Abstract
The minimal inhibitory concentration (MIC) values of penicillin, ampicillin, tetracycline, chloramphenicol, and streptomycin for 500 gonococcal strains were studied for product-moment coefficient correlations. The strains were isolated from male patients with acute gonococcal urethritis attending the Venereal Disease Clinic in Addis Ababa.
The MICs of all pairs except the ampicillin/streptomycin pair were positively correlated, with r = 0.20-0.53, P<0.001. The ampicillin/streptomycin pair was not correlated, r = 0.06, P>0.05.
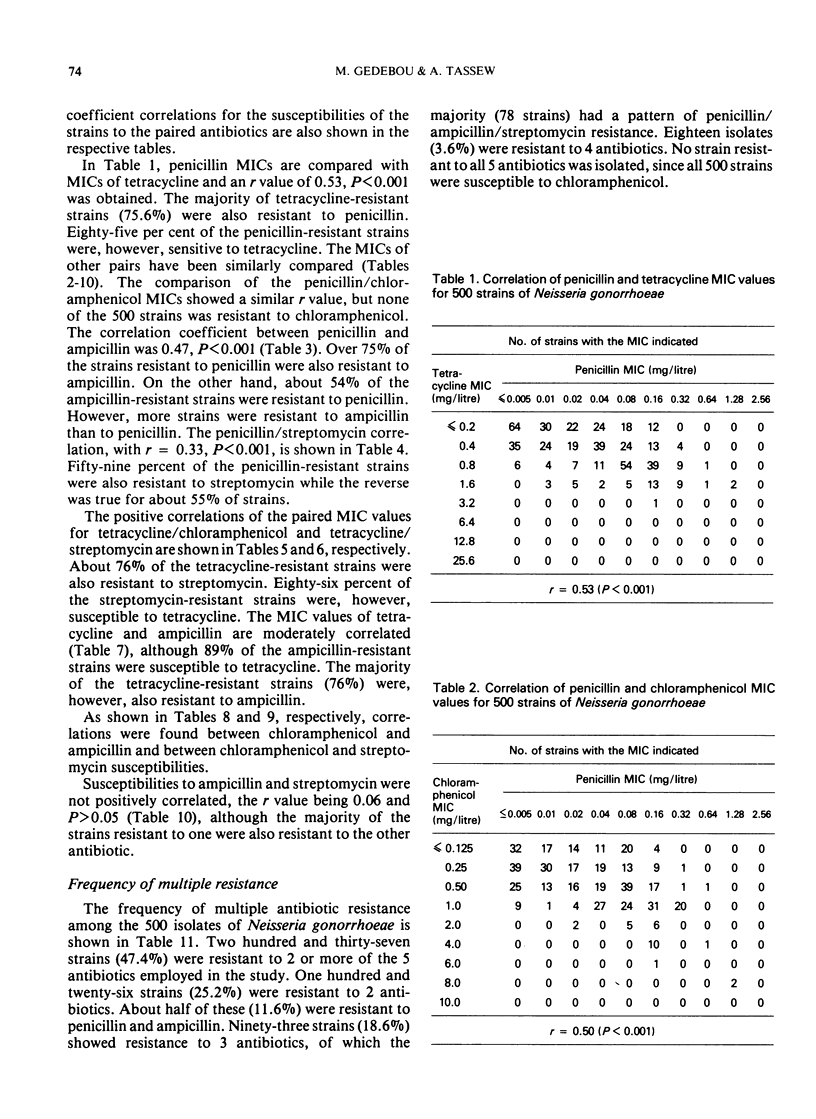
