Abstract
Aryloxyphenoxypropionates, inhibitors of the plastid acetyl-CoA carboxylase (ACC) of grasses, also inhibit Toxoplasma gondii ACC. Clodinafop, the most effective of the herbicides tested, inhibits growth of T. gondii in human fibroblasts by 70% at 10 μM in 2 days and effectively eliminates the parasite in 2–4 days at 10–100 μM. Clodinafop is not toxic to the host cell even at much higher concentrations. Parasite growth inhibition by different herbicides is correlated with their ability to inhibit ACC enzyme activity, suggesting that ACC is a target for these agents. Fragments of genes encoding the biotin carboxylase domain of multidomain ACCs of T. gondii, Plasmodium falciparum, Plasmodium knowlesi, and Cryptosporidium parvum were sequenced. One T. gondii ACC (ACC1) amino acid sequence clusters with P. falciparum ACC, P. knowlesi ACC, and the putative Cyclotella cryptica chloroplast ACC. Another sequence (ACC2) clusters with that of C. parvum ACC, probably the cytosolic form.
Toxoplasma gondii is a protozoan parasite of the phylum Apicomplexa that causes infections in immunocompromised patients, such as patients with transplants, cancer, or AIDS, and in congenitally infected infants. Other serious diseases are caused by related apicomplexan parasites such as Plasmodium (malaria) and Cryptosporidium in humans and Eimeria in animals. One of the unique features of apicomplexan parasites is the presence of a nonphotosynthetic plastid (1–8) containing its own 35-kilobase genome, which is essential for parasite survival. Antibiotics that apparently target plastid protein synthesis specifically block parasite replication (9, 10). It has been postulated that the apicomplexan plastid was acquired by endosymbiosis of an alga (2, 3, 7). The plastid is bounded by four membranes, and its genome shows substantial sequence similarity to algal and other chloroplast DNA (2, 3, 6, 7). Although the metabolic functions of this degenerate plastid remain largely unknown, it is possible that it provides a site for fatty acid biosynthesis (8, 11). Thiolactomycin, an inhibitor of fatty acid elongation, prevents growth of Plasmodium falciparum (5). Several herbicides have already been shown to restrict apicomplexan parasite growth without toxicity to mammalian cells (11, 12).
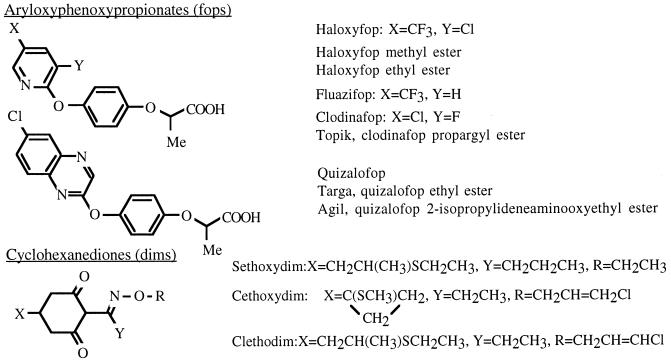
In plants, genes encoding enzymes for fatty acid synthesis, including three of the four subunits of the chloroplast ACC of dicotyledenous plants, are present in the nuclear genome. Their protein products are imported into chloroplasts, where they assemble and function in the first step of fatty acid synthesis. In monocotyledenous plants, such as wheat, both the chloroplast form and the cytoplasmic form of ACC consist of a single large polypeptide, with the biotin carboxylase (BC) domain located near the N terminus. The monocot chloroplast enzyme is the selective target of aryloxyphenoxypropionate (fops) and cyclohexanedione (dims) herbicides (Fig. 1; see ref. 13 for review). The detailed molecular mechanism of inhibition is not known, but the ACC that is localized in plastids of grasses is extremely sensitive to these herbicides. All of the multisubunit chloroplast enzymes of dicot plants and bacteria as well as the ACCs from man, chicken, rat, and yeast are resistant.
Figure 1.
