Abstract
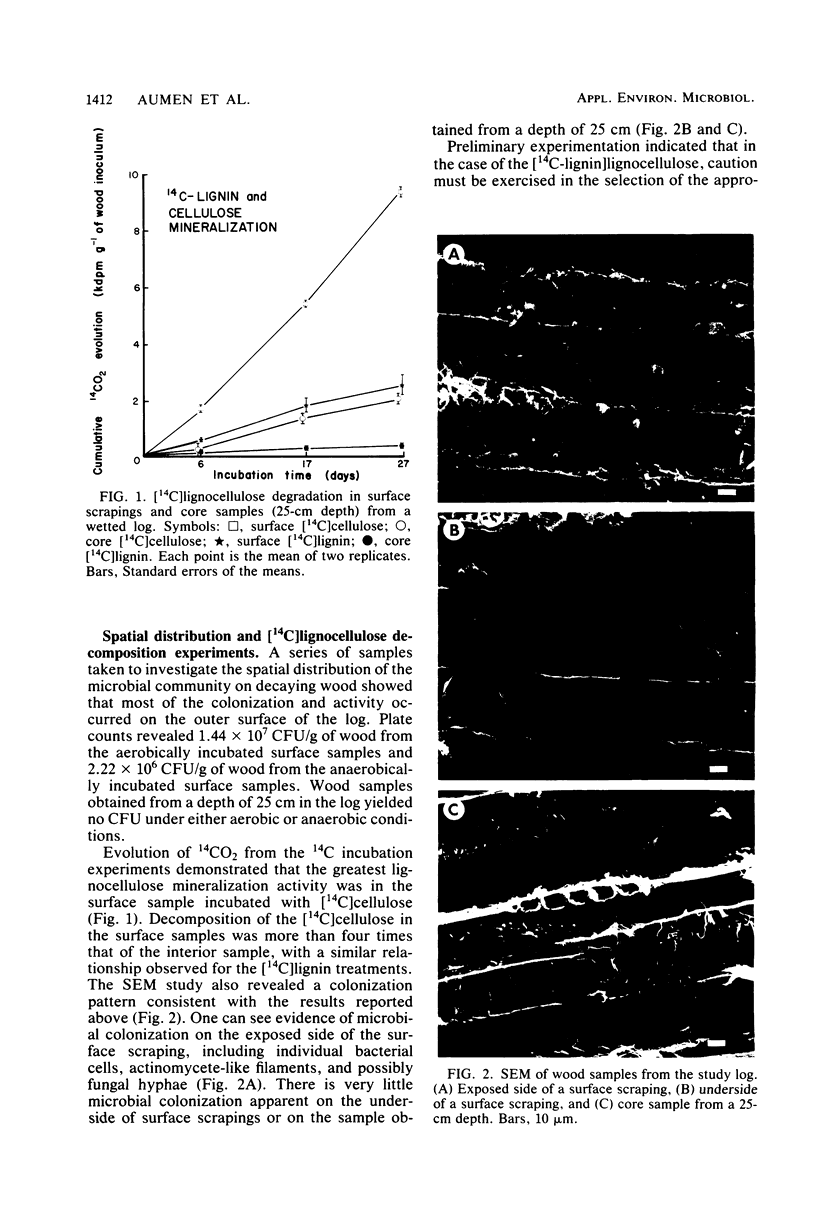
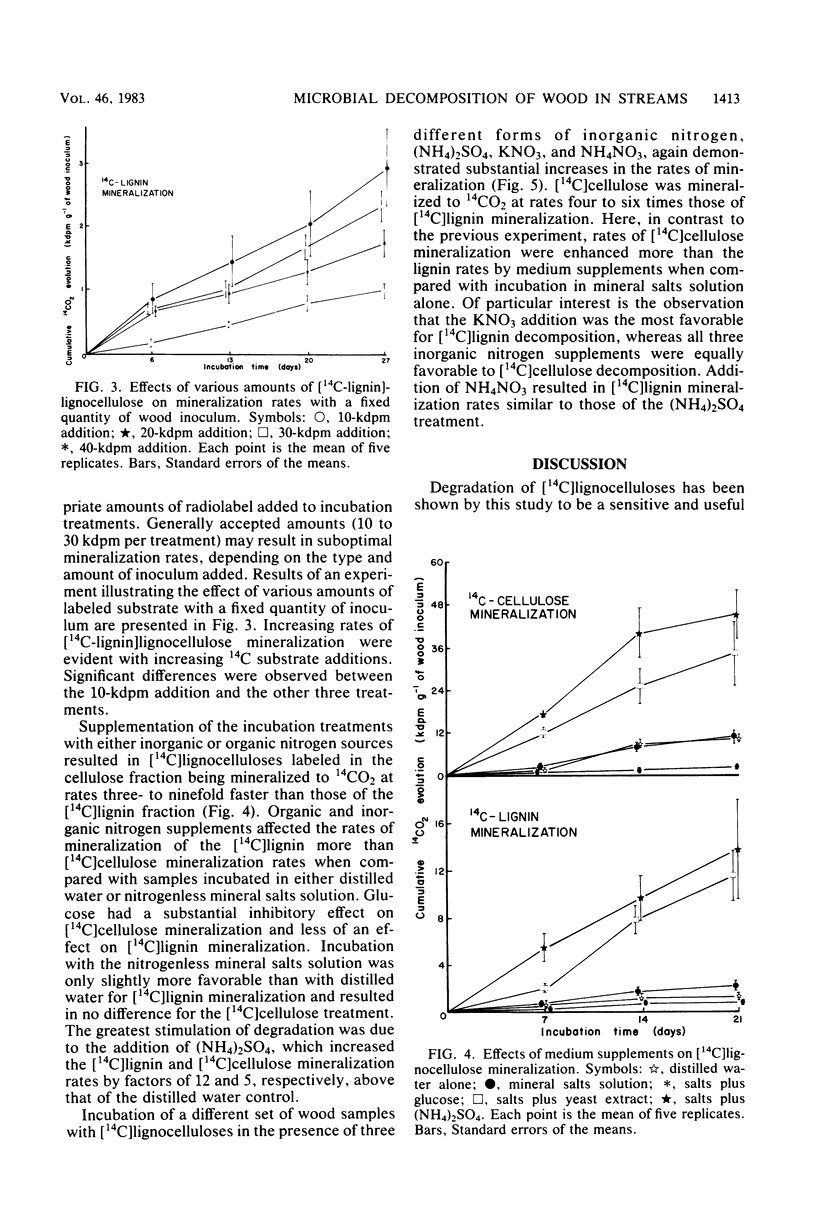
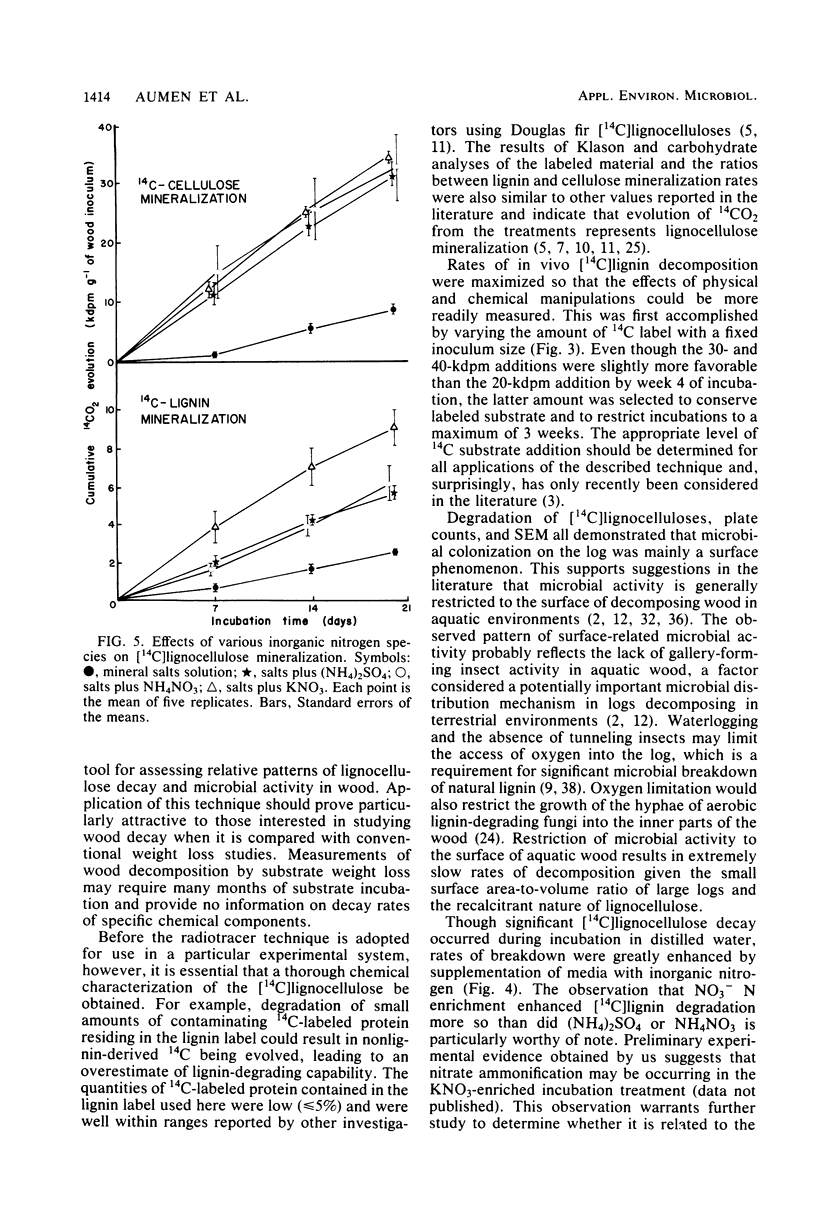
The distribution and lignocellulolytic activity of the microbial community was determined on a large log of Douglas fir (Pseudotsuga menziesii) in a Pacific Northwest stream. Scanning electron microscopy, plate counts, and degradation of [14C]lignocelluloses prepared from Douglas fir and incubated with samples of wood taken from the surface and within the log revealed that most of the microbial colonization and lignocellulose-degrading activity occurred on the surface. Labeled lignocellulose and surface wood samples were incubated in vitro with nutrient supplements to determine potential limiting factors of [14C]lignocellulose degradation. Incubations carried out in a nitrogenless mineral salts and trace elements solution were no more favorable to degradation than those carried out in distilled water alone. Incubations supplemented with either (NH4)2SO4 or organic nitrogen sources showed large increases in the rates of mineralization over incubations with mineral salts and trace elements alone, with the greatest effect being observed from an addition of (NH4)2SO4. Subsequent incubations with (NH4)2SO4, KNO3, and NH4NO3 revealed that KNO3 was the most favorable for lignin degradation, whereas all three supplements were equally favorable for cellulose degradation. Supplementation with glucose repressed both lignin and cellulose mineralization. The results reported in this study indicate that nitrogen limitation of wood decomposition may exist in streams of the Pacific Northwest. The radiotracer technique was shown to be a sensitive and useful tool for assessing relative patterns of lignocellulose decay and microbial activity in wood, along with the importance of thoroughly characterizing the experimental system before its general acceptance.