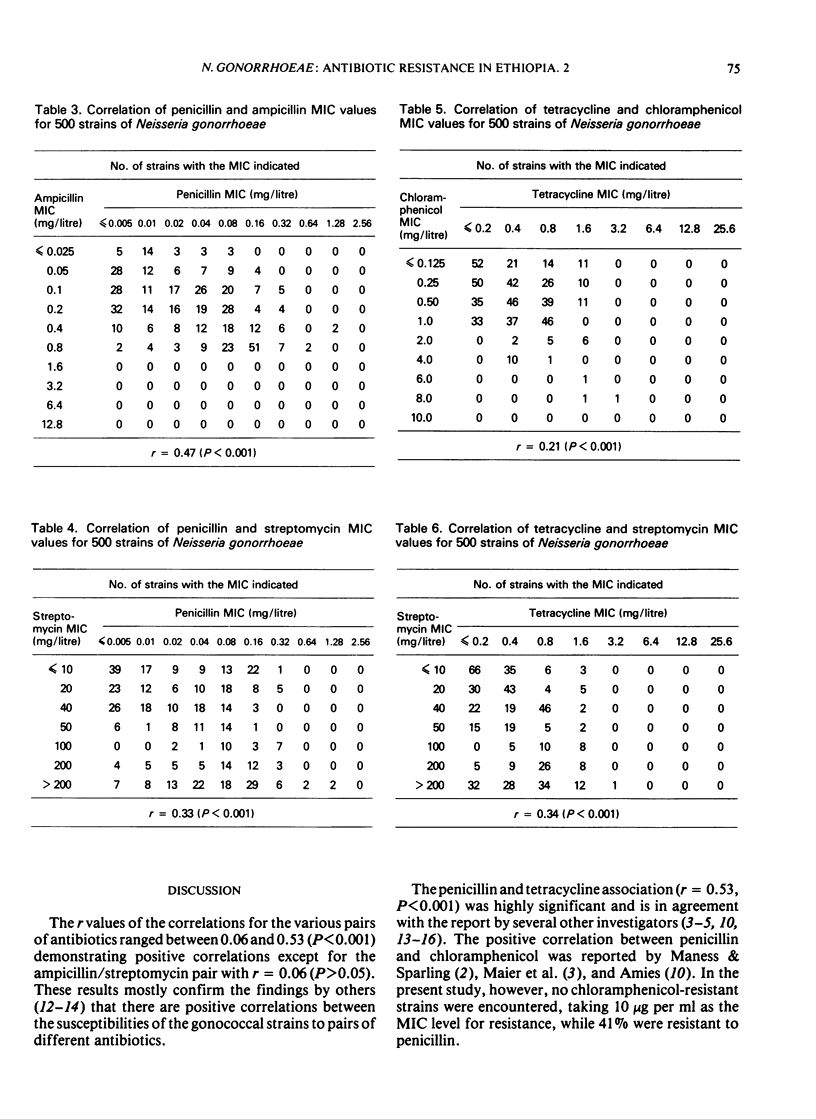
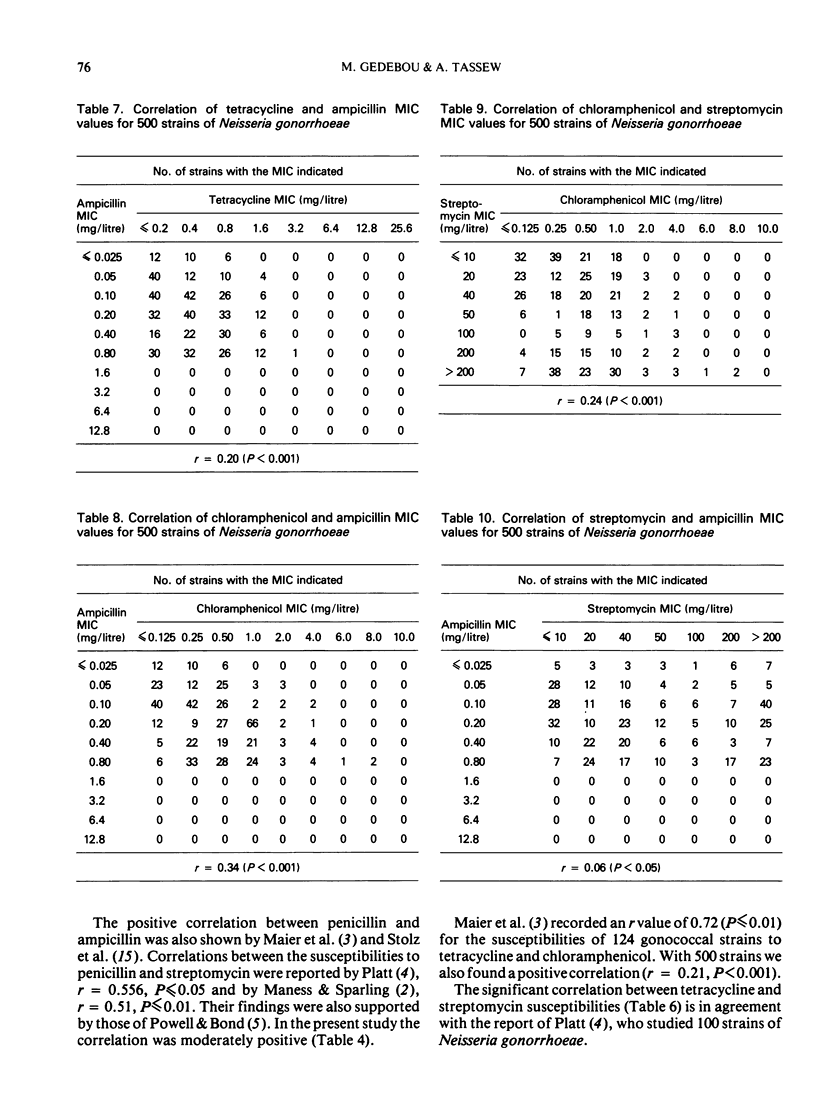
Despite the significant correlation between the susceptibilities of strains to penicillin and tetracycline, 85% of the penicillin-resistant strains were sensitive to tetracycline. However, 75.6% of tetracycline-resistant strains were also resistant to penicillin. Similar differences in percentages of resistance and susceptibility of strains also occurred between tetracycline and ampicillin as well as between tetracycline and streptomycin pairs, while pair correlations were also noted.
