Abstract
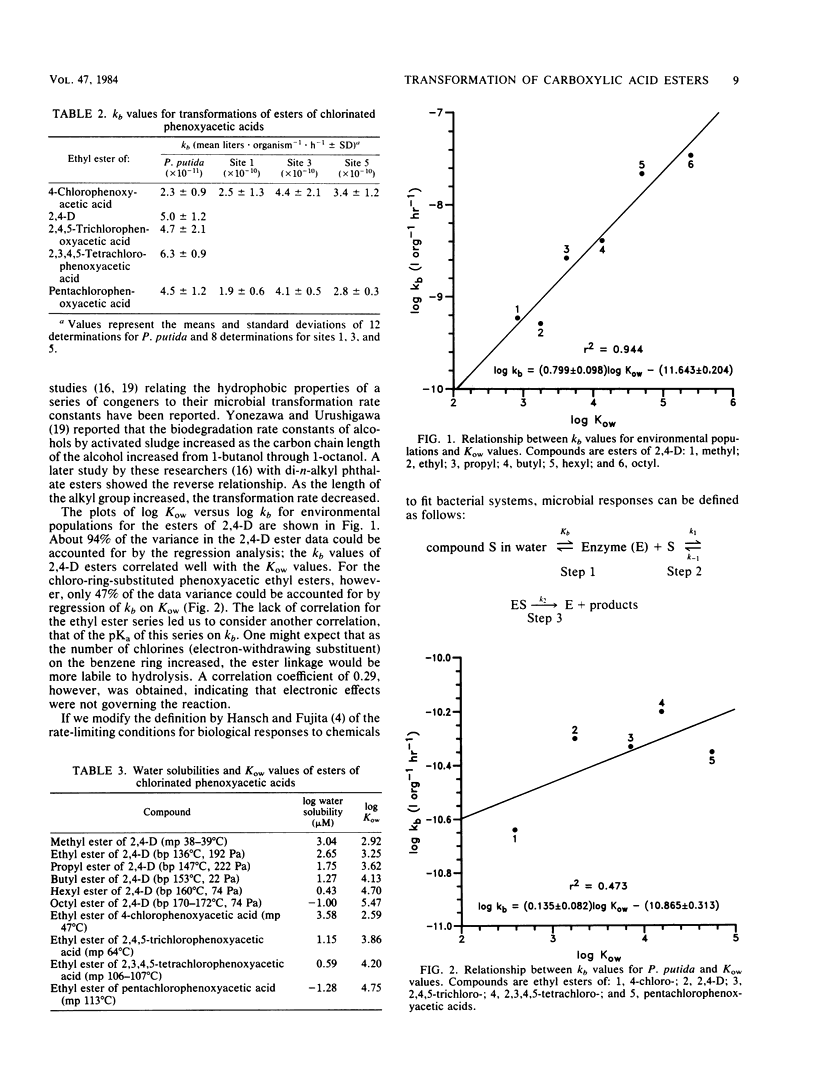
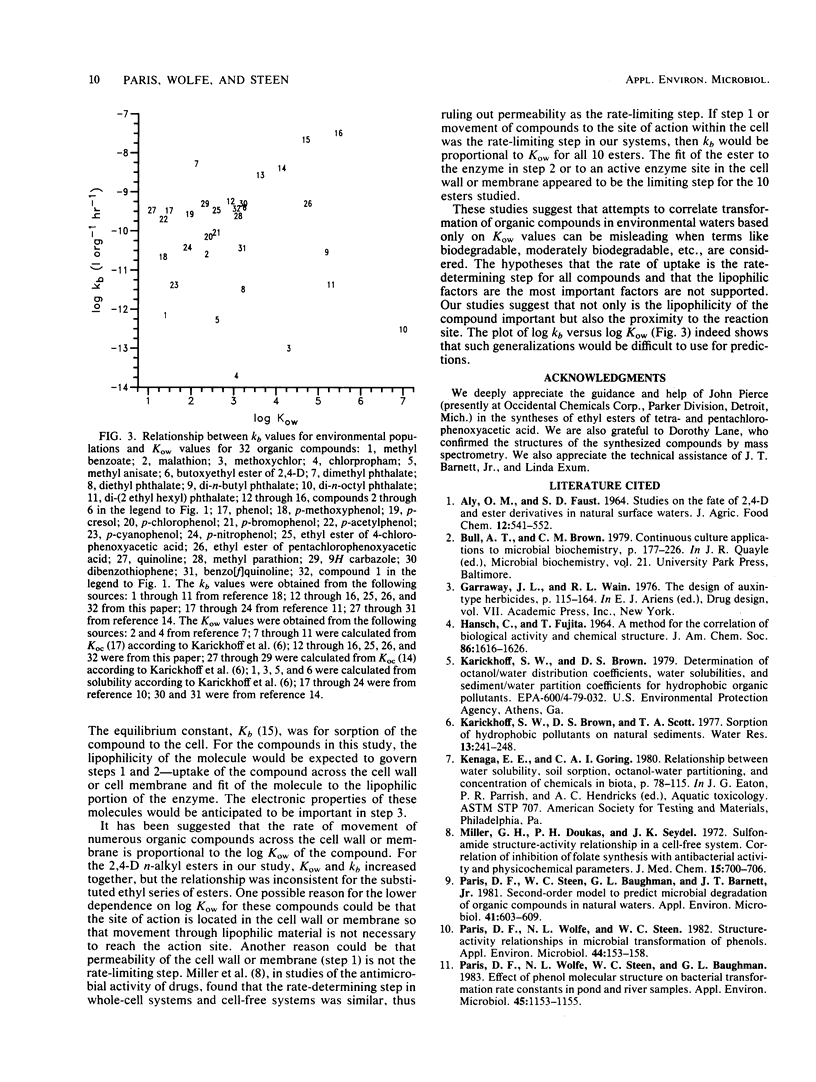
Two groups of compounds were selected for microbial transformation studies. In the first group were carboxylic acid esters having a fixed aromatic moiety and an increasing length of the alkyl component. Ethyl esters of chlorine-substituted carboxylic acids were in the second group. Microorganisms from environmental waters and a pure culture of Pseudomonas putida U were used. The bacterial populations were monitored by plate counts, and disappearance of the parent compound was followed by gas-liquid chromatography as a function of time. The products of microbial hydrolysis were the respective carboxylic acids. Octanol-water partition coefficients (Kow) for the compounds were measured. These values spanned three orders of magnitude, whereas microbial transformation rate constants (kb) varied only 50-fold. The microbial rate constants of the carboxylic acid esters with a fixed aromatic moiety increased with an increasing length of alkyl substituents. The regression coefficient for the linear relationships between log kb and log Kow was high for group 1 compounds, indicating that these parameters correlated well. The regression coefficient for the linear relationships for group 2 compounds, however, was low, indicating that these parameters correlated poorly.