Structure of aryloxyphenoxypropionate and cyclohexanedione herbicides.
Materials and Methods
Inhibition of T. gondii ACC Activity by Herbicides.
A total of 2 × 109 tachyzoites of the T. gondii RH strain were collected from peritoneal cavities of 25-g female Swiss–Webster mice (Taconic Labs, Germantown, NY) after 2 days of infection, separated from host cells by passage through a 3-μm filter, and lysed as described (11). Low molecular weight material was then removed by gel filtration on Sephadex G50 as described for ACC expressed in yeast (14). Average acetyl-CoA-dependent (at least 50-fold stimulation) incorporation of 14C from labeled bicarbonate (2 μCi per assay; 1 Ci = 37 GBq) into acid-stable malonyl-CoA (100% control) was 4,700 cpm per assay (1,100–7,800 cpm in different experiments, depending on protein amount and preparation). Determination of ACC activity, its inhibition by herbicides, and biotinylated peptide analysis was as described (15). Herbicides were added as 10-fold concentrated solutions in 10% (vol/vol) DMSO in the Sephadex column buffer. Aryloxyphenoxypropionate esters were added as 1 mM solutions in DMSO.
Inhibition of T. gondii Growth by Herbicides.
Human foreskin fibroblasts were grown in 96-well tissue culture plates in Iscoves’ modified Dulbecco’s medium containing l-glutamine and penicillin/streptomycin at 37°C in 100% humidity and a 5% CO2 environment. In the inhibition assay, confluent monolayers of fibroblasts were infected with T. gondii tachyzoites of the RH strain, and herbicides were added 1 hour later. T. gondii growth was assessed by incorporation of tritiated uracil (2.5 μCi per well) added during the last 18–24 hours of the 2-day treatment. Average tritium incorporation in the absence of inhibitors (100% control) was 37,000 cpm per well (23,000–64,000 cpm in different experiments). In the toxicity assay, fibroblast growth in the presence of herbicides was assessed by incorporation of tritiated thymidine (2.5 μCi per well) added for the last 18–24 hours of the 2-day treatment. Average tritium incorporation in the absence of inhibitors (100% control) was 3,700 cpm per well (1,900–5,800 cpm in different experiments). Herbicides were dissolved in DMSO and used in tissue culture at a 1:100 dilution. Tachyzoite passage in mouse peritoneum and the in vitro studies was as described (11, 16). Parasite growth was assessed microscopically at ×10 and ×400 magnification after 6 days of culture, after fixation and staining as described (16). Fibroblasts were grown in four-chamber slides (Lab-Tek) and infected with tachyzoites of T. gondii. Clodinafop was added 1 hour after infection and again after 2 days of culture (with fresh medium). After 2 more days, medium was removed; cultures were washed with warm (37°C) Hanks’ balanced salt solution; and medium without inhibitor was added for the last 2 days of culture.
Gene Cloning and Sequence Analysis.
Single-strand cDNA, prepared from total RNA from T. gondii tachyzoites by using random hexamer primers, was used to amplify a fragment of the BC coding sequence by using the degenerate primers described previously (TCGAATTCGTNATNATHAARGC and GCTCTAGAGKRTGYTCNACYTG; ref. 17). Amplification was for 40 cycles—each 1 min at 95°C, 1 min at 42–46°C, and 2 min at 68°C—by using the High Fidelity PCR System (Roche Molecular Biochemicals). MgCl2 concentration was 1.5 mM. PCR products were cloned into the Promega vector pGEM-T Easy and sequenced. A T. gondii [strain RH (EP)] genomic library was obtained from the AIDS Reagent Program (www.aidsreagent.org). Seven and five positive clones were found with ACC1 and ACC2 probes, respectively, among ≈3.2 × 104 plaques, as described (18). Gene fragments encoding the BC domain are reported here (GenBank accession numbers AF157612 and AF157613, respectively). Fragments of ACC genes were PCR-cloned and sequenced from C. parvum, P. falciparum, and P. knowlesi by using the primers and conditions described above (GenBank accession numbers AF157614, AF157615, and AF157616, respectively). Sequencing was carried out by the University of Chicago Cancer Center DNA Facility. Introns were revealed by comparing genomic and cDNA sequences or by aligning the coding capacity of genomic DNA with amino acid sequences of ACCs from other organisms. Sequence alignment and the phylogenetic tree (neighbor-joining method, distances corrected for multiple substitutions) were created by using clustal x (19), with the Anabaena 7,120 BC domain sequence as an outgroup.
