Abstract
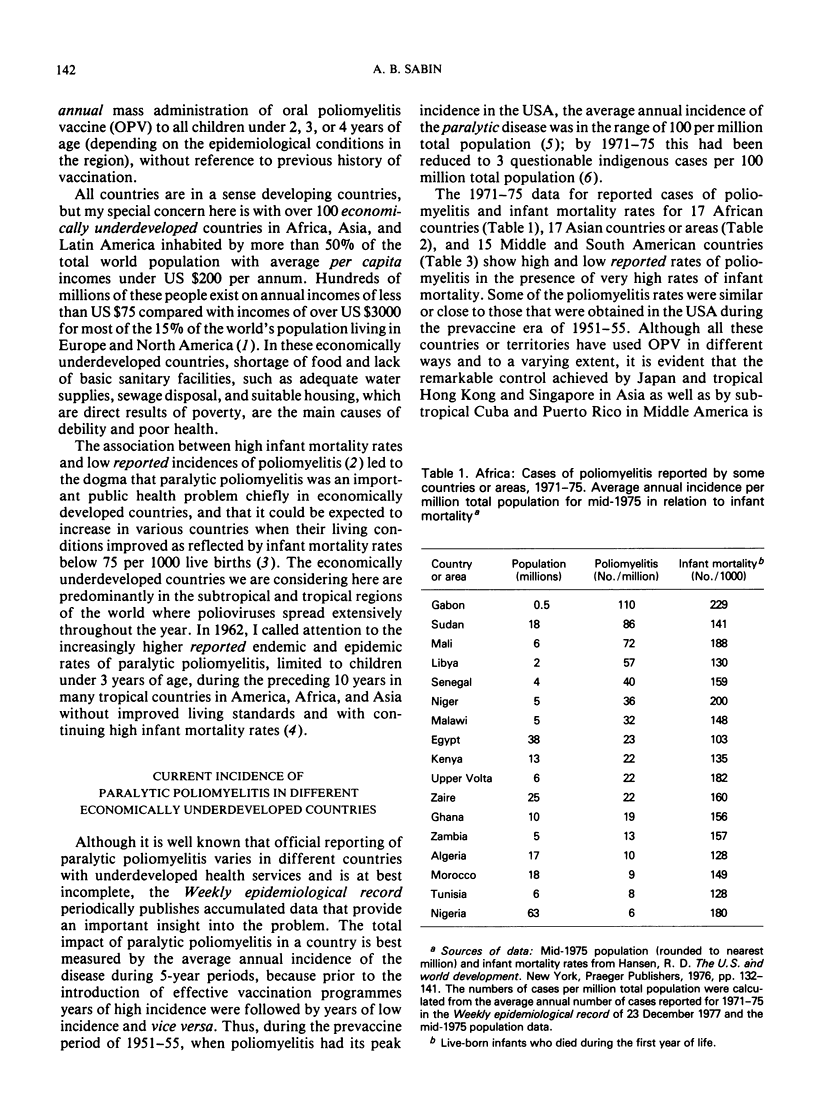
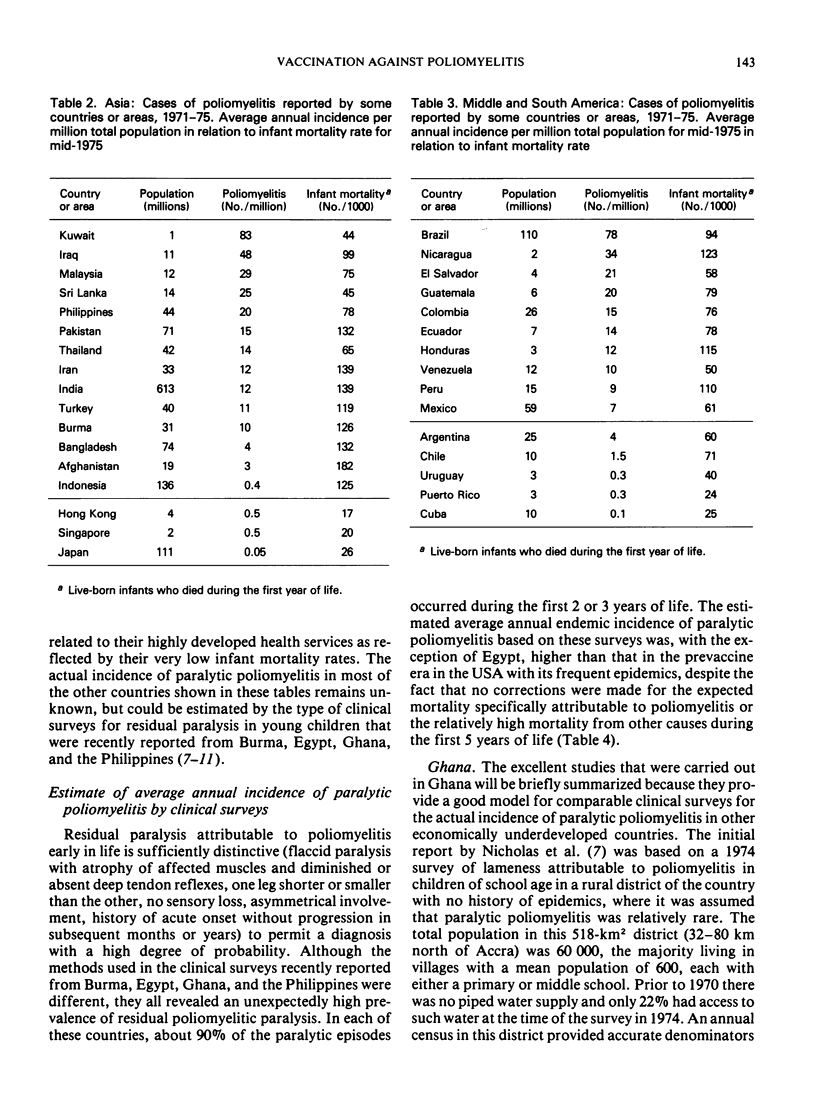
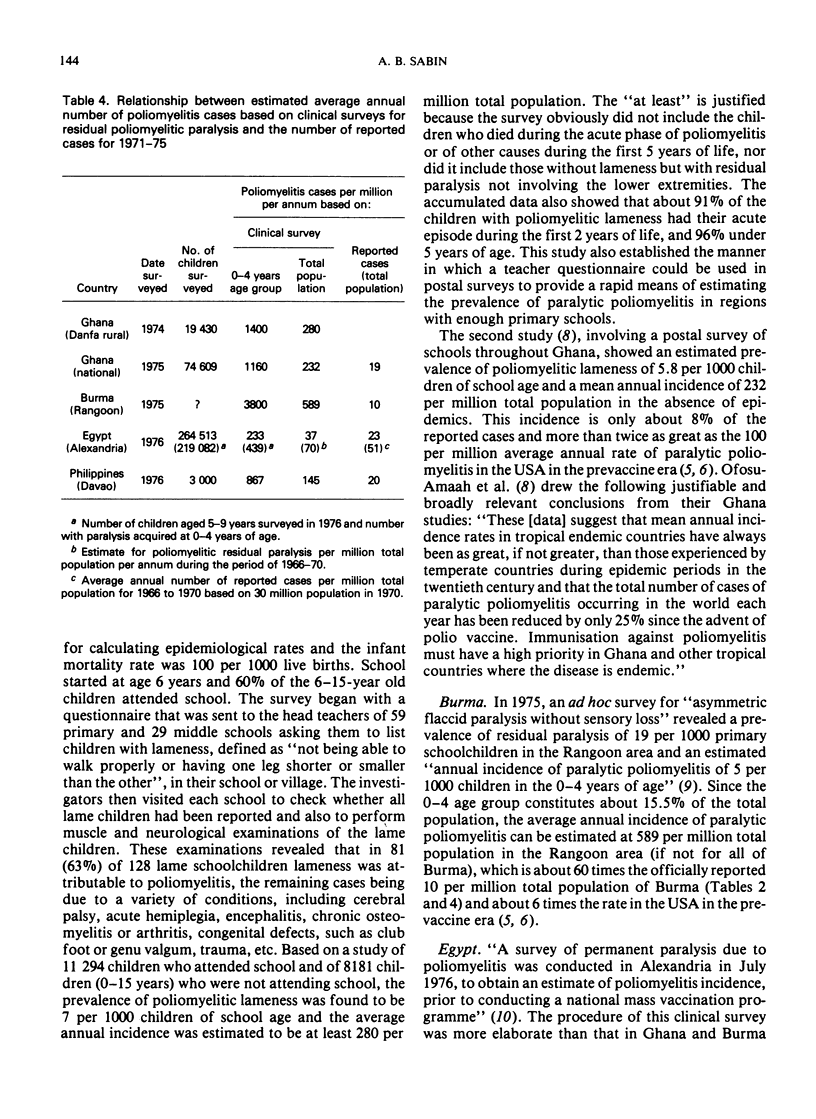
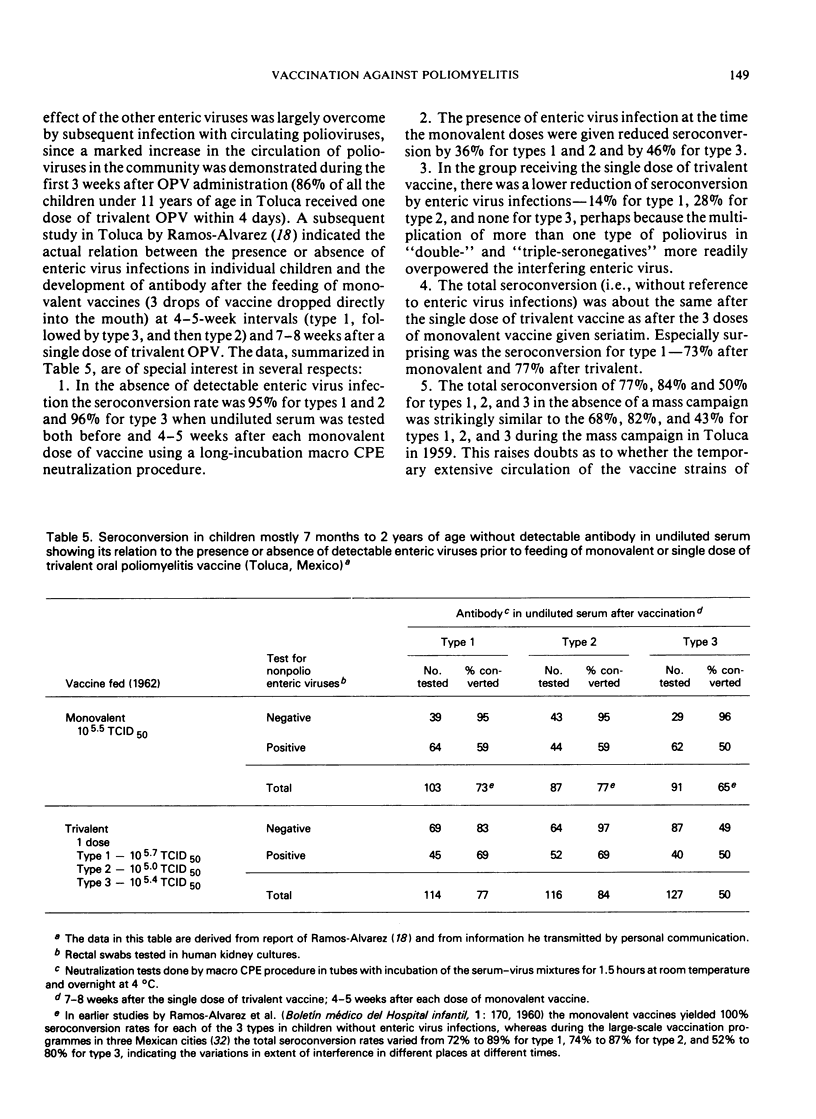
Poliomyelitis lameness surveys in children of school age recently reported from Burma, Egypt, Ghana, and the Philippines have indicated an estimated, average annual endemic incidence of paralytic poliomyelitis similar to or higher than the overall average annual rate in the USA during the peak years in the prevaccine era. Contrary to oft-expressed dogma, high rates of paralytic poliomyelitis are occurring annually in regions with high infant mortality rates, continuing undernutrition, and absence of basic sanitary facilities. Recent data indicate that prolonged breast feeding does not impede the effectiveness of oral poliovirus vaccine (OPV). A high prevalence of nonpoliovirus enteric infections can modify, delay, and lower the frequency of seroconversion after OPV, but these effects are overcome by multiple doses. The problem of eliminating paralytic poliomyelitis from economically underdeveloped countries depends on administrative rather than immunological or