Abstract
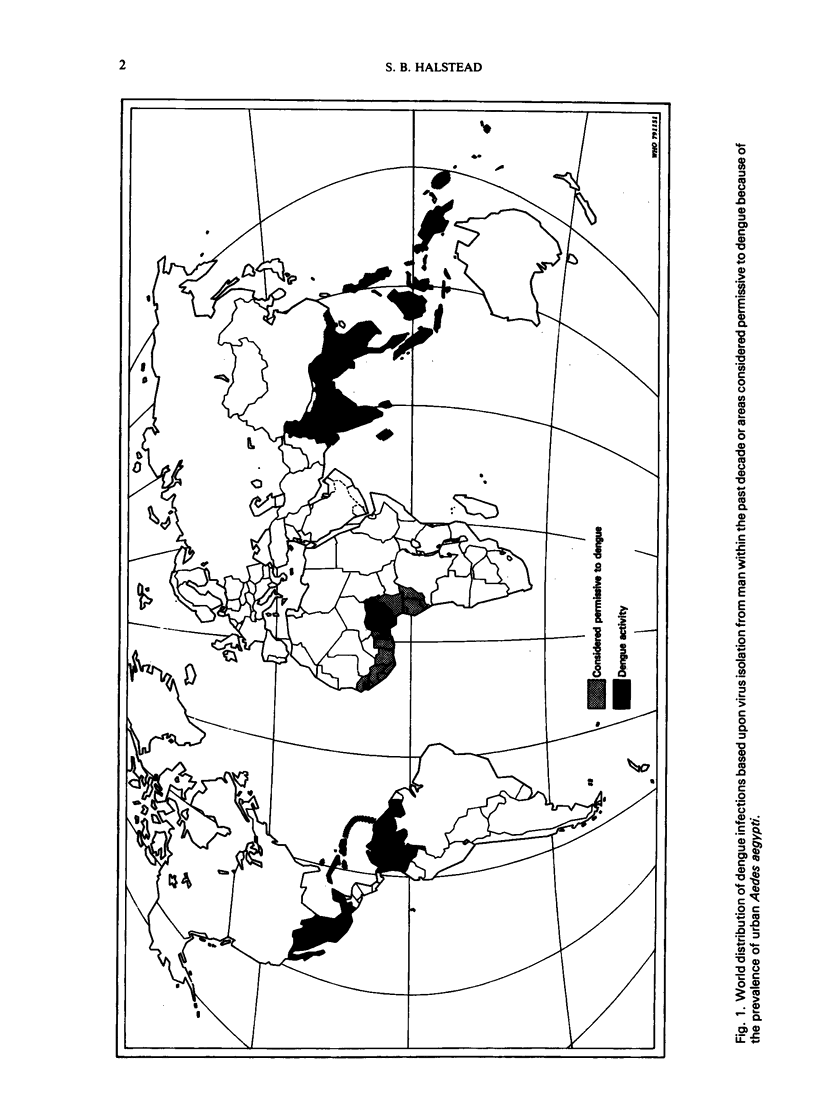
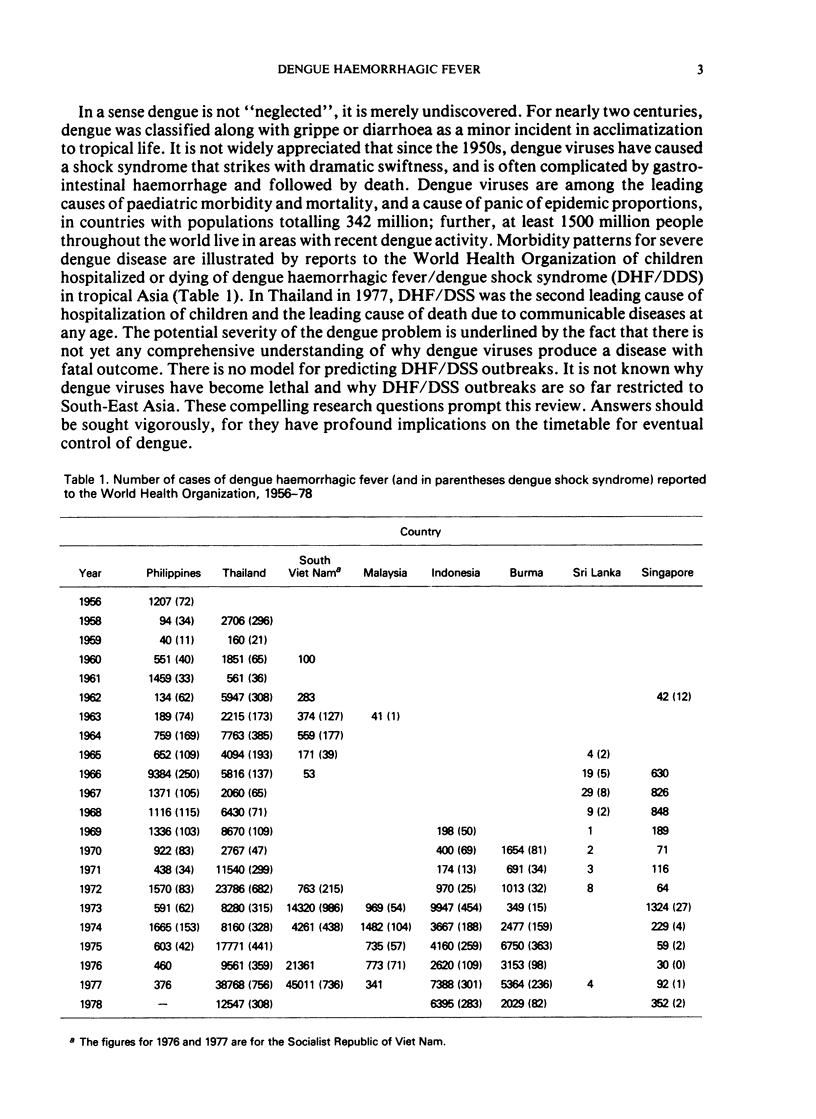
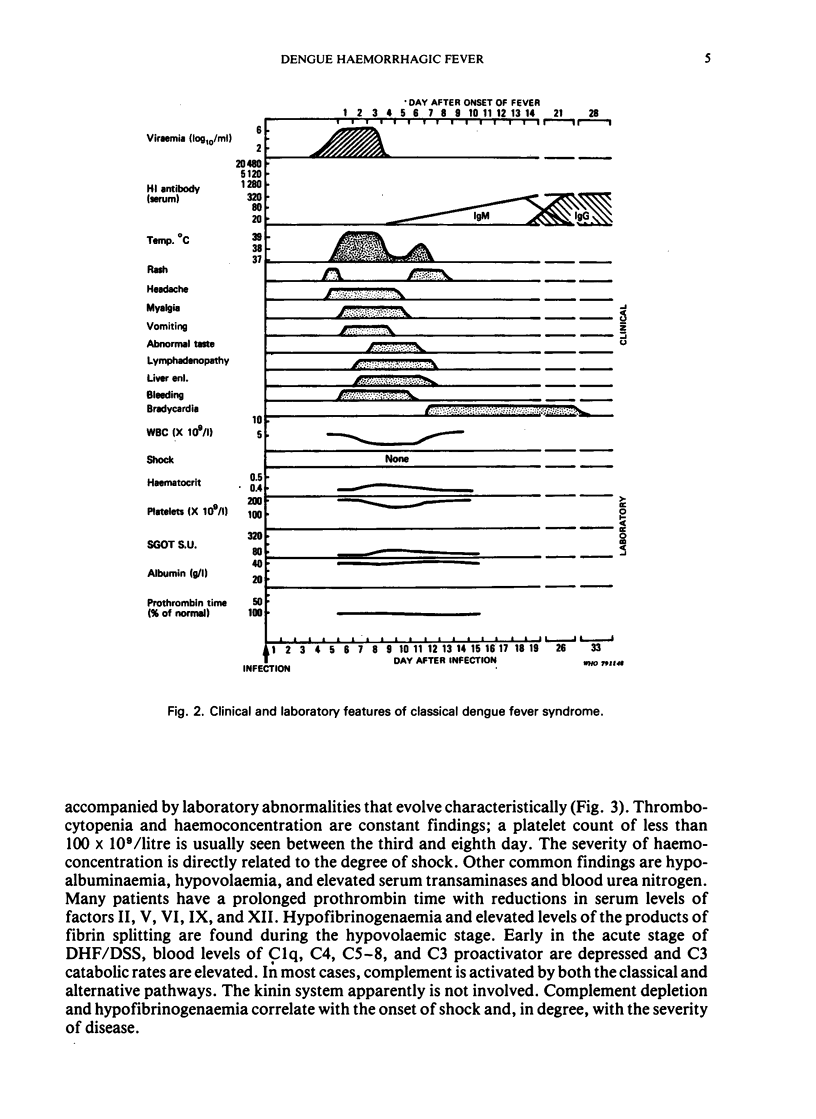
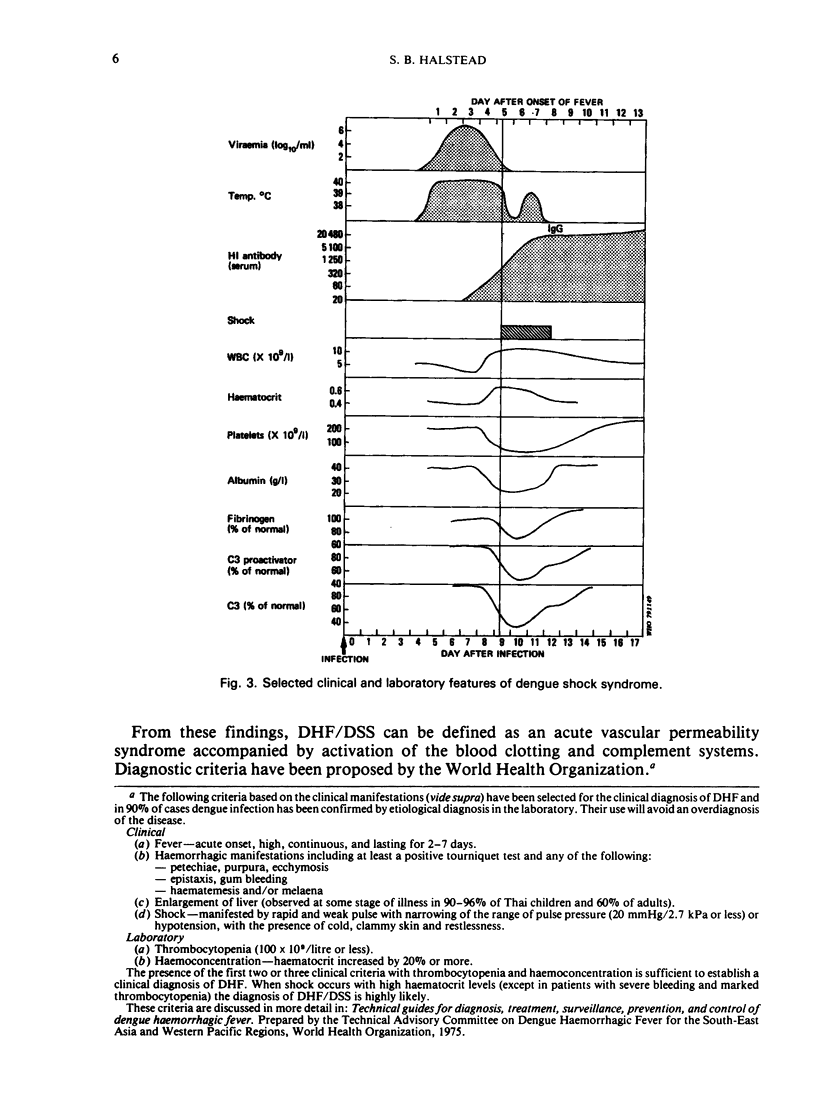
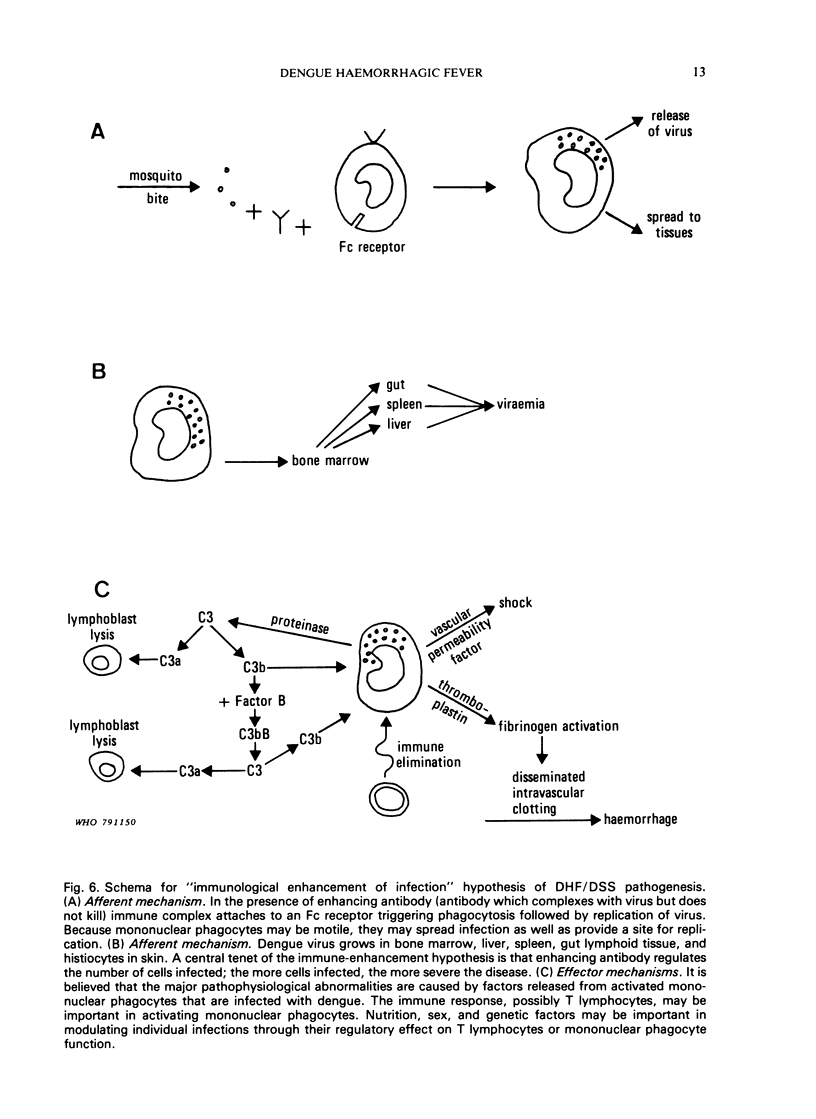
Dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS) is an enigmatic and growing public health problem which is confined at present to countries of South-East Asia. Since 1956, over 350 000 patients have been hospitalized and nearly 12 000 deaths have been reported. Dengue viruses, a group of four flaviviruses, are transmitted to man by Aedes aegypti. Currently, dengue viruses are actively transmitted in 61 countries which circle the globe in the tropical zone and have a combined population of 1500 million. Because the precise antecedents to DHF/DSS are unknown, the public health hazard posed by this syndrome is potentially worldwide. Epidemiological studies in South-East Asia clearly link DHF/DSS to individuals who have had a previous dengue infection or who have acquired maternal dengue antibody. Such antibody may serve as an opsonin, enhancing dengue virus infection of mononuclear phagocytes—the type of cell in man to which dengue infection may be confined. Antibody-mediated infection of these cells is the central concept in the hypothesis of immune infection enhancement. This hypothesis provides a conceptual framework for design of future research. There is an urgent need for a comprehensive identification of ”risk factors” in DHF/DSS. This research could be approached by undertaking comparative prospective epidemiological studies in dengue-endemic areas with and without DHF/DSS. Although important progress is being made in the development of attenuated dengue vaccines for each dengue type, a clearer understanding of the pathogenesis of DHF/DSS may be required to provide guidelines for safe and lasting immunoprophylaxis in man.