Results and Discussion
Herbicide Inhibition of T. gondii ACC.
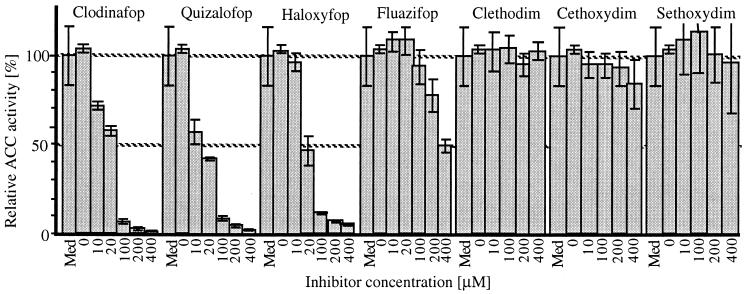
ACC activity is conveniently measured in vitro by the incorporation of the carboxyl group from bicarbonate into an acid-stable form by using protein extracts after Sephadex G50 filtration (14). Substantial acetyl-CoA-dependent activity was observed in extracts from tachyzoites of T. gondii (11) isolated from infected mice (Fig. 2). No ACC activity could be detected in control extracts of macrophages from uninfected mice, the usual minor contaminant of the parasite preparation (data not shown). Two major biotin-containing proteins were revealed with streptavidin after electrophoresis of the extract proteins (data not shown). One band at 240 kDa corresponds to the expected size for a multidomain ACC, whereas another at 130 kDa corresponds to the size expected for pyruvate carboxylase (e.g., yeast M16595, human U04641).
Figure 2.
Inhibition of T. gondii ACC activity by herbicides. Med, activity in medium without inhibitor or DMSO, 100%. Structures of the herbicides tested are shown in Fig. 1.
Fig. 1 shows the structures of the fops and dims that were tested on the ACC-containing protein extracts of T. gondii described above. Three of the four fops were striking inhibitors, whereas none of the dims had significant inhibitory effect in the enzyme assay. There was 50% inhibition at 20 μM and 90% inhibition at 100 μM by Clodinafop, Quizalofop, and Haloxyfop (Fig. 2).
Herbicide Inhibition of T. gondii Growth.
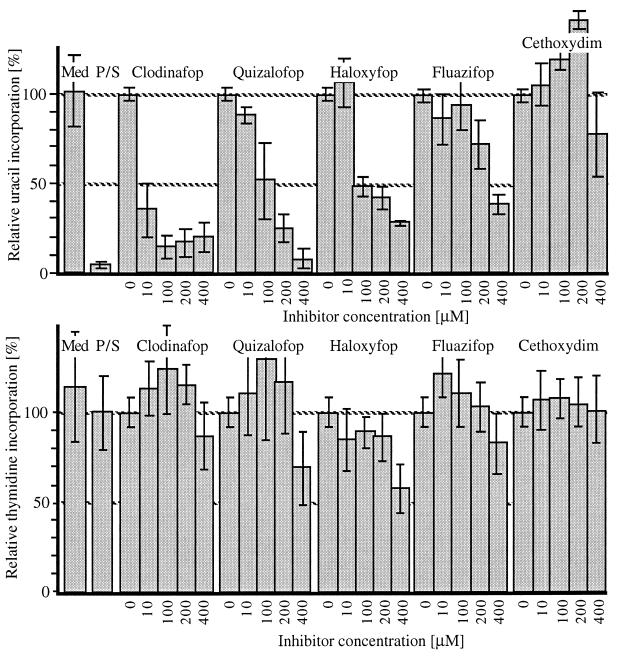
The effect of the herbicides on uninfected fibroblasts and on T. gondii growth and replication was tested as described (11) by using incorporation of radiolabeled thymidine into growing fibroblasts to determine toxicity for the host cell and incorporation of radiolabeled uracil and microscopic inspection to measure T. gondii growth. Antiparasite activity and toxicity for four fops and one dim are shown in Fig. 3. Pyrimethamine and sulfadiazine, drugs that are known inhibitors of folate synthesis, were included as a positive control (11). This combination of agents inhibited uracil incorporation by T. gondii by >95% without toxicity for fibroblasts (Fig. 3).
