Abstract
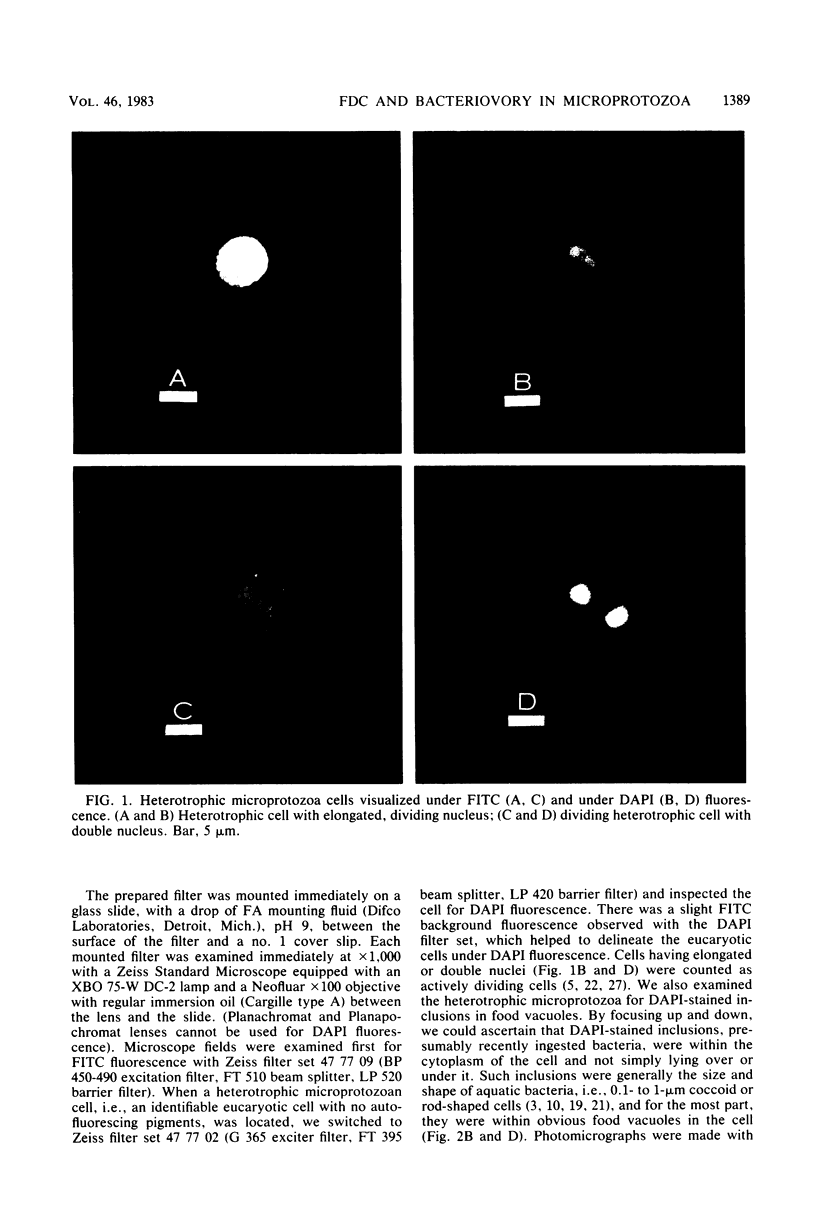
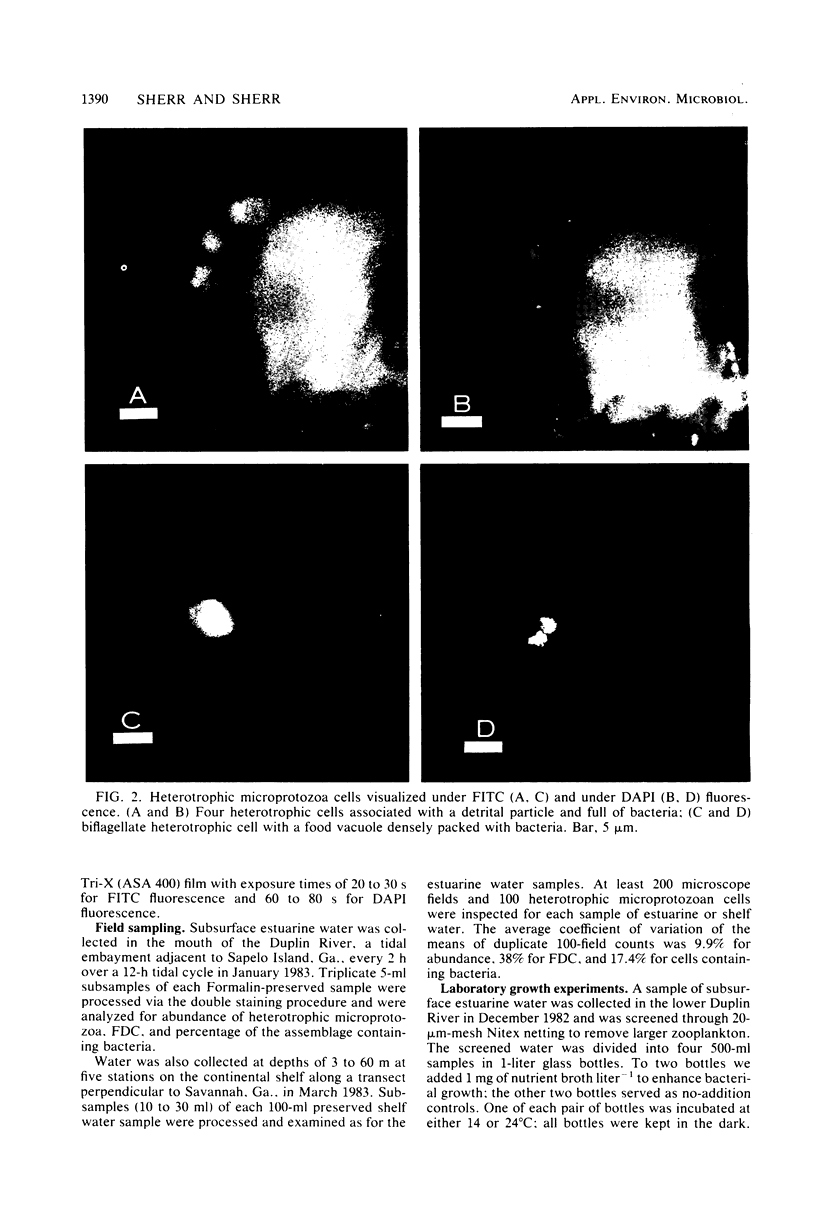
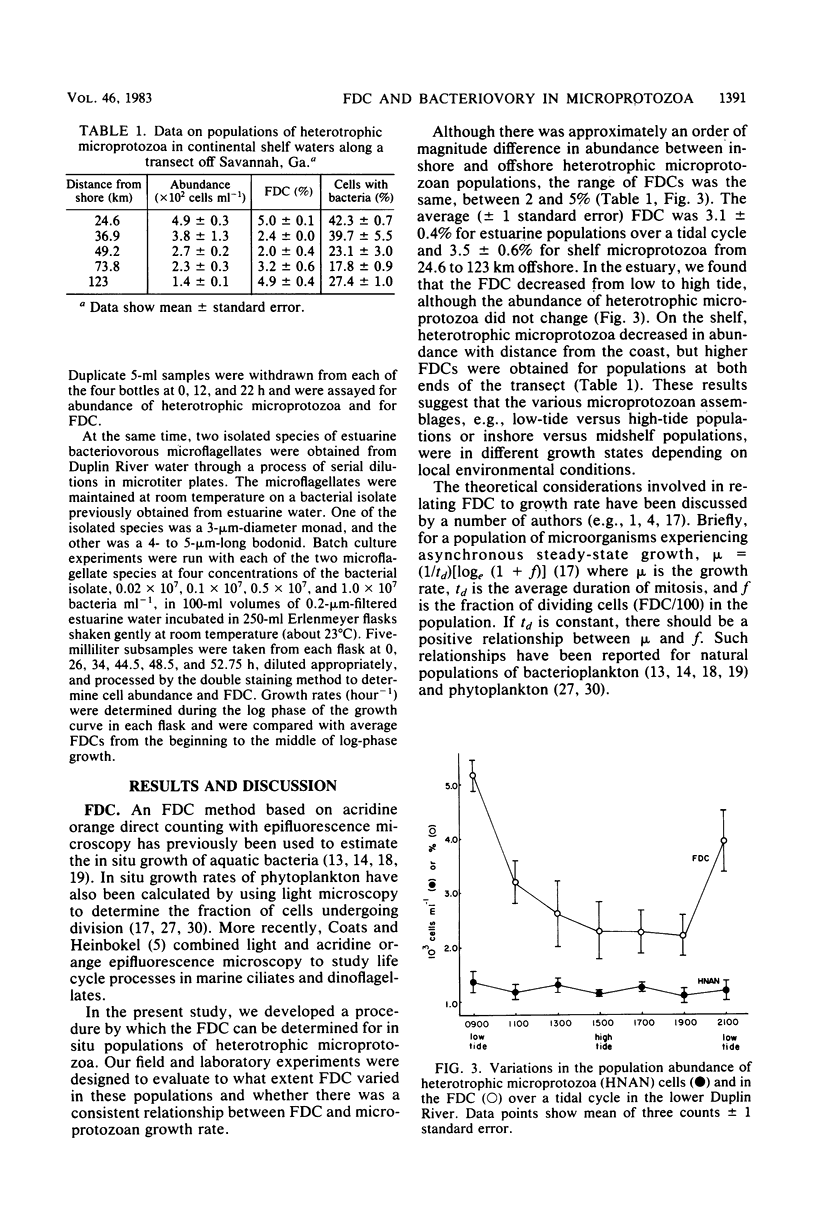
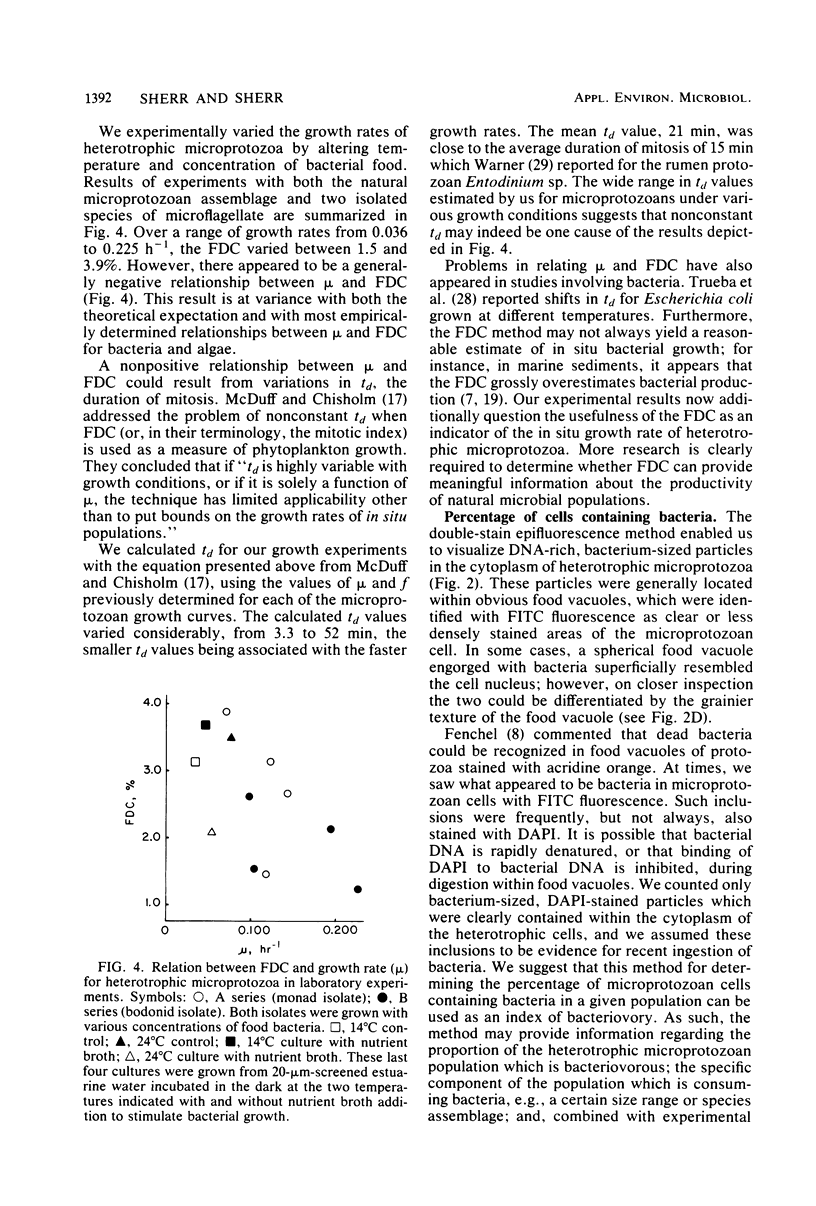
We have developed a double-staining procedure for use with epifluorescence microscopy which allows the detection both of dividing cells and of ingested bacteria in food vacuoles of heterotrophic microprotozoa. Microprotozoan cells are stained sequentially with the DNA-specific fluorochrome DAPI (4′,6-diami-dino-2-phenylindole) and the nonspecific protein stain fluorescein isothiocyanate. During microscopic examination, heterotrophic microprotozoan cells are first located with fluorescein isothiocyanate fluorescence and then epifluorescence filter sets are switched to permit inspection under DAPI fluorescence of the cell nuclei and of the contents of food vacuoles. Among in situ populations of estuarine microprotozoa sampled over a tidal cycle, we found from 2.2 to 5.2% of the heterotrophic cells