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker K. H. Effects of selected assay parameters on measurement of lignocellulose mineralization with a radiolabeled substrate. Appl Environ Microbiol. 1983 Mar;45(3):1129–1131. doi: 10.1128/aem.45.3.1129-1131.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barder M. J., Crawford D. L. Effects of carbon and nitrogen supplementation on lignin and cellulose decomposition by a Streptomyces. Can J Microbiol. 1981 Aug;27(8):859–863. doi: 10.1139/m81-136. [DOI] [PubMed] [Google Scholar]
- Crawford D. L., Crawford R. L. Microbial degradation of lignocellulose: the lignin component. Appl Environ Microbiol. 1976 May;31(5):714–717. doi: 10.1128/aem.31.5.714-717.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. L., Crawford R. L., Pometto A. L. Preparation of specifically labeled C-(lignin)- and C-(cellulose)-lignocelluloses and their decomposition by the microflora of soil. Appl Environ Microbiol. 1977 Jun;33(6):1247–1251. doi: 10.1128/aem.33.6.1247-1251.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. L., Floyd S., Pometto A. L., 3rd, Crawford R. L. Degradation of natural and Kraft lignins by the microflora of soil and water. Can J Microbiol. 1977 Apr;23(4):434–440. doi: 10.1139/m77-064. [DOI] [PubMed] [Google Scholar]