No chloramphenicol-resistant strain was isolated. But correlations were found between the MIC values of chloramphenicol and those of the other 4 antibiotics.
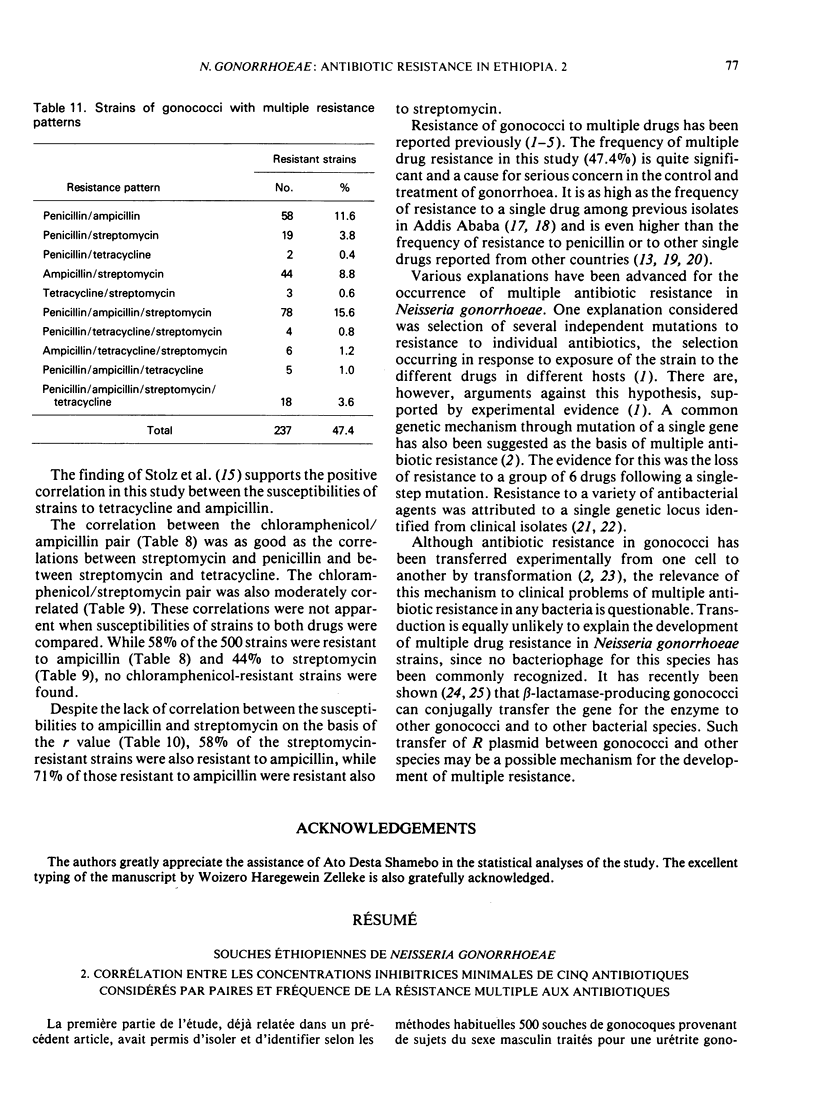
Forty-seven percent of the 500 strains were resistant to 2 or more antibiotics. One hundred and twenty-six strains (25.2%) were resistant to 2 antibiotics, about half of which were resistant to penicillin and ampicillin. Resistance to 3 antibiotics was shown in 18.6% of strains, the majority being resistant to penicillin, ampicillin, and streptomycin. Eighteen strains (3.6%) were resistant to 4 antibiotics, while no strain was found resistant to all 5 antibiotics.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amies C. R. Sensitivity of Neisseria gonorrhoeae to penicillin and other antibiotics. Studies carried out in Toronto during the period 1961 to 1968. Br J Vener Dis. 1969 Sep;45(3):216–222. doi: 10.1136/sti.45.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge R. W., Wallace C. K. Neisseria gonorrhoeae in Ethiopia: I. Antibiotic sensitivity pattern. Ethiop Med J. 1975 Jan;13(1):19–22. [PubMed] [Google Scholar]
- Eisenstein B. I., Sox T., Biswas G., Blackman E., Sparling P. F. Conjugal transfer of the gonococcal penicillinase plasmid. Science. 1977 Mar 11;195(4282):998–1000. doi: 10.1126/science.402693. [DOI] [PubMed] [Google Scholar]
- Gedebou M., Tassew A. Neisseria gonorrhoeae isolates from Ethiopia 1. In vitro susceptibility patterns to five antibiotics. Bull World Health Organ. 1980;58(1):67–71. [PMC free article] [PubMed] [Google Scholar]
- Gundersen T., Odegaard K., Gjessing H. C. Treatment of gonorrhoea by one oral dose of ampicillin and probenecid combined. Br J Vener Dis. 1969 Sep;45(3):235–237. doi: 10.1136/sti.45.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier T. W., Beilstein H. R., Zubrzycki L. Multiple antibiotic resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1974 Jul;6(1):22–28. doi: 10.1128/aac.6.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier T. W., Warner P., Zubryzycki L., Chila M. Identification of drug resistance loci in various clinical isolates of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1977 Sep;12(3):444–446. doi: 10.1128/aac.12.3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier T. W., Zubrzycki L., Coyle M. B. Genetic analysis of drug resistance in Neisseria gonorrhoeae: identification and linkage relationships of loci controlling drug resistance. Antimicrob Agents Chemother. 1975 May;7(5):676–681. doi: 10.1128/aac.7.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness M. J., Sparling P. F. Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae. J Infect Dis. 1973 Sep;128(3):321–330. doi: 10.1093/infdis/128.3.321. [DOI] [PubMed] [Google Scholar]