Full text
PDF


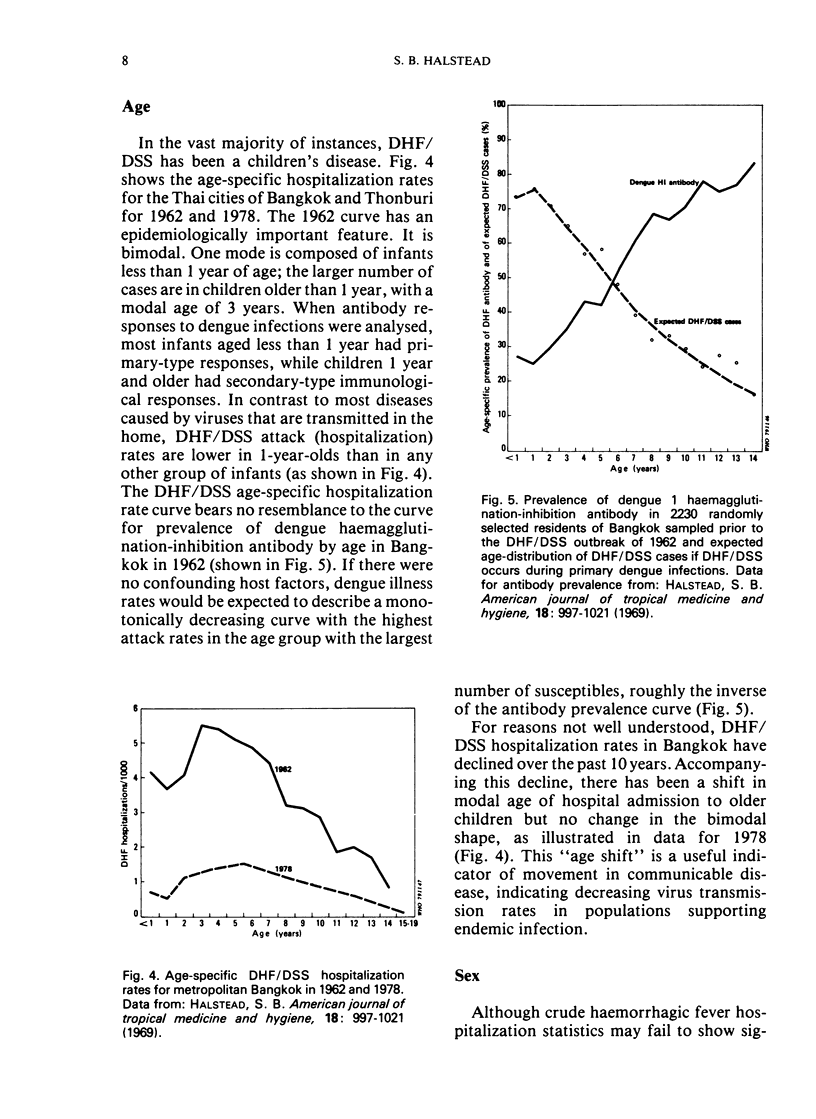
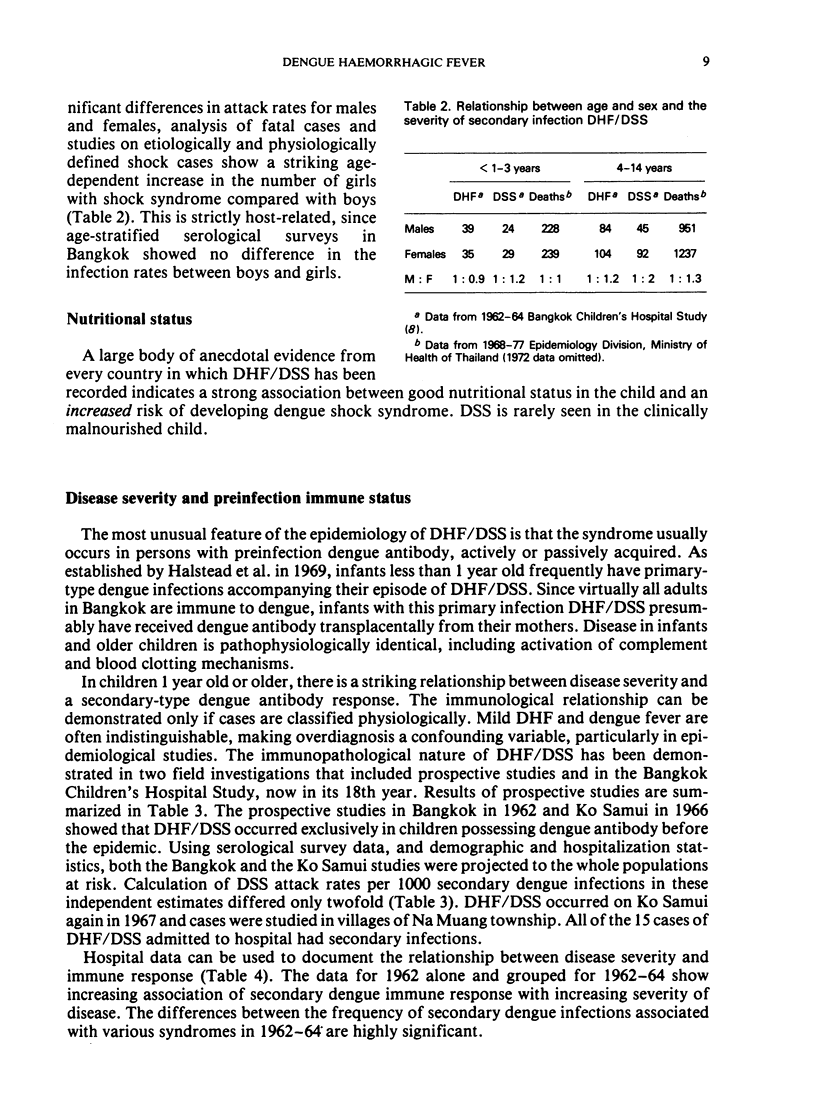
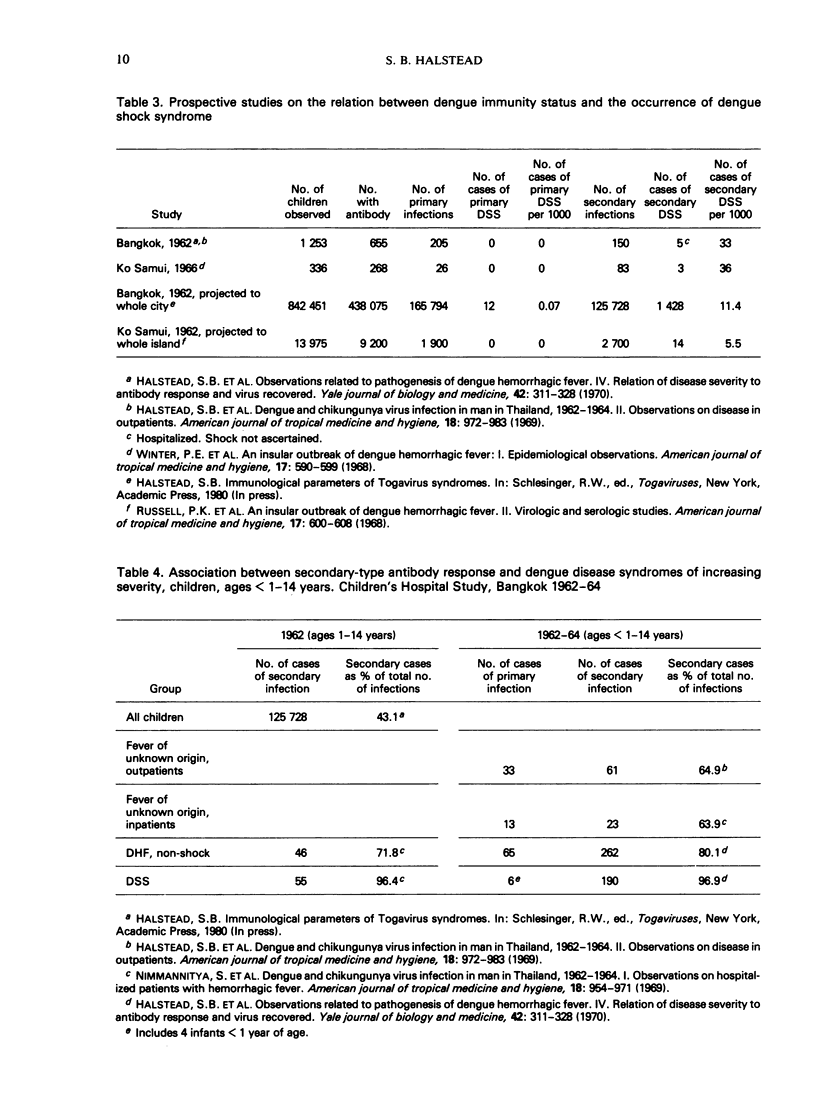
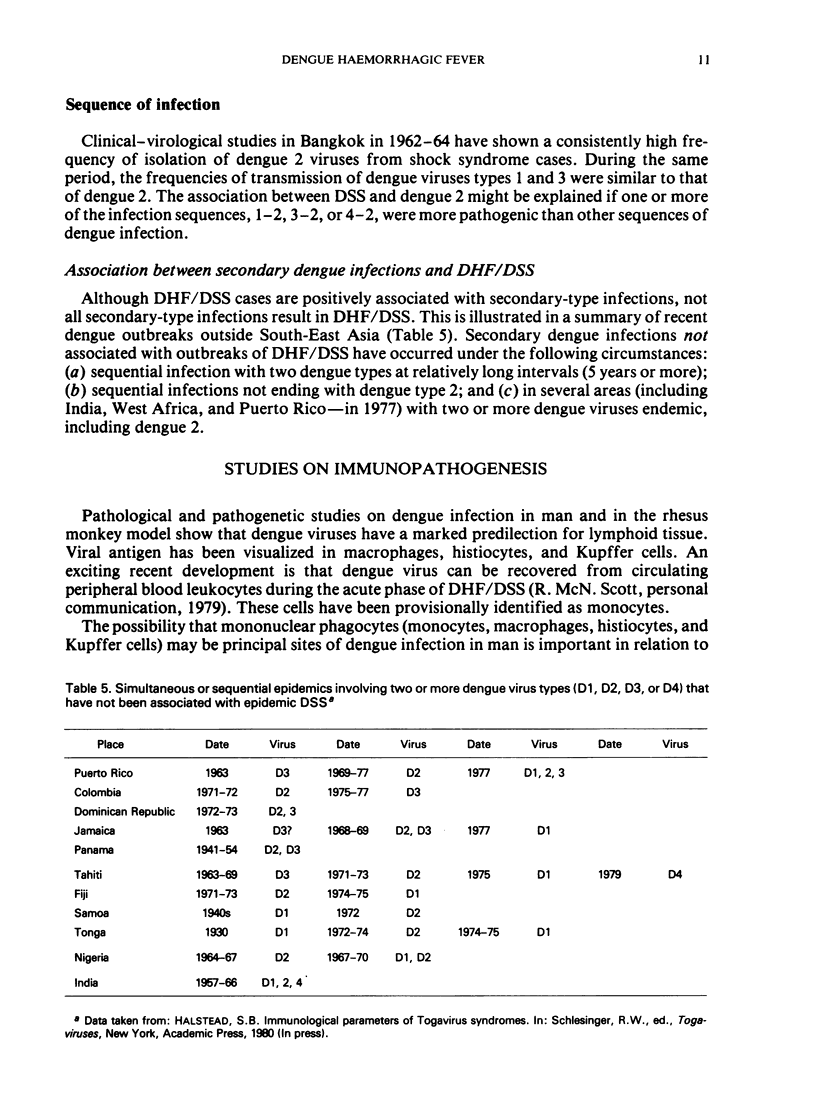









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eckels K. H., Brandt W. E., Harrison V. R., McCown J. M., Russell P. K. Isolation of a temperature-sensitive dengue-2 virus under conditions suitable for vaccine development. Infect Immun. 1976 Nov;14(5):1221–1227. doi: 10.1128/iai.14.5.1221-1227.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis. 1979 Oct;140(4):527–533. doi: 10.1093/infdis/140.4.527. [DOI] [PubMed] [Google Scholar]
- Halstead S. B. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis. 1979 Oct;140(4):527–533. doi: 10.1093/infdis/140.4.527. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., Nimmannitya S., Cohen S. N. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J Biol Med. 1970 Apr;42(5):311–328. [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., Nimmannitya S., Cohen S. N. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J Biol Med. 1970 Apr;42(5):311–328. [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., Nimmannitya S., Margiotta M. R. Dengue d chikungunya virus infection in man in Thailand, 1962-1964. II. Observations on disease in outpatients. Am J Trop Med Hyg. 1969 Nov;18(6):972–983. doi: 10.4269/ajtmh.1969.18.972. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., Nimmannitya S., Margiotta M. R. Dengue d chikungunya virus infection in man in Thailand, 1962-1964. II. Observations on disease in outpatients. Am J Trop Med Hyg. 1969 Nov;18(6):972–983. doi: 10.4269/ajtmh.1969.18.972. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977 Jul 1;146(1):201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., Shotwell H., Casals J. Studies on the pathogenesis of dengue infection in monkeys. II. Clinical laboratory responses to heterologous infection. J Infect Dis. 1973 Jul;128(1):15–22. doi: 10.1093/infdis/128.1.15. [DOI] [PubMed] [Google Scholar]
- Marchette N. J., Halstead S. B., Chow J. S. Replication of dengue viruses in cultures of peripheral blood leukocytes from dengue-immune rhesus monkeys. J Infect Dis. 1976 Mar;133(3):274–282. doi: 10.1093/infdis/133.3.274. [DOI] [PubMed] [Google Scholar]
- Nimmannitya S., Halstead S. B., Cohen S. N., Margiotta M. R. Dengue and chikungunya virus infection in man in Thailand, 1962-1964. I. Observations on hospitalized patients with hemorrhagic fever. Am J Trop Med Hyg. 1969 Nov;18(6):954–971. doi: 10.4269/ajtmh.1969.18.954. [DOI] [PubMed] [Google Scholar]
- Russell P. K., Yuill T. M., Nisalak A., Udomsakdi S., Gould D. J., Winter P. E. An insular outbreak of dengue hemorrhagic fever. II. Virologic and serologic studies. Am J Trop Med Hyg. 1968 Jul;17(4):600–608. doi: 10.4269/ajtmh.1968.17.600. [DOI] [PubMed] [Google Scholar]
- Winter P. E., Yuill T. M., Udomsakdi S., Gould D., Nantapanich S., Russell P. K. An insular outbreak of dengue hemorrhagic fever. I. Epidemiologic observations. Am J Trop Med Hyg. 1968 Jul;17(4):590–599. doi: 10.4269/ajtmh.1968.17.590. [DOI] [PubMed] [Google Scholar]