epidemiological factors, although a specially concentrated effort is needed in countries where most of the cases occur during the first two years of life and where paralytic polioviruses are propagating throughout the year in a large proportion of the infant population. Under such circumstances, expanded routine infant immunization programmes, which include OPV but reach at best only 20-40% of the total infant population, who receive only one or a few doses of vaccines requiring multiple doses, cannot be expected to eliminate paralytic poliomyelitis as an important public health problem. Injections of multiple doses of quadruple vaccine (DPT + inactivated poliomyelitis vaccine) would not only greatly increase the cost of routine immunizations but would not achieve more or as much as feeding OPV at the time of the DPT injections. Mass administration of OPV each year on 2 days of the year 2 months apart, to all children under 2, 3, or 4 years of age (depending on the epidemiological situation), without reference to the number of OPV doses they may have had before, can be expected to yield optimum results in countries with small numbers of professional health personnel and many other year-round problems.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRECHT R. M., BIGWOOD DE Jr LEVY W. C., QUINLIVAN J. J., ROGERS E. F., WESTMAN E. R. Oral poliovirus vaccination program in central New York State, 1961. Public Health Rep. 1963 May;78:403–412. [PMC free article] [PubMed] [Google Scholar]
- Deforest A., Parker P. B., DiLiberti J. H., Yates H. T., Jr, Sibinga M. S., Smith D. S. The effect of breast-feeding on the antibody response of infants to trivalent oral poliovirus vaccine. J Pediatr. 1973 Jul;83(1):93–95. doi: 10.1016/s0022-3476(73)80323-3. [DOI] [PubMed] [Google Scholar]
- Dömök I., Balayan M. S., Fayinka O. A., Skrtić N., Soneji A. D., Harland P. S. Factors affecting the efficacy of live poliovirus vaccine in warm climates. Efficacy of type 1 Sabin vaccine administered together with antihuman gamma-globulin horse serum to breast-fed and artificially fed infants in Uganda. Bull World Health Organ. 1974;51(4):333–347. [PMC free article] [PubMed] [Google Scholar]