- Moses J. M., Desai M. S., Bhosle C. B., Trasi M. S. Present pattern of antibiotic sensitivity of gonococcal strains isolated in Bombay. Br J Vener Dis. 1971 Aug;47(4):273–278. doi: 10.1136/sti.47.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt D. J. Prevalence of multiple antibiotic resistance in Neisseria gonorrhoeae. Br J Vener Dis. 1976 Dec;52(6):384–386. doi: 10.1136/sti.52.6.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plorde J. J., Kidan T. G., Wright L. J. Penicillin sensitivity of gonococci in Ethiopia. Br J Vener Dis. 1973 Jun;49(3):260–262. doi: 10.1136/sti.49.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. T., Bond J. H. Multiple antibiotic resistance in clinical strains of Neisseria gonorrhoeae isolated in South Carolina. Antimicrob Agents Chemother. 1976 Oct;10(4):639–645. doi: 10.1128/aac.10.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYN A. Sensitivity of N. gonorrhoeae to antibiotics. Br J Vener Dis. 1961 Jun;37:145–157. doi: 10.1136/sti.37.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyn A., Bentzon M. W. Relationships between the sensitivities in vitro of Neisseria gonorrhoeae to spiramycin, penicillin, streptomycin, tetracycline, and erythromycin. Br J Vener Dis. 1969 Sep;45(3):223–227. doi: 10.1136/sti.45.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyn A., Benzon M. W. A study of the relationships between the sensitivities of Neisseria gonorrhoeae to sodium penicillin G, four semi-synthetic penicillins, spiramycin, and fusidic acid. Br J Vener Dis. 1968 Jun;44(2):140–150. doi: 10.1136/sti.44.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Falkow S. Conjugal transfer of R plasmids in Neisseria gonorrhoeae. Nature. 1977 Apr 14;266(5603):630–631. doi: 10.1038/266630a0. [DOI] [PubMed] [Google Scholar]
- Ronald A. R., Eby J., Sherris J. C. Susceptibility of Neisseria gonorrhoeae to penicillin and tetracycline. Antimicrob Agents Chemother (Bethesda) 1968;8:431–434. [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Sparling P. F. Transfer of antibiotic resistance in mixed cultures of Neisseria gonorrhoeae. J Infect Dis. 1974 Dec;130(6):660–663. doi: 10.1093/infdis/130.6.660. [DOI] [PubMed] [Google Scholar]
- Sparling P. F. Antibiotic resistance in Neisseria gonorrhoeae. Med Clin North Am. 1972 Sep;56(5):1133–1144. doi: 10.1016/s0025-7125(16)32339-2. [DOI] [PubMed] [Google Scholar]
- Stolz E., Zwart H. G., Michel M. F. Sensitivity to ampicillin, penicillin, and tetracyline of gonococci in Rotterdam. Br J Vener Dis. 1974 Jun;50(3):202–207. doi: 10.1136/sti.50.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watko L. P., Brownlow W. J. Antibiotic susceptibility of Neisseria gonorrhoeae isolated in the Western Pacific in 1971. Br J Vener Dis. 1975 Feb;51(1):34–37. doi: 10.1136/sti.51.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C., Rose D. L., Tramont E. C. Increased antibiotic resistance of Neisseria gonorrhoeae in Korea. Antimicrob Agents Chemother. 1976 Apr;9(4):716–718. doi: 10.1128/aac.9.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]



