Abstract
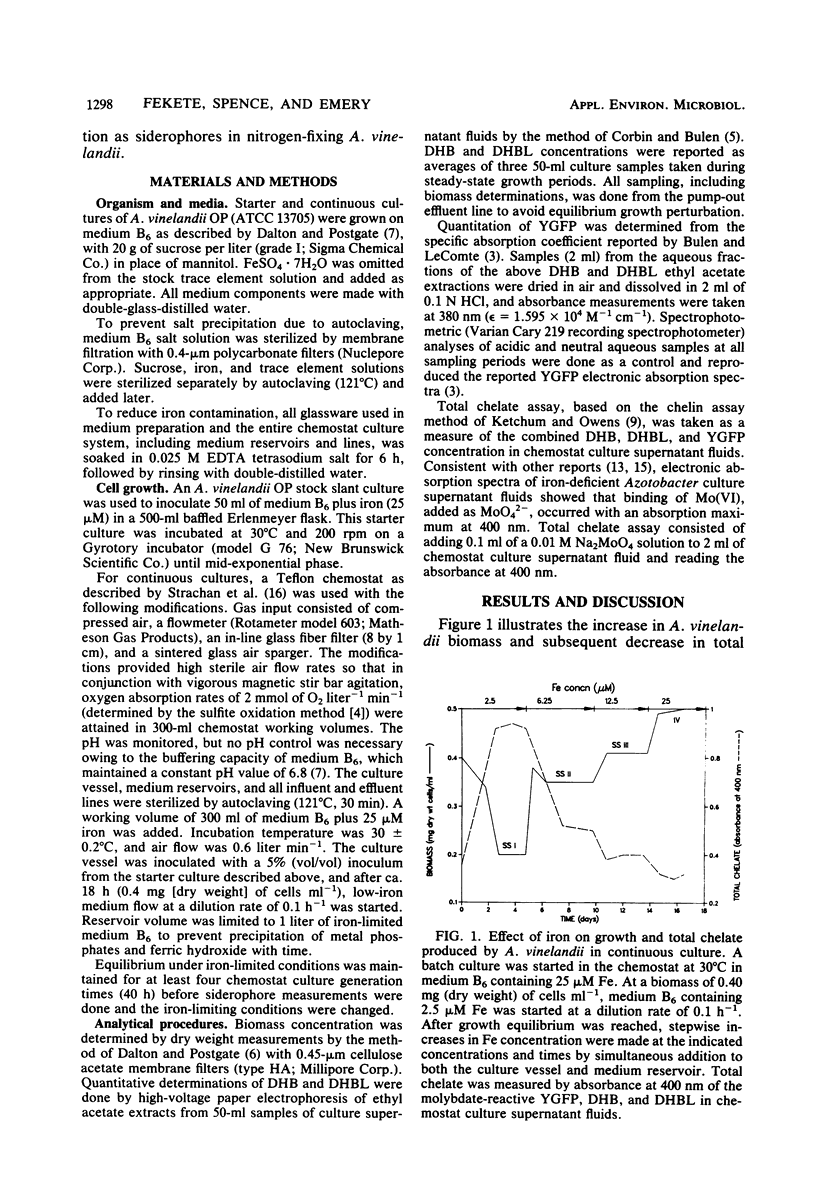
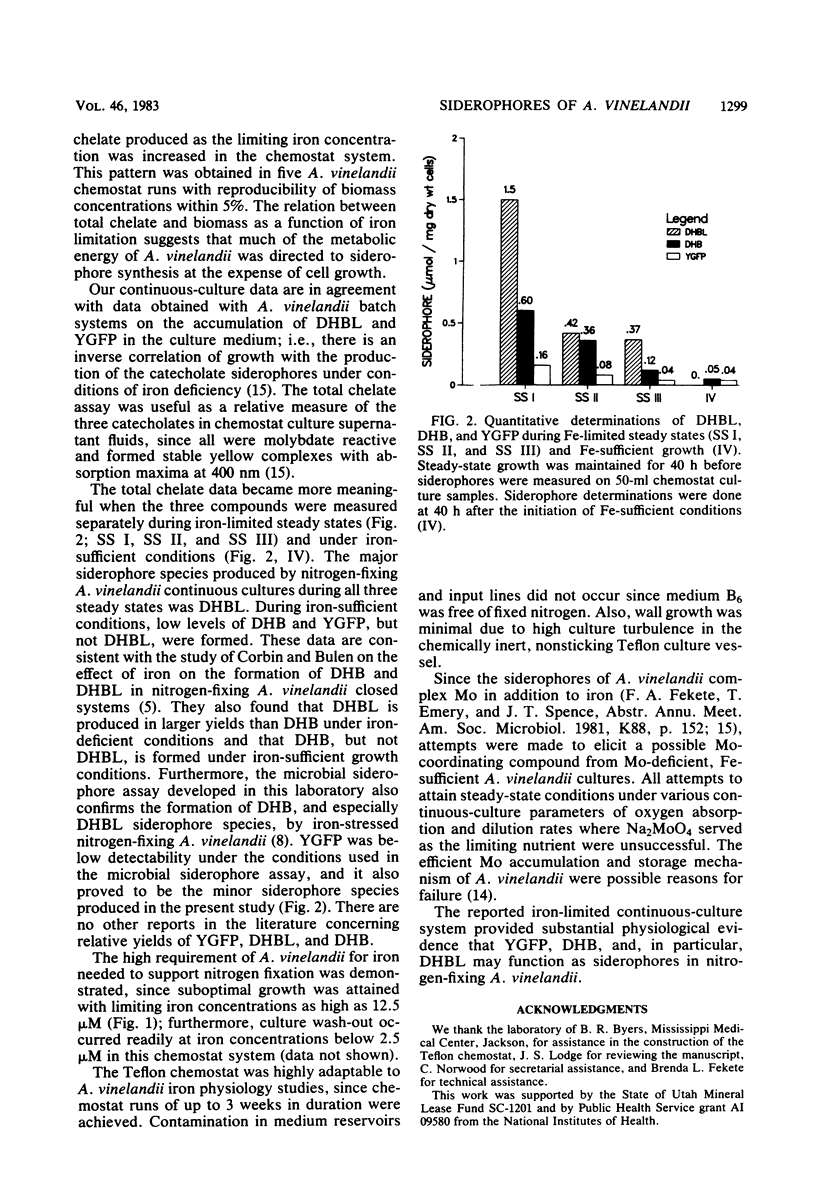
Azotobacter vinelandii requires a high complement of iron and an efficient iron acquisition system to support nitrogen fixation. To circumvent problems inherent in batch culture trace metal studies, continuous cultures were used to measure the response of A. vinelandii to iron stress. Iron was found to be growth limiting for nitrogen-fixing A. vinelandii at a concentration as high as 12.5 μM; iron was growth sufficient at 25 μM. Iron-stressed A. vinelandii in continuous culture formed 2,3-hydroxybenzoic acid (DHB), 2-N,6-N-di-(2,3-dihydroxybenzoyl)-l-lysine (DHBL), and a chromophoric yellow-green fluorescent peptide (YGFP). At a fixed dilution rate of 0.1 h−1, steady-state growth occurred at growth-limiting iron concentrations. DHB and DHBL were quantitatively measured during iron-limited steady states and iron-sufficient states by Arnow colorimetric assays. YGFP was determined by absorbance measurements taken at 380 nm, and the concentration was calculated from the reported specific absorption coefficient. Biomass increased and DHBL, DHB, and YGFP concentrations decreased as the concentration of growth-limiting iron was increased in the culture vessel and medium reservoirs. DHBL was the major siderophore and YGFP was the minor siderophore species produced during iron-limited equilibrium growth. A low level of DHB and YGFP, but no DHBL, was formed under iron-sufficient conditions. These results provide further physiological evidence that DHB, YGFP, and especially DHBL may function as siderophores in nitrogen-fixing A. vinelandii.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S., DeVoe I. W. Iron in Neisseria meningitidis: minimum requirements, effects of limitation, and characteristics of uptake. J Bacteriol. 1978 Oct;136(1):35–48. doi: 10.1128/jb.136.1.35-48.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULEN W. A., LECOMTE J. R. Isolation and properties of a yellow-green fluorescent peptide from azotobacter medium. Biochem Biophys Res Commun. 1962 Dec 19;9:523–528. doi: 10.1016/0006-291x(62)90119-5. [DOI] [PubMed] [Google Scholar]