- John T. J., Devarajan L. V., Luther L., Vijayarathnam P. Effect of breast-feeding on seroresponse of infants to oral poliovirus vaccination. Pediatrics. 1976 Jan;57(1):47–53. [PubMed] [Google Scholar]
- John T. J., Jayabal P. Oral polio vaccination of children in the tropics. I. The poor seroconversion rates and the absence of viral interference. Am J Epidemiol. 1972 Oct;96(4):263–269. doi: 10.1093/oxfordjournals.aje.a121457. [DOI] [PubMed] [Google Scholar]
- LEPOW M. L., WARREN R. J., GRAY N., INGRAM V. G., ROBBINS F. C. Effect of Sabin Type 1 poliomyelitis vaccine administered by mouth to newborn infants. N Engl J Med. 1961 May 25;264:1071–1078. doi: 10.1056/NEJM196105252642102. [DOI] [PubMed] [Google Scholar]
- MILLER D. G., OPTON E. M., HORSTMANN D. M. The 1961 Middletown Oral Poliovirus Vaccine Program. VIII. Immunological effectiveness of the two dose schedule. Yale J Biol Med. 1962 Apr;34:505–511. [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L. Advantages and disadvantages of killed and live poliomyelitis vaccines. Bull World Health Organ. 1978;56(1):21–38. [PMC free article] [PubMed] [Google Scholar]
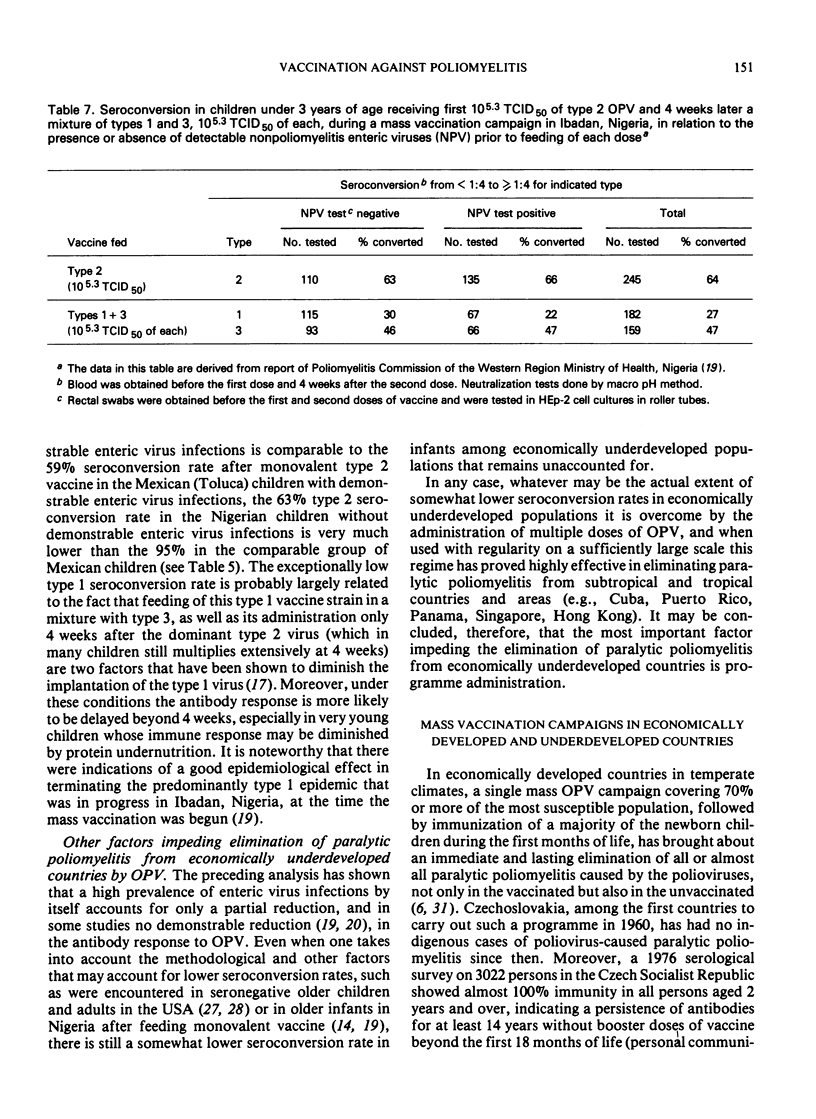