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Miller G. H., Doukas P. H., Seydel J. K. Sulfonamide structure-activity relationship in a cell-free system. Correlation of inhibition of folate synthesis with antibacterial activity and physicochemical parameters. J Med Chem. 1972 Jul;15(7):700–706. doi: 10.1021/jm00277a002. [DOI] [PubMed] [Google Scholar]
- PAYNE W. J., FEISAL V. E. Bacterial utilization of dodecyl sulfate and dodecyl benzene sulfonate. Appl Microbiol. 1963 Jul;11:339–344. doi: 10.1128/am.11.4.339-344.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Steen W. C., Baughman G. L., Barnett J. T. Second-order model to predict microbial degradation of organic compounds in natural waters. Appl Environ Microbiol. 1981 Mar;41(3):603–609. doi: 10.1128/aem.41.3.603-609.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Wolfe N. L., Steen W. C., Baughman G. L. Effect of phenol molecular structure on bacterial transformation rate constants in pond and river samples. Appl Environ Microbiol. 1983 Mar;45(3):1153–1155. doi: 10.1128/aem.45.3.1153-1155.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Wolfe N. L., Steen W. C. Structure-activity relationships in microbial transformation of phenols. Appl Environ Microbiol. 1982 Jul;44(1):153–158. doi: 10.1128/aem.44.1.153-158.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