- Crawford D. L. Lignocellulose decomposition by selected streptomyces strains. Appl Environ Microbiol. 1978 Jun;35(6):1041–1045. doi: 10.1128/aem.35.6.1041-1045.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federle T. W., Vestal J. R. Lignocellulose mineralization by arctic lake sediments in response to nutrient manipulation. Appl Environ Microbiol. 1980 Jul;40(1):32–39. doi: 10.1128/aem.40.1.32-39.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett W. F., Connors W. J., Kirk T. K., Zeikus J. G. Microbial decomposition of synthetic C-labeled lignins in nature: lignin biodegradation in a variety of natural materials. Appl Environ Microbiol. 1977 Jan;33(1):43–51. doi: 10.1128/aem.33.1.43-51.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser P., Kirk T. K., Zeikus J. G. Ligninolytic enzyme system of Phanaerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol. 1978 Sep;135(3):790–797. doi: 10.1128/jb.135.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Connors W. J., Bleam R. D., Hackett W. F., Zeikus J. G. Preparation and microbial decomposition of synthetic [14C]ligins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2515–2519. doi: 10.1073/pnas.72.7.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccubbin A. E., Hodson R. E. Mineralization of detrital lignocelluloses by salt marsh sediment microflora. Appl Environ Microbiol. 1980 Oct;40(4):735–740. doi: 10.1128/aem.40.4.735-740.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid I. D. Effects of Nitrogen Sources on Cellulose and Synthetic Lignin Degradation by Phanerochaete chrysosporium. Appl Environ Microbiol. 1983 Mar;45(3):838–842. doi: 10.1128/aem.45.3.838-842.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H. Biochemistry and genetics of nitrate reductase in bacteria. Adv Microb Physiol. 1976;14(11):315–375. doi: 10.1016/s0065-2911(08)60230-1. [DOI] [PubMed] [Google Scholar]