Figure 3.
Inhibition of T. gondii growth by herbicides. (A) Inhibition of T. gondii growth measured by [3H]uracil incorporation after 2 days of culture in the presence of herbicide. (B) Effect of herbicides on growth of human foreskin fibroblasts measured by [3H]thymidine incorporation after 2 days of culture in the presence of inhibitor. Incorporation in the presence of 1% DMSO, 100%. Mean values with standard deviations are from measurements in at least three independent experiments, each with duplicate or triplicate determinations. Med, medium without inhibitor or DMSO. P/S, pyrimethamine (100 ng/ml) plus sulfadiazine (25 ng/ml).
Consistent with the data for inhibition of ACC activity in vitro, the activity of the fops and the dim on T. gondii growth in fibroblasts was in the same concentration range after 2 days in culture. Clodinafop was even more active in this assay than in the enzyme assay, giving 70% inhibition at 10 μM (Fig. 3). In some experiments, Sethoxydim showed a modest inhibitory activity at higher concentrations (data not shown). With regard to toxicity, fops were mildly toxic only at the highest concentration tested, 400 μM. Both the fops and the dims are widely used as herbicides. As such, they have been tested extensively for safety by their developers (e.g., The Pesticide Manual, British Crop Protection Council, www.bcpc.org).
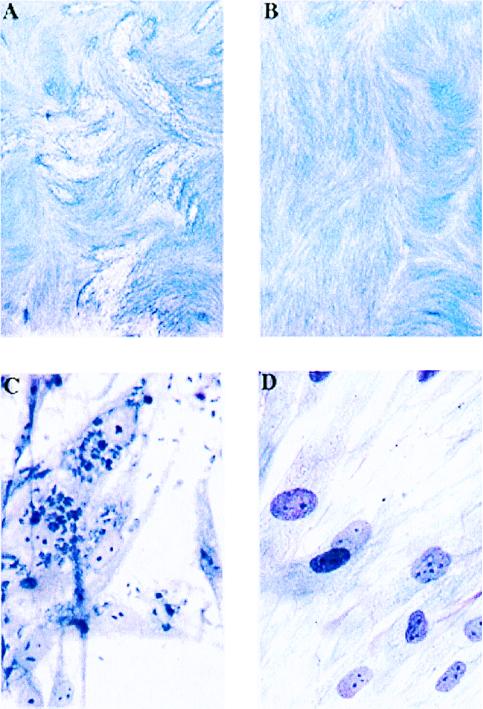
In separate experiments, the effect of Clodinafop on T. gondii was assessed by using light microscopy. First, micrographs of infected fibroblasts cultured for 2 days in the presence of Clodinafop at 10 and 100 μM, when compared with control uninfected and infected cells without the herbicide, showed a substantial reduction of the number of Toxoplasma tachyzoites at 10 μM Clodinafop and almost complete elimination of parasites at 100 μM (data not shown). The effectiveness of Clodinafop at 10 μM was enhanced by longer culture and appeared to be irreversible (Fig. 4). In the latter experiment, parasite-infected fibroblasts were cultured for 6 days. Clodinafop was added 1 hour after infection, and medium containing Clodinafop was replaced again after 2 days of culture. Finally, for the last 2 days of culture, fresh medium without Clodinafop was added. After the 4-day exposure to 10 μM Clodinafop followed by the 2-day recovery, no parasites were observed in the culture.
