.84-89.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy J. B., Young L. Y., Reinhard M. Methanogenic decomposition of ferulic Acid, a model lignin derivative. Appl Environ Microbiol. 1980 Feb;39(2):436–444. doi: 10.1128/aem.39.2.436-444.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. The metabolism of p-hydroxybenzoate by Rhodopseudomonas palustris and its regulation. Arch Mikrobiol. 1967;59(1):143–148. doi: 10.1007/BF00406325. [DOI] [PubMed] [Google Scholar]
- RIBBONS D. W., EVANS W. C. Oxidative metabolism of protocatechuic acid by certain soil pseudomonads: a new ring-fission mechanism. Biochem J. 1962 Jun;83:482–492. doi: 10.1042/bj0830482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford T., Dalsis D. E. Analysis of formaldehyde in shrimp by high-pressure liquid chromatography. J Agric Food Chem. 1982 May-Jun;30(3):600–602. doi: 10.1021/jf00111a047. [DOI] [PubMed] [Google Scholar]
- Ribbons D. W. Stoicheiometry of O-demethylase activity in Pseudomonas aeruginosa. FEBS Lett. 1970 May 25;8(2):101–104. doi: 10.1016/0014-5793(70)80235-6. [DOI] [PubMed] [Google Scholar]
- Tack B. F., Chapman P. J., Dagley S. Metabolism of gallic acid and syringic acid by Pseudomonas putida. J Biol Chem. 1972 Oct 25;247(20):6438–6443. [PubMed] [Google Scholar]
- Taylor B. F., Campbell W. L., Chinoy I. Anaerobic degradation of the benzene nucleus by a facultatively anaerobic microorganism. J Bacteriol. 1970 May;102(2):430–437. doi: 10.1128/jb.102.2.430-437.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