- Brill W. J. Biochemical genetics of nitrogen fixation. Microbiol Rev. 1980 Sep;44(3):449–467. doi: 10.1128/mr.44.3.449-467.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. L., Bulen W. A. The isolation and identification of 2,3-dihydroxybenzoic acid and 2-N,6-N-di-92,3-dihydroxybenzoyl)-L-lysine formed by iron-deficient Azotobacter vinelandii. Biochemistry. 1969 Mar;8(3):757–762. doi: 10.1021/bi00831a002. [DOI] [PubMed] [Google Scholar]
- Dalton H., Postgate J. R. Effect of oxygen on growth of Azotobacter chroococcum in batch and continuous cultures. J Gen Microbiol. 1968 Dec;54(3):463–473. doi: 10.1099/00221287-54-3-463. [DOI] [PubMed] [Google Scholar]
- Fekete F. A., Spence J. T., Emery T. A rapid and sensitive paper electrophoresis assay for the detection of microbial siderophores elicited in solid-plating culture. Anal Biochem. 1983 Jun;131(2):516–519. doi: 10.1016/0003-2697(83)90207-5. [DOI] [PubMed] [Google Scholar]
- Ketchum P. A., Owens M. S. Production of molybdenum-coordinating compound by Bacillus thuringiensis. J Bacteriol. 1975 May;122(2):412–417. doi: 10.1128/jb.122.2.412-417.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., von Tigerstrom M. Iron- and molybdenum-repressible outer membrane proteins in competent Azotobacter vinelandii. J Bacteriol. 1982 Jul;151(1):237–242. doi: 10.1128/jb.151.1.237-242.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienkos P. T., Brill W. J. Molybdenum accumulation and storage in Klebsiella pneumoniae and Azotobacter vinelandii. J Bacteriol. 1981 Feb;145(2):743–751. doi: 10.1128/jb.145.2.743-751.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan R. C., Aranha H., Lodge J. S., Arceneaux J. E., Byers B. R. Teflon chemostat for studies of trace metal metabolism in Streptococcus mutans and other bacteria. Appl Environ Microbiol. 1982 Jan;43(1):257–260. doi: 10.1128/aem.43.1.257-260.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