Figure 4.
Inhibition of T. gondii growth in human foreskin fibroblasts after culture in the presence of Clodinafop. (A and C) T. gondii in fibroblasts in the absence of inhibitor. (B and D) Absence of T. gondii in fibroblasts cultured for 6 days, with 10 μM Clodinafop for the first 4 days. Parasite growth was assessed microscopically at ×10 (A and B) and ×400 (C and D) magnification.
The form of fops used as herbicides in the field are esters, which are converted to free acids by plant esterases. The true inhibitor of ACC is the free acid (13). Two esters of Haloxyfop, two esters of Quizalofop, and one ester of Clodinafop (Topik) were found to have no effect on T. gondii ACC activity in crude extracts and were relatively inactive in the 2-day uracil incorporation assay described in Fig. 3, except for Topik, which was as active as the free acid (data not shown). This result suggests a significant level of hydrolysis of Topik, but not of the other esters, in T. gondii.
Apicomplexan Genes Encoding ACC.
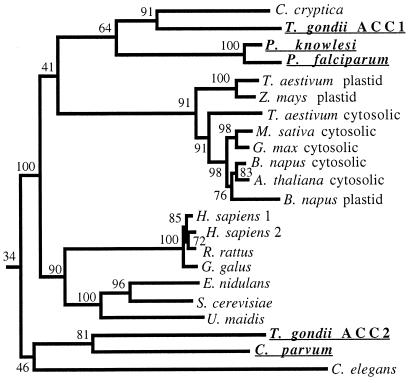
Single-stranded cDNA prepared from total RNA extracted from T. gondii tachyzoites was used as a template for the PCR amplification of a 440-bp fragment encoding the BC domain of ACC, by using primers and conditions described before for wheat ACC (17). Several independent PCRs yielded five different products. Two of these appeared to encode parts of multidomain ACCs. Genomic clones encoding the entire BC domain were then isolated from a genomic library by using the PCR-cloned fragments as probes, and these were sequenced (Fig. 5). Similarly, the sequence of a part of the BC domain of ACCs of P. knowlesi, P. falciparum, and C. parvum were determined from PCR-cloned gene fragments.
Figure 5.
Amino acid sequence comparison of the BC domains of T. gondii ACC1 and ACC2 with other ACCs, including those of apicomplexa. Sequence alignment and the phylogenetic tree (Neighbor-Joining Method, distances corrected for multiple substitutions) were created by using clustal x (19), with the Anabaena 7120 BC domain sequence as an outgroup. The length of the alignment is 550 amino acids, but shorter sequences from C. parvum, P. falciparum, and P. knowlesi ACCs were deduced from partial genomic sequences. Numbers at the nodes indicate bootstrap values (%). The sequences reported in this paper are underlined, and all are available from GenBank.
Fig. 5 shows a phylogenetic analysis based on amino acid sequence comparisons of the two candidate ACCs from T. gondii with those of other BC domains. Three apicomplexan sequences (T. gondii, P. knowlesi, and P. falciparum) cluster together with Cyclotella cryptica ACC, an enzyme thought to be present in the diatom chloroplast (20). We suggest that this isozyme, to be called ACC1 in T. gondii, is the plastid form. The other ACC, to be called ACC2 in T. gondii, clusters with the ACC of C. parvum. These two ACCs are probably cytosolic forms. This assignment awaits analysis of the 5′ portion of the cDNA, where a sequence encoding a signal/transit peptide ought to be found for the plastid isozyme (5).
The origin and evolutionary history of the genes cannot be reconstructed from the limited sequence information of the apicomplexan ACCs without such data for other closely related species. The partial genomic sequences revealed differences in intron number and location between the BC domains of ACC1 and ACC2 of T. gondii. The corresponding domains of the ACC genes from the other apicomplexa lack introns.