- Nicholas D. D., Kratzer J. H., Ofosu-Amaah S., Belcher D. W. Outside Europe. Is poliomyelitis a serious problem in developing countries?--the Danfa experience. Br Med J. 1977 Apr 16;1(6067):1009–1012. doi: 10.1136/bmj.1.6067.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofosu-Amaah S., Kratzer J. H., Nicholas D. D. Is poliomyelitis a serious problem in developing countries?--lameness in Ghanaian schools. Br Med J. 1977 Apr 16;1(6067):1012–1014. doi: 10.1136/bmj.1.6067.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUL J. R. Endemic and epidemic trends of poliomyelitis in Central and South America. Bull World Health Organ. 1958;19(4):747–758. [PMC free article] [PubMed] [Google Scholar]
- Ramos-Alvarez M., Bessudo L., Sabin A. B. Paralytic syndromes associated with noninflammatory cytoplasmic or nuclear neuronopathy. Acute paralytic disease in Mexican children, neuropathologically distinguishable from Landry-Guillain-Barré syndrome. JAMA. 1969 Feb 24;207(8):1481–1492. [PubMed] [Google Scholar]
- SABIN A. B., MICHAELS R. H., ZIRING P., KRUGMAN S., WARREN J. Effect of oral poliovirus vaccine in newborn children. II. Intestinal resistance and antibody response at 6 months in children fed type I vaccine at birth. Pediatrics. 1963 Apr;31:641–650. [PubMed] [Google Scholar]