in a recognizable stage of division (nuclei elongated or double). Batch culture growth experiments were also carried out both with natural populations and with two isolated species of estuarine microprotozoa. In these experiments, the frequency of dividing cells ranged from 1.2 to 3.8% and appeared to be negatively correlated with growth rate. Microprotozoan populations sampled in continental shelf waters off Savannah, Ga., had mean frequencies of dividing cells ranging from 2.0 to 5.0%. A large fraction of cells in heterotrophic microprotozoan populations (an average of 27.4 ± 1.0% in estuarine water and of 30.1 ± 4.8% in shelf water) had DAPI-stained inclusions, presumably recently ingested bacteria, in their food vacuoles.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. D. Microbial growth rates in nature. Bacteriol Rev. 1971 Mar;35(1):39–58. doi: 10.1128/br.35.1.39-58.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron D. A., Davis P. G., Madin L. P., Sieburth J. M. Heterotrophic bacteria and bacterivorous protozoa in oceanic macroaggregates. Science. 1982 Nov 19;218(4574):795–797. doi: 10.1126/science.218.4574.795. [DOI] [PubMed] [Google Scholar]
- Caron D. A. Technique for enumeration of heterotrophic and phototrophic nanoplankton, using epifluorescence microscopy, and comparison with other procedures. Appl Environ Microbiol. 1983 Aug;46(2):491–498. doi: 10.1128/aem.46.2.491-498.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K. T., Nilson E. H., Case M. J., Marr A. G., Hungate R. E. Estimation of growth rate from the mitotic index. Appl Microbiol. 1973 May;25(5):778–780. doi: 10.1128/am.25.5.778-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagström A., Larsson U., Hörstedt P., Normark S. Frequency of dividing cells, a new approach to the determination of bacterial growth rates in aquatic environments. Appl Environ Microbiol. 1979 May;37(5):805–812. doi: 10.1128/aem.37.5.805-812.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. B., Shafer D., Ryan T., Pope D. H., Lowery H. K. Bacterioplankton in antarctic ocean waters during late austral winter: abundance, frequency of dividing cells, and estimates of production. Appl Environ Microbiol. 1983 May;45(5):1622–1632. doi: 10.1128/aem.45.5.1622-1632.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell S. Y., Christian R. R. Frequency of dividing cells as an estimator of bacterial productivity. Appl Environ Microbiol. 1981 Jul;42(1):23–31. doi: 10.1128/aem.42.1.23-31.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueba F. J., van Spronsen E. A., Traas J., Woldringh C. L. Effects of temperature on the size and shape of Escherichia coli cells. Arch Microbiol. 1982 May;131(3):235–240. doi: 10.1007/BF00405885. [DOI] [PubMed] [Google Scholar]
- WARNER A. C. Some factors influencing the rumen microbial population. J Gen Microbiol. 1962 Apr;28:129–146. doi: 10.1099/00221287-28-1-129. [DOI] [PubMed] [Google Scholar]