One of the other PCR products encodes a BC domain similar to that of pyruvate carboxylase. The deduced amino acid sequences encoded by the remaining two PCR products were similar to the BC domains of rat ACC and prokaryotic-type biotin-dependent carboxylases, respectively. These fragments were assumed to encode the host mouse ACC and a carboxylase from a bacterial contaminant. In addition to the 240-kDa ACC polypeptide, Western blots of our T. gondii protein extracts probed with streptavidin revealed a putative pyruvate carboxylase of about 130 kDa but no bacterial-type biotin carboxyl carrier protein subunit of ACC with expected apparent size of 20–30 kDa (e.g., ref. 17).
There is a very strong correlation between the sensitivity of ACC activity and Toxoplasma growth inhibition by the 12 different compounds tested in this study (Figs. 1–3, and data not shown). This result links the Toxoplasma growth phenotype with the effect of the compounds on ACC enzymatic activity. We do not know the basis for the sensitivity of some of the multidomain ACCs to fops and dims or why some, like the T. gondii ACC activity reported here, are sensitive to fops but more resistant to dims.
Compounds in the fop family differ in their properties as well, with a clear correlation between activity and structure, e.g., the relatively low inhibitory activity of Fluazifop. We cannot determine from their sequences why the apicomplexan ACC and the ACC from grasses show different patterns of sensitivity to dims and fops nor why the ACCs of animals and the plant cytosol are resistant. Experiments in progress have narrowed the target for herbicide sensitivity of plastid ACC to a region encompassing a stretch of 400 amino acids of the carboxyltransferase domain. These experiments use yeast gene replacement strains, in which chimeric genes encoding wheat ACCs replace the yeast ACC1 gene (ref. 14; T. Nikolskaya, O. Zagnitko, G. Tevzadze, R.H., and P.G., unpublished results). Such yeast strains are herbicide-sensitive if they contain a gene encoding sensitive ACC. The genes encoding T. gondii ACCs will allow us to clarify which of the isozymes is targeted to the plastid. The majority of the ACC activity in our T. gondii protein extracts is inhibited by fops and therefore are likely to represent the plastid form.
Earlier studies of herbicide action on plants and yeast gene-replacement strains showed that inhibition of ACC activity in sensitive species leads to metabolite depletion to such an extent that the organism cannot grow. The results reported here indicate that T. gondii can also be targeted by ACC inhibitors. This result reflects the essential contribution of ACC to the pathway of de novo fatty acid synthesis and forms the basis for the use of ACC inhibitors as herbicides in agriculture and their potential future use as treatment for apicomplexan diseases.
After this work was completed, we learned of a study in which two herbicide inhibitors of the nonmevalonic acid pathway for carotenoid biosynthesis were shown to be effective against P. falciparum in human red blood cells and in whole mice (21). The target enzymes seem to be located in the apicoplast.
Acknowledgments
We thank Peter Carlson for providing unpublished results, E. Mui for technical assistance in preparing lysates of T. gondii for the enzyme assays, D. Mack and M. Kirisits for T. gondii cDNA, J. Barnwell for P. knowlesi and P. falciparum DNA, S. Tzipori and A. Fairfield for C. parvum DNA, and G. Bowie for help with the micrographs. Herbicides were kindly provided by Dow Elanco, now Dow Agro, Indianapolis, IN, and Novartis, Research Triangle Park, NC. This work was supported by National Institutes of Health Grants AI 16945, 27530, and 49228, and the Harris and Frances Block Research Fund. R.M. is the Jules and Doris Stein Research to Prevent Blindness Professor at University of Chicago.