- SABIN A. B. Poliomyelitis in the tropics. Increasing incidence and prospects for control. Trop Geogr Med. 1963 Mar;15:38–44. [PubMed] [Google Scholar]
- SABIN A. B. Present position of immunization against poliomyelitis with live virus vaccines. Br Med J. 1959 Mar 14;1(5123):663–680. doi: 10.1136/bmj.1.5123.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin A. B. Poliomyelitis vaccination. Evaluation and direction in continuing application. Am J Clin Pathol. 1978 Jul;70(1 Suppl):136–140. [PubMed] [Google Scholar]
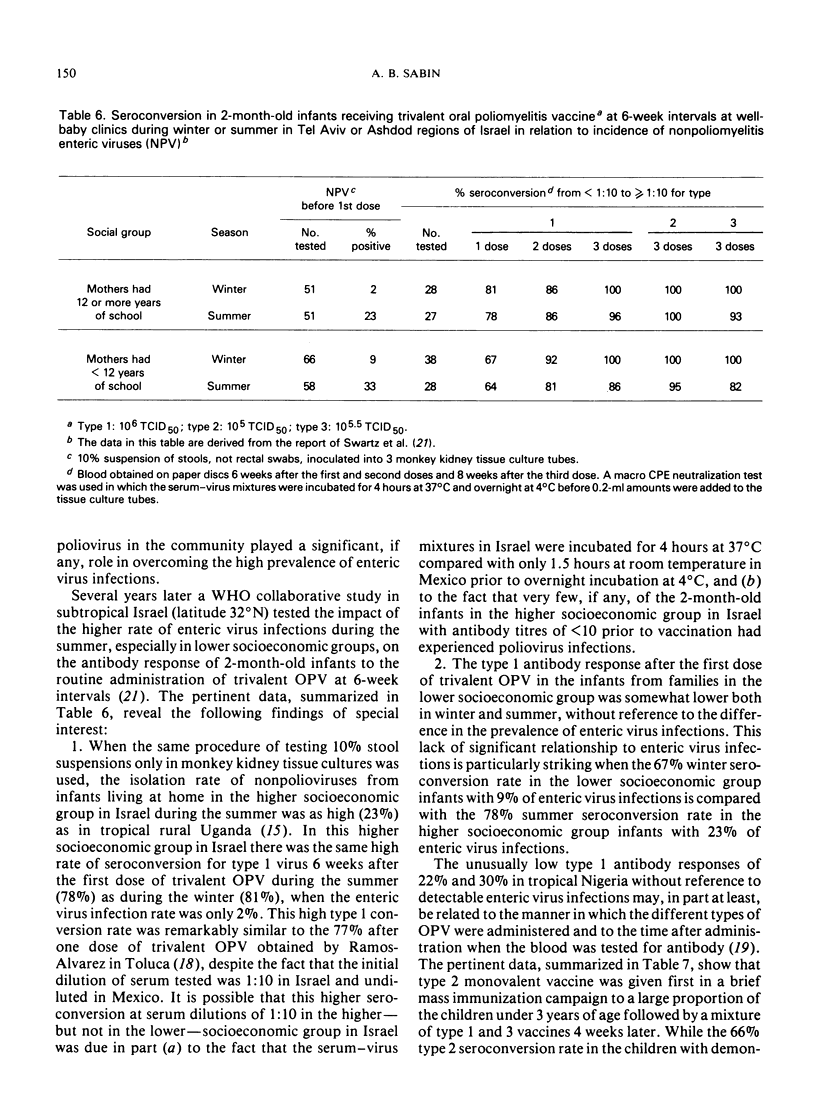
- Swartz T. A., Skalska P., Gerichter C. G., Cockburn W. C. Routine administration of oral polio vaccine in a subtropical area. Factors possibly influencing sero-conversion rates. J Hyg (Lond) 1972 Dec;70(4):719–726. doi: 10.1017/s0022172400022567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi A., Ragona G., Biondi W., Rocchi G., Archetti I. Seroimmunity to polioviruses in an urban population of Italy. Bull World Health Organ. 1976;54(3):275–278. [PMC free article] [PubMed] [Google Scholar]