Abbreviations
- ACC
acetyl-CoA carboxylase
- BC
biotin carboxylase
Footnotes
References
- 1.Hackstein J H P, Mackenstedt U, Mehlhorn H, Meijerink J P P, Schubert H, Leunissen J A M. Parasitol Res. 1995;81:207–216. doi: 10.1007/BF00937111. [DOI] [PubMed] [Google Scholar]
- 2.Wilson I. In: Series H: Cell Biology, NATO ASI. Smith J, editor. Vol. 78. Berlin: Springer; 1993. pp. 51–60. [Google Scholar]
- 3.Williamson D H, Gardner M J, Preiser P, Moore D J, Rangarchari K, Wilson R J M. Mol Gen Genet. 1994;243:249–252. doi: 10.1007/BF00280323. [DOI] [PubMed] [Google Scholar]
- 4.McFadden G I, Reith M E, Mulholland J, Lang-Unnsch N. Nature (London) 1996;381:482. doi: 10.1038/381482a0. [DOI] [PubMed] [Google Scholar]
- 5.Waller R F, Keeling P J, Donald R G K, Striepen B, Handman E, Lang-Unnasch N, Cowman A F, Besra G S, Roos D S, McFadden G I. Proc Natl Acad Sci USA. 1998;95:12352–12357. doi: 10.1073/pnas.95.21.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson R J M, Williamson D H. Microbiol Mol Biol Rev. 1997;61:1–16. doi: 10.1128/mmbr.61.1.1-16.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohler S, Delwiche C F, Denny P W, Tinley L G, Webster P, Wilson R J M, Palmer J D, Roos D S. Nature (London) 1997;275:1485–1489. doi: 10.1126/science.275.5305.1485. [DOI] [PubMed] [Google Scholar]
- 8.Fichera M E, Roos D S. Nature (London) 1997;390:407–409. doi: 10.1038/37132. [DOI] [PubMed] [Google Scholar]
- 9.Beckers C J M, Roos D S, Donald R G K, Luft B J, Schwab J C, Cao Y, Joiner K A. J Clin Invest. 1995;95:367–376. doi: 10.1172/JCI117665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfefferkorn E R, Nothnagel R F, Borotz S E. Antimicrob Agents Chemother. 1992;36:1091–1096. doi: 10.1128/aac.36.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts F, Roberts C W, Johnson J J, Kyle D E, Krell T, Coggins J R, Coombs G H, Milhous W K, Tzipori S, Ferguson D J, et al. Nature (London) 1998;393:801–805. doi: 10.1038/31723. [DOI] [PubMed] [Google Scholar]
- 12.Stokkermans T J W, Schwartzman J D, Keenan K, Morrissette N S, Tilney L G, Roos D S. Exp Parasitol. 1996;84:355–370. doi: 10.1006/expr.1996.0124. [DOI] [PubMed] [Google Scholar]
- 13.Golz A, Focke M, Lichtenthaler H K. J Plant Physiol. 1994;143:426–433. [Google Scholar]
- 14.Joachimiak M, Tevzadze G, Podkowinski J, Haselkorn R, Gornicki P. Proc Natl Acad Sci USA. 1997;94:9990–9995. doi: 10.1073/pnas.94.18.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gornicki P, Haselkorn R. Plant Mol Biol. 1993;22:547–552. doi: 10.1007/BF00015984. [DOI] [PubMed] [Google Scholar]
- 16.Mack D, McLeod R. Antimicrob Agents Chemother. 1984;26:26–30. doi: 10.1128/aac.26.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gornicki P, Podkowinski J, Scappino L A, DiMaio J, Ward E, Haselkorn R. Proc Natl Acad Sci USA. 1994;91:6860–6864. doi: 10.1073/pnas.91.15.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Podkowinski J, Sroga G E, Haselkorn R, Gornicki P. Proc Natl Acad Sci USA. 1996;93:1870–1874. doi: 10.1073/pnas.93.5.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson J D, Higgins D G, Gibson T J. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roessler P G, Ohlrogge J B. J Biol Chem. 1993;268:19254–19259. [PubMed] [Google Scholar]
- 21.Joamaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Turbachova I, Ebert M, Zeidler J, Lichtenthaler H K, et al. Science. 1999